Abstract
The aim of this report was to investigate whether the diagnosis of feline leukemia virus (FeLV) infection by serology might be feasible and useful. Among the various viral proteins, the FeLV env-gene product (SU) and the envelope transmembrane protein p15E were considered promising candidates for the serological diagnosis of FeLV infection. Thus, we evaluated p15E and three other FeLV antigens, namely, a recombinant env-gene product, whole FeLV, and a short peptide from the FeLV transmembrane protein, for their potential to detect FeLV infection. To evaluate possible exposure of cats to FeLV, we tested serum and plasma samples from experimentally and naturally infected and vaccinated cats for the presence of antibodies to these antigens by enzyme-linked immunosorbent assays (ELISAs). The serological results were compared with the p27 and proviral real-time PCR results. We found that p15E displayed a diagnostic sensitivity of 95.7% and a specificity of 100% in experimentally infected cats. In naturally infected cats, p15E showed a diagnostic sensitivity of 77.1% and a specificity of 85.6%. Vaccinated cats displayed minimal antibody levels to p15E, suggesting that anti-p15E antibodies indicate infection rather than vaccination. The other antigens turned out to be too unspecific. The lower specificity in cats exposed to FeLV under field conditions may be explained by the fact that some cats become infected and seroconvert in the absence of detectable viral nucleic acids in plasma. We conclude that p15E serology may become a valuable tool for diagnosing FeLV infection; in some cases, it may replace PCR.
INTRODUCTION
Infection with the feline leukemia virus (FeLV) (1) is of veterinary relevance (2, 3), although its importance differs in most study populations (4, 5). The disease outcome in infected cats is usually defined according to the presence of provirus and viral antigen in the blood (6–8). However, it is highly unpredictable because it is dependent on factors like the virus subtype (9) and the age (10) and the general condition of the cat.
The diagnosis of FeLV infection is mainly based on the detection of virus or viral antigen in the plasma, serum, or whole blood. The most common serological tests detect the presence of either p27 antigen by an enzyme-linked immunosorbent assay (ELISA) (11) or FeLV structural antigens in the cytoplasm of infected leukocytes and platelets by an immunofluorescence antibody test (IFA) (12, 13). Moreover, Western blot analysis detects the presence of specific FeLV antibodies. Alternatively, nonserological methods include virus isolation (29) or PCR to detect the proviral (FeLV DNA) load or viral (FeLV RNA) load (15–17). However, due to the laborious and/or cost-intensive character of most of these methods, they are not all suitable for clinical use.
It is known that infected cats are able to elicit antibodies against different components of FeLV (18–22). However, until now the detection of antibodies to FeLV had limited significance for several reasons: first, there is no evidence that reliable antibody detection can predict FeLV infection; second, it is not known which antibodies are suitable; and third, the existence of endogenous FeLV (enFeLV) is widespread in cat populations in that every cell in every single cat harbors multiple copies of enFeLV (23, 24). As enFeLV is not completely tolerated by the immune system, antibodies which are indistinguishable from antibodies to exogenous FeLV are elicited (25). Only a few techniques, e.g., real-time PCR, are able to distinguish between endogenous and exogenous FeLV (26). Thus, FeLV antibodies so far have been not considered to be useful as diagnostic parameters. Moreover, several studies failed to detect a sufficient antibody response against various epitopes of FeLV. Fontenot and coworkers (27) analyzed the reactivity of a predicted FeLV transmembrane immunodominant domain (Imd-TM peptide) and investigated its potential as a diagnostic reagent in serology. It was revealed that this peptide displayed only negligible levels of reactivity using sera from FeLV-infected cats, rendering the Imd-TM peptide as not qualified for FeLV diagnosis. Langhammer and coworkers (25) produced recombinant FeLV p15E and showed that cats infected with FeLV developed antibodies against p15E, although the reactions in ELISAs were low. Epitope mapping revealed a variety of epitopes recognized by sera from FeLV-infected animals, including epitopes detected by sera from p15E-immunized cats, but weaker. They concluded that natural FeLV infection results in a weak induction of binding antibodies specific for p15E and a low induction of neutralizing antibodies. However, Lutz and coworkers (22) qualitatively and quantitatively compared the antibody levels to different FeLV components in naturally infected cats and found that p15E exhibited strong antigenicity. They observed that cats that became immune or viremic after infection displayed elevated levels of antibodies to p15E. They concluded that antibodies to p15E indicate FeLV infection but may have little involvement in virus neutralization. With these results in mind, we hypothesized that the FeLV transmembrane (TM) envelope protein p15E and other viral proteins may have the potential to be a useful diagnostic tool in serology. We therefore evaluated p15E, a recombinant FeLV env-gene product (p45), whole virus (FL-74), and a short synthetic peptide (EPK211) derived from the TM unit of the FeLV envelope protein. Using indirect ELISAs, we systematically screened sera from naturally and experimentally infected and immunized cats. For each sample, the results of provirus PCR, p27 in plasma, and immunization status were known.
