Abstract
A 52-year-old woman with relapsed acute lymphoblastic leukemia was diagnosed with Mycobacterium massiliense pneumonia after 4 months of chemotherapy. She developed M. massiliense bacteremia 1 month later. This is the first report of a proven case of M. massiliense bacteremia as a consequence of M. massiliense pneumonia.
CASE REPORT
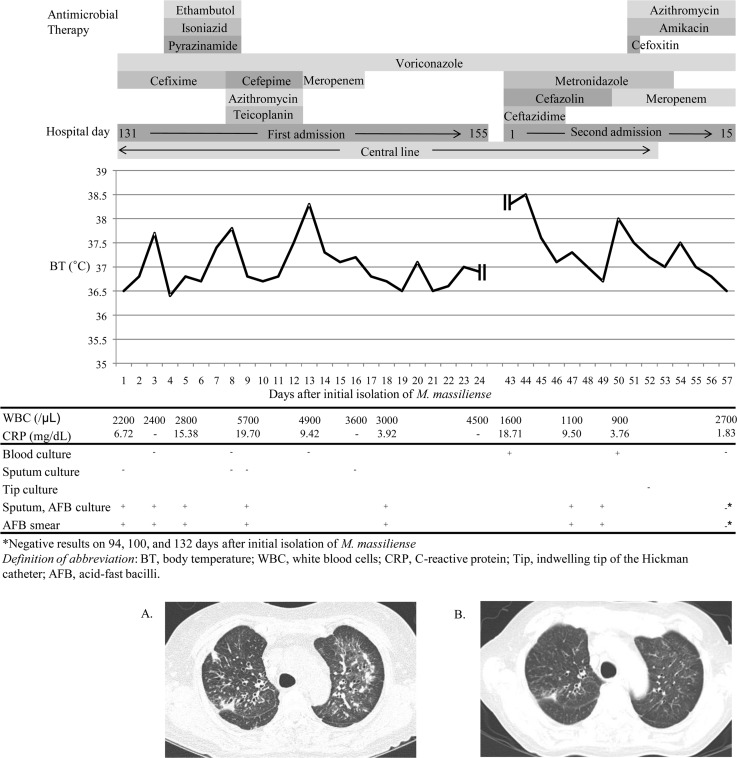
In November 2012, a 52-year-old woman with acute lymphoblastic leukemia diagnosed in November 2011 was admitted with relapsed disease. The patient was fitted with a Hickman catheter and treated for 3 months with alternating cycles of a combination of cyclophosphamide (300 mg/m2/day), vincristine (2 mg/day), doxorubicin (50 mg/m2/day), and dexamethasone (40 mg/day), followed by a combination of fludarabine (30 mg/m2/day), cytarabine (2 g/m2/day), granulocyte colony-stimulating factor (5 μg/kg/day), and idarubicin (10 mg/m2/day), as induction chemotherapy. During chemotherapy, pancytopenia was sustained for 3 months. On hospital day 14 (HD14), the patient's blood values were as follows: neutrophils, 9/μl; hemoglobin, 8.4 g/dl; and platelets, 53,000/μl. The patient had negative blood culture results 20 times at intervals of 2 days to 2 weeks from HD12 to HD140. Blood cultures were performed as a set using Bactec Plus Aerobic/F vials and Bactec Lytic/10 Anaerobic/F vials (BD Diagnostics, Sparks, MD) and were incubated in a Bactec FX blood culture system (BD Diagnostics). There was no evidence of abnormalities on transthoracic echocardiography (TTE), performed on HD20, HD47, and HD76. From HD76, the patient gradually developed an aggravated cough and sputum. Ill-defined diffuse centrilobular nodules and patchy infiltration in both lungs were detected by high-resolution computed tomography (HRCT) performed on HD133. The blood neutrophil count was 1,332/μl, and the C-reactive protein (CRP) was elevated (6.71 mg/dl). The patient's clinical course since then is shown in Fig. 1. Sputum and blood cultures for ordinary bacteria, PCR assays for the detection of Chlamydophila pneumoniae, Legionella pneumophila, and Mycoplasma pneumoniae by using the Seeplex pneumobacter detection system (Seegene, Seoul, South Korea), and PCR assays for 16 respiratory viruses by using the Anyplex II RV16 detection system (Seegene) were all negative. A sputum acid-fast bacillus (AFB) smear was positive, but real-time PCR analysis of a sputum specimen for the detection of Mycobacterium tuberculosis complex using the Cobas TaqMan MTB test (Roche Diagnostics, Mannheim, Germany) was negative. It was assumed from the AFB result and HRCT findings that the patient had tuberculosis; therefore, ethambutol, isoniazid, and pyrazinamide were administered empirically for 5 days from HD134, but on finding that the M. tuberculosis PCR assay was negative, this treatment was discontinued. AFB cultures from three consecutive sputum specimens all grew colonies of the Mycobacterium abscessus group, which was identified using a GenoType Mycobacterium assay (Hain Diagnostika, Nehren, Germany). Radiological and microbiological findings were consistent with nontuberculous mycobacterial (NTM) lung disease according to the diagnostic criteria of the American Thoracic Society (1), and no other bacterial, viral, or fungal causes were detected. TTE on HD137 revealed no evidence of endocarditis. The patient was discharged on HD155 with a neutrophil count of 3,606/μl. In April 2013, she was readmitted due to febrile neutropenia. On admission, her neutrophil count, hemoglobin level, and platelet count were 683/μl, 8.4 g/dl, and 97,000/μl, respectively, and her body temperature was 38.3°C. Cultures of a set of blood samples taken from the central line and of two sets of samples taken from peripheral blood grew a Gram-positive rod in the aerobic vials, with detection times of 80.7 h for the central line culture and 82.2 h and 95 h for the peripheral blood cultures. The organism appeared as tiny white colonies on blood agar plates after incubation for 2 days at 37°C. The colony morphologies of the sputum and blood isolates were similarly smooth (Fig. 2). The blood isolate had been identified as the M. abscessus group and, therefore, to identify the source of the bacteremia, species identification by PCR sequencing of hsp65 and rpoB (2) was performed on two blood isolates and seven sputum isolates. The following isolates and sequences were used as references: M. abscessus sensu stricto (ATCC 19977; GenBank accession number AY498743.1), Mycobacterium massiliense (CIP 108297; GenBank accession number HQ450848.1), and Mycobacterium bolletii (CIP 108541; GenBank accession number FJ607778.1). Both the blood and sputum isolates had the same sequences as that of M. massiliense CIP 108297. The initial isolates from blood and sputum were typed by pulsed-field gel electrophoresis (PFGE) of XbaI- and DraI-restricted genomic DNA (3). As a control, three isolates of M. massiliense and two of M. abscessus sensu stricto collected in early 2013 from other patients who were epidemiologically unrelated to this case were included. The pulsotypes of two isolates from this patient were identical to each other but different from those of five isolates from other patients (Fig. 2). A drug susceptibility test was performed on the M. massiliense isolates from blood and sputum by using Sensititre RAPMYCO plates (Trek Diagnostic Systems, East Grinstead, United Kingdom). Both were susceptible to amikacin and clarithromycin, but the blood isolate was also linezolid susceptible, whereas the sputum isolate was linezolid intermediate. Inducible macrolide resistance was not detected during prolonged incubation for 14 days. PCR for erm(41) revealed a 2-bp deletion of nucleotides 64 and 65 and a 276-bp deletion at a downstream locus, which was consistent with the identification of M. massiliense (4). Cultures of a set of blood samples taken from the Hickman catheter and of two sets of samples taken from peripheral blood on HD8 of the second admission were also positive for M. massiliense in the aerobic vials, with detection times of 84.9 h for the central line blood and 82.3 h and 85.4 h for the peripheral blood. The catheter was removed on HD10 of the second admission, and a culture of the tip was negative for any organisms after incubation for 2 days. Sputum AFB smears and cultures on HD5 and HD7 of the second admission were positive for M. massiliense. After treatment for 1 week with azithromycin (250 mg/day), amikacin (15 mg/kg/day), and cefoxitin (12 g/day), the fever subsided. On HD15 of the second admission, blood cultures were negative, and the CRP level had decreased to 1.83 mg/dl. As a result, the patient was transferred to a local hospital with improvement of her complete blood count (neutrophils 1,890/μl; hemoglobin 9.4 g/dl; and platelets, 34,000/μl). On follow-up at an outpatient clinic in June 2013, the patient was symptom free. Sputum AFB cultures and blood cultures were negative, and HRCT revealed less extensive centrilobular nodules and patchy infiltration in both lungs.
FIG 1.
Clinical course, antimicrobial therapy, and laboratory data of the patient. Chest computed tomography showed (A) bilateral interstitial infiltration on day 3 and (B) an improved state on day 78.
FIG 2.