MATERIALS AND METHODS
Antigens for ELISA.
We evaluated four antigen preparations.
(i) p15E.
The whole TM subunit without the membrane-spanning helix part of the viral envelope protein of FeLV was cloned and purified in our laboratories. Briefly, the amino acid sequences 446 to 582 and 626 to 642 of FeLV subtype A (FeLV-A) (GenBank accession no. AAA93093.1) were cloned into the pET-16b expression vector (Novagen; Merck Millipore, Switzerland) and expressed in Escherichia coli BL21-CodonPlus (DE3)-RIL cells (Stratagene). The fusion protein containing p15E was purified by affinity chromatography. Afterward, it was dialyzed against 7.5 mM Tris-HCl buffer, pH 8.0. The protein product was verified with Western blot analysis using a primary in-house monoclonal mouse ascites antibody in a dilution of 1:6,000 to specifically detect p15E and a secondary goat anti-mouse peroxidase (PO)-conjugated antibody (Jackson ImmunoResearch Laboratories, Inc., PA) in a dilution of 1:1,000. The purity of p15E, i.e., the presence of potential E. coli residuals in p15E, was determined using three different E. coli sera tested with ELISAs (Fig. 1); see the Enzyme-Linked Immunosorbent Assays section.
FIG 1.
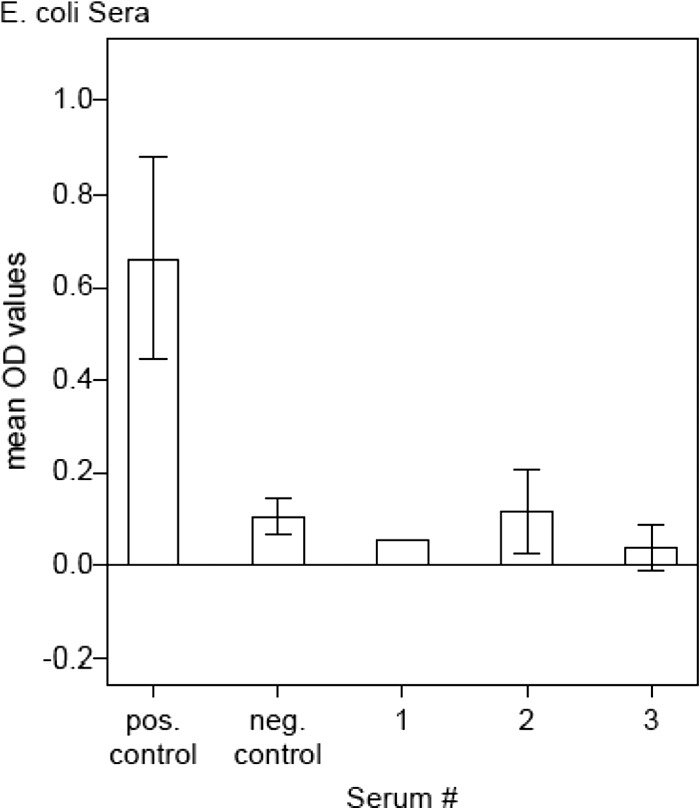
Purity of recombinant p15E. Mean OD values of a positive and a negative cat control serum sample and three E. coli serum samples (nos. 1 to 3) tested with antibody ELISAs using the recombinant p15E cloning product as antigen. Compared to the positive and negative controls, E. coli samples 1 to 3 reached only low OD levels (<0.117).