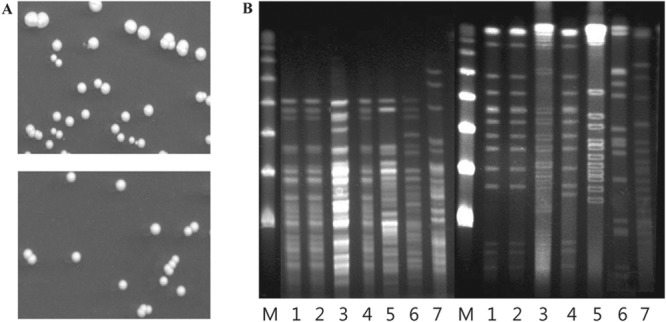
Colony morphologies and PFGE analysis of M. massiliense isolates. (A) Both sputum (top) and blood (bottom) isolates grew smooth white colonies, 2 mm in diameter, on Middlebrook 7H10 agar after incubation for 7 days at 37°C. (B) PFGE analysis of genomic DNA from bacterial isolates after digestion with XbaI (left set of lanes) and DraI (right set of lanes). The patient's sputum (lanes 1) and blood (lanes 2) isolates show identical profiles, while three M. massiliense isolates (lanes 3, 4, and 5) and two M. abscessus sensu stricto isolates (lanes 6 and 7) from epidemiologically unrelated patients each had distinct profiles. Lanes M, a lambda ladder PFGE marker (New England BioLabs, Ipswich, MA) was used as a size marker.
This is the first report of a microbiologically proven case of M. massiliense bacteremia as a consequence of a lung infection with the same strain. Bacteremia caused by an NTM infection is usually associated with central lines (5–7) or with iatrogenic infection by injection (8). There are several reports of M. abscessus group bacteremia associated with lung diseases (9–13). The lung is one of the most frequently involved sites in disseminated NTM infection in immunocompromised patients (7, 14) and is often speculated to be a portal of bacteremia in M. abscessus group bacteremia. Although M. massiliense was isolated from cultures of respiratory specimens as well as from blood cultures in a few cases of bacteremia (9–11), the lung was already affected by other causes, such as tuberculosis or ANCA-related vasculitis or aspergillosis. Therefore, M. massiliense could colonize (9, 11) or disseminate to the lung secondary to bacteremia (10). In the present case, NTM lung infection was diagnosed first in a patient without underlying lung disease, and bacteremia was diagnosed 1 month later. Immunosuppression is a major risk factor for both lung infection and disseminated NTM infection (9–11). Cell-mediated immunity is a major defense mechanism against NTM infections, in which gamma interferon (IFN-γ)-activated macrophages play a key role (15). The underlying disease and recent chemotherapy in the patient described here are likely to have compromised cell-mediated immunity, thereby contributing to the development of bacteremia. All isolates from the blood and respiratory specimens belonged to a smooth colony type (16) and had identical hsp65, rpoB, and erm(41) sequences. The PFGE pulsotype indicated that they arose from the same M. massiliense clone. The lung lesion preceded bacteremia and was the most likely route of invasion. However, as the patient's pulmonary infection was nosocomial and her central line was in place for 6 months, colonization of the central line could have been the portal for bacteremia, since coincidental exposure of the skin and respiratory tract to a common infectious source is possible in an acute health care setting (14). M. massiliense is an environmental Mycobacterium, and a contaminated medical environment or product is therefore a plausible source for nosocomial outbreaks or disseminated infections (12, 17). The finding that all three sets of blood cultures were positive twice was concordant with continuous and intravascular bacteremia. However, all blood cultures were negative before M. massiliense was isolated from sputum cultures, and M. massiliense was first detected in blood on HD1 of the second admission, 1 month after NTM lung disease was diagnosed. The negative culture from the tip of the central line and the <2-h difference in the time to detection for the vial from the central line blood and those from peripheral blood using the Bactec FX system are evidence against central line-associated bacteremia (18). Therefore, a diagnosis of M. massiliense bacteremia as a consequence of M. massiliense pneumonia was made.
The patient's bacteremia resolved, and the lung lesion responded well to therapy with azithromycin, amikacin, and cefoxitin. Pulmonary diseases involving M. massiliense have a better outcome than those with M. abscessus sensu stricto (19, 20). However, M. massiliense sepsis was fatal in the first two reported cases (9, 10). Although M. massiliense has been isolated from respiratory specimens from a few bacteremia cases (10, 11), M. massiliense is a frequent colonizer of immunosuppressed patients (7, 14, 21). Virulence causing fatal outcomes in cases of bacteremia has not yet been resolved. A recent report of a fatal case of acute respiratory failure in an immunocompetent elderly man in South Korea infected by M. massiliense (21) also indicates the virulence of M. massiliense in lung infection. Sepsis due to bacterial pneumonia most commonly occurs with more virulent organisms, such as Streptococcus pneumoniae (22). This case proved that M. massiliense is virulent enough to cause dissemination from pulmonary infection, at least in an immunocompromised host. In South Korea, the M. abscessus group is the second most frequent cause of NTM lung diseases after members of the Mycobacterium avium-Mycobacterium intracellulare complex (23) in immunocompromised hosts as well as immunocompetent hosts (24). M. massiliense is highly prevalent, accounting for nearly half of cases of M. abscessus group lung disease in South Korea (25). Therefore, it is a major concern that M. massiliense possibly causes bacteremia following chronic lung infection.
This case indicates that bacteremia is possible in cases of M. massiliense lung infection at least in immunocompromised patients. Although rapidly growing NTM can be detected in blood cultures (13, 26), they are possibly missed in routine blood culture due to insufficient incubation times (13, 27). Prolonged blood culture or terminal subculture would be useful to detect M. massiliense bacteremia sensitively. In conclusion, clinical microbiologists should be aware of the possibility of bacteremia and examine blood cultures more carefully in cases of M. massiliense lung infection in immunocompromised patients.
Footnotes
Published ahead of print 19 March 2014
REFERENCES
- 1.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367–416. 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 2.Macheras E, Roux AL, Ripoll F, Sivadon-Tardy V, Gutierrez C, Gaillard JL, Heym B. 2009. Inaccuracy of single-target sequencing for discriminating species of the Mycobacterium abscessus group. J. Clin. Microbiol. 47:2596–2600. 10.1128/JCM.00037-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace RJ, Zhang Y, Brown BA, Fraser V, Mazurek GH, Maloney S. 1993. DNA large restriction fragment patterns of sporadic and epidemic nosocomial strains of Mycobacterium chelonae and Mycobacterium abscessus. J. Clin. Microbiol. 31:2697–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastian S, Veziris N, Roux A-L, Brossier F, Gaillard J-L, Jarlier V, Cambau E. 2011. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob. Agents Chemother. 55:775–781. 10.1128/AAC.00861-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su SH, Chen YH, Tsai TY, Huang SC, Lin CY, Chen TC, Lu PL. 2013. Catheter-related Mycobacterium abscessus bacteremia manifested with skin nodules, pneumonia, and mediastinal lymphadenopathy. Kaohsiung J. Med. Sci. 29:50–54. 10.1016/j.kjms.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 6.El Helou G, Viola GM, Hachem R, Han XY, Raad II. 2013. Rapidly growing mycobacterial bloodstream infections. Lancet Infect. Dis. 13:166–174. 10.1016/S1473-3099(12)70316-X [DOI] [PubMed] [Google Scholar]
- 7.Redelman-Sidi G, Sepkowitz KA. 2010. Rapidly growing mycobacteria infection in patients with cancer. Clin. Infect. Dis. 51:422–434. 10.1086/655140 [DOI] [PubMed] [Google Scholar]
- 8.Liu R, To KK, Teng JL, Choi GK, Mok KY, Law KI, Tso EY, Fung KS, Wu TC, Wu AK, Fung SH, Wong SC, Trendell-Smith NJ, Yuen KY. 2013. Mycobacterium abscessus bacteremia after receipt of intravenous infusate of cytokine-induced killer cell therapy for body beautification and health boosting. Clin. Infect. Dis. 57:981–991. 10.1093/cid/cit443 [DOI] [PubMed] [Google Scholar]
- 9.Tortoli E, Gabini R, Galanti I, Mariottini A. 2008. Lethal Mycobacterium massiliense sepsis, Italy. Emerg. Infect. Dis. 14:984–985. 10.3201/eid1406.080194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobashi Y, Mouri K, Obase Y, Miyashita N, Nakanaga K, Oka M. 2011. Pulmonary Mycobacterium massiliense disease with septicemia during immunosuppressive treatment. Intern. Med. 50:1069–1073. 10.2169/internalmedicine.50.4733 [DOI] [PubMed] [Google Scholar]
- 11.Hamamoto T, Yuki A, Naoi K, Kawakami S, Banba Y, Yamamura T, Hikota R, Watanabe J, Kimura F, Nakanaga K, Hoshino Y, Ishii N, Shimazaki H, Nakanishi K, Tamai S. 2012. Bacteremia due to Mycobacterium massiliense in a patient with chronic myelogenous leukemia: case report. Diagn. Microbiol. Infect. Dis. 74:183–185. 10.1016/j.diagmicrobio.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 12.Kuo YM, Cheng A, Wu PC, Hsieh SC, Hsieh SM, Hsueh PR, Yu CL. 2011. Disseminated Mycobacterium abscessus infection and showerheads, Taiwan. Emerg. Infect. Dis. 17:2077–2078. 10.3201/eid1711.110050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki T, Ito W, Takeda M, Kobayashi N, Uek S, Sato K, Nakamura M, Tomita N, Kayaba H, Chihara J. 2011. Detection of Mycobacterium abscessus from blood cultures during treatment of interstitial pneumonia: a case study. Rinsho Byori 59:852–857 [PubMed] [Google Scholar]