(ii) p45.
The nonglycosylated recombinant variant of the gp70 surface unit (vaccine antigen) of the envelope protein of FeLV-A was purchased from Virbac Schweiz AG (Glattbrugg, Switzerland). This antigenic suspension (Leucogen) is adjuvanted with 0.1 ml of a 3% aluminum hydroxide gel and 10 μg of purified extract of Quillaja saponaria.
(iii) Whole virus (FL-74).
Sucrose gradient-purified whole FeLV was available in our laboratories. Whole virus originated from FL-74 cells, a lymphoblastoid cell line chronically infected with FeLVABC (28).
(iv) EPK211.
This 15-amino acid-long synthetic peptide (Ac-GWFEGWFNRSPWFTT-NH2) mimics a short part of the carboxy-terminal region of the FeLV TM subunit of the viral envelope protein. It was expressed by solid-phase peptide synthesis and purified by analytical high-performance liquid chromatography (purity >85%) in the laboratories of EspiKem Srl (Florence, Italy).
Serum and plasma samples.
We used serum and plasma samples from different laboratory animals as well as from privately owned animals.
The samples from the first study (9/group) done in 2006 (8) are defined as follows. Group 1 served as unvaccinated control animals. Group 2 was vaccinated with Eurifel (now known as Purevax; Merial, Lyon, France). Eurifel is a nonadjuvanted canarypox-vectored live vaccine (ALVAC) containing FeLV-A env, gag, and part of pol. Group 3 received Fel-O-Vax LV-K IV (Fort Dodge, IA, USA), which is a polyvalent killed whole-virus FeLV vaccine. Vaccines were injected twice subcutaneously. Four weeks after the second vaccination, each cat was challenged with FeLV-A/Glasgow-1 (14).
The samples from the second vaccination study (15/group) (H. Lutz and R. Hofmann-Lehmann, unpublished data) are defined as follows. Group 1 served as an unvaccinated control group. Group 2 received Fevaxyn FeLV (Solvay Animal Health, Inc., MN, USA), which consists of inactivated (or killed) antigen. Group 3 was vaccinated with Fel-O-Vax LV-K IV (Fort Dodge), and group 4 received Leucogen (Virbac). Vaccines were injected twice subcutaneously. Four weeks after the second vaccination, each cat was challenged with FeLV-A/Glasgow-1 (14). In both studies, blood samples were collected prior to vaccination and on the day of challenge exposure. Thereafter, samples were collected weekly until week 15. Furthermore, we used plasma samples from the unvaccinated control groups from week 8 or 10 postchallenge from Pfizer (Kent, United Kingdom), Merial (Lyon, France), and Virbac (Glattbrugg, Switzerland) (n = 47). For longitudinal effects on antibodies, we used sera from the unvaccinated but challenged control groups from week 8. Moreover, we used the plasma samples from naive, unchallenged, and unvaccinated specific-pathogen-free (SPF) young cats (n = 94) from the different vaccination studies and from old unchallenged SPF cats (n = 10) available in our laboratories from an earlier study. To test the reactivity of vaccinated cats, we used all groups from the vaccination studies mentioned above and tested the samples after several vaccine applications but before challenge with FeLV-A.
Additionally, serum samples from privately owned cats (n = 294) in Switzerland were used. The samples already existed in our laboratories and were collected from April 2004 until January 2005 (8). The veterinarians who had submitted these samples to us also provided us with the vaccination records of the cats. For all of the samples we tested, PCR (provirus) and ELISA (p27) results were known. For all experiments involving research animals, the laboratory director (H. Lutz) had obtained from the state veterinary office the necessary animal permits. All experiments were done in full agreement with all relevant legal requirements.
Enzyme-linked immunosorbent assays.