- 14.Chou CH, Chen HY, Chen CY, Huang CT, Lai CC, Hsueh PR. 2011. Clinical features and outcomes of disseminated infections caused by non-tuberculous mycobacteria in a university hospital in Taiwan, 2004–2008. Scand. J. Infect. Dis. 43:8–14. 10.3109/00365548.2010.519345 [DOI] [PubMed] [Google Scholar]
- 15.Jonsson B, Ridell M, Wold AE. 2013. Phagocytosis and cytokine response to rough and smooth colony variants of Mycobacterium abscessus by human peripheral blood mononuclear cells. APMIS 121:45–55. 10.1111/j.1600-0463.2012.02932.x [DOI] [PubMed] [Google Scholar]
- 16.Kim BJ, Yi SY, Shim TS, Do SY, Yu HK, Park YG, Kook YH. 2012. Discovery of a novel hsp65 genotype within Mycobacterium massiliense associated with the rough colony morphology. PLoS One 7:e38420. 10.1371/journal.pone.0038420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HJ, Cho Y, Lee S, Kook Y, Lee D, Lee J, Park BJ. 2012. Mycobacterium massiliense outbreak after intramuscular injection, South Korea. Epidemiol. Infect. 140:1880–1887. 10.1017/S0950268811002809 [DOI] [PubMed] [Google Scholar]
- 18.Raad I, Hanna HA, Alakech B, Chatzinikolaou I, Johnson MM, Tarrand J. 2004. Differential time to positivity: a useful method for diagnosing catheter-related bloodstream infections. Ann. Intern. Med. 140:18–25. 10.7326/0003-4819-140-1-200401060-00007 [DOI] [PubMed] [Google Scholar]
- 19.Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Shin SJ, Huitt GA, Daley CL, Kwon OJ. 2011. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am. J. Respir. Crit. Care Med. 183:405–410. 10.1164/rccm.201003-0395OC [DOI] [PubMed] [Google Scholar]
- 20.Harada T, Akiyama Y, Kurashima A, Nagai H, Tsuyuguchi K, Fujii T, Yano S, Shigeto E, Kuraoka T, Kajiki A, Kobashi Y, Kokubu F, Sato A, Yoshida S, Iwamoto T, Saito H. 2012. Clinical and microbiological differences between Mycobacterium abscessus and Mycobacterium massiliense lung diseases. J. Clin. Microbiol. 50:3556–3561. 10.1128/JCM.01175-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi KH, Yu HM, Jeong JS, Kim SR, Lee YC. 2013. A fatal case of acute respiratory failure caused by Mycobacterium massiliense. Tuberc. Respir. Dis. (Seoul) 74:79–81. 10.4046/trd.2013.74.2.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, Torres A, Whitney CG. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44(Suppl 2):S27–S72. 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh WJ, Kwon OJ, Lee KS. 2005. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J. Korean Med. Sci. 20:913–925. 10.3346/jkms.2005.20.6.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh WJ, Kwon OJ, Lee KS. 2002. Nontuberculous mycobacterial pulmonary diseases in immunocompetent patients. Korean J. Radiol. 3:145–157. 10.3348/kjr.2002.3.3.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HY, Kook Y, Yun YJ, Park CG, Lee NY, Shim TS, Kim BJ, Kook YH. 2008. Proportions of Mycobacterium massiliense and Mycobacterium bolletii strains among Korean Mycobacterium chelonae-Mycobacterium abscessus group isolates. J. Clin. Microbiol. 46:3384–3390. 10.1128/JCM.00319-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin JH, Kim HR, Lee JN. 2005. Clinical significance and species identification of rapidly growing mycobacteria isolated from routine blood cultures. Korean J. Lab. Med. 25:162–167 [Google Scholar]
- 27.Kasuga E, Matsumoto T, Oana K, Shiohara M, Okabe T, Yamauchi K, Honda T, Ota H, Kawakami Y. 2007. Evaluation of BacT/Alert 3D SA bottles for accurate detection of mycobacteremia with special reference to Mycobacterium abscessus. Eur. J. Med. Res. 12:43–46 [PubMed] [Google Scholar]