ELISAs were based essentially on methods described earlier (30, 31). Anti-FeLV p45 and anti-FeLV whole-virus (FL-74) antibodies were measured by ELISAs as previously described (22, 32). p45 and whole virus were used at final concentrations of 100 ng/well. For EPK211 and p15E, ELISAs were newly established to find optimal signal-to-noise ratios. Before coating, the antigen (100 μg/ml) was boiled for 1 min at 95°C in a 10% sodium dodecyl sulfate (SDS) (Sigma-Aldrich) solution and then diluted in 0.1 M carbonate coating buffer, pH 9.6 (Na2CO3 water-free) (Sigma-Aldrich), to final concentrations of 0.25 ng/μl (antigen) and 0.005% (SDS). Then 100 μl of this solution was added to each well of a flat-bottom 96-well ELISA plate (TPP, Trasadingen, Switzerland) and incubated for 3 h at 37°C. Plates were either used after incubation or stored at −20°C. The assay was performed as follows. In order to block the remaining empty spaces in the wells to avoid unspecific binding, 1% bovine serum albumin (BSA) (Sigma-Aldrich) in P3x buffer, pH 7.4 (0.15 M sodium chloride, 1 mM EDTA, 0.05 M Tris-base, 0.1% BSA, 0.1% Tween 20), was added and incubated for 1 h (37°C). Wells were washed three times with ELISA wash buffer, pH 7.4 (0.15 M sodium chloride, 0.2% Tween 20). Serum and plasma samples were diluted 1:200 in P3x, and 100 μl/well was added to the wells. After incubation for 1 h (37°C) and three washing steps, a goat anti-cat IgG (H+L) PO-conjugated secondary antibody (Milan Analytica AG, Rheinfelden, Switzerland) diluted 1:3,000 in P3x was added (100 μl/well). After another 1 h of incubation (37°C) and three washing steps, 100 μl of a substrate solution consisting of 0.2 M citric acid, pH 4.0 (98 vol%), 2% hydrogen peroxide (1 vol%), and 40 mM ABTS [2, 2′-azino-di(3-ethylbenzthiazoline-6-sulfonate)] (1 vol%), (all chemicals were from Sigma-Aldrich) was added. After 10 min (room temperature), optical density (OD) values were measured at 415 nm with a spectrophotometer (SpectraMax Plus 384; Molecular Devices, Sunnyvale, CA). Assays run on different days were compared by using 2 control sera as internal standards with every assay; one had a strong reactivity to FeLV and the other was negative.
p15E purity was tested with an ELISA under the same conditions mentioned above using three different rabbit sera (kindly provided by M. Wittenbrink) containing antibodies against specific epitopes of E. coli. A secondary goat anti-rabbit IgG (H+L) PO-conjugated antibody (Milan Analytica) was used to detect any signal. For the negative and positive controls, cat sera and an AffiniPure goat anti-cat IgG (H+L) PO-conjugated secondary antibody (Milan Analytica) was used.
PCR.
Exogenous FeLV provirus sequences present in blood leukocytes were detected and quantitated by a real-time PCR under conditions described earlier (26). This PCR amplifies a 74-bp sequence within the FeLV U3 long terminal repeat (LTR) portion specific for exogenous FeLV.
Data analysis and statistics.
ELISA OD values were used as standardized OD values: (OD value [sample] − OD value [negative control]/(OD value [positive control] − OD value [negative control]).
Software package NCSS 2007 version 07.1.20 (NCSS LLC, Kaysville, UT, USA) and PASW statistics software version 18.0.2 (Polar Engineering and Consulting, Nikiski, AK) were used for the receiver operating characteristic (ROC) analyses, and Win Episcope 2.0 software (Borland International Inc., CA, USA) was used for calculation of the kappa values. To compare the differences in the mean values between the old SPF cats and the young SPF cats, one-way analysis of variance (ANOVA) and the Welch/Brown-Forsythe test was used.
RESULTS
Purity of recombinant p15E.
Unlike the other three antigens that were already available for antibody ELISA, the FeLV transmembrane protein p15E had to be specifically synthesized and purified in our laboratories. After cloning in E. coli cells and demonstration of the product's presence by Western blot analysis, p15E was highly purified and tested for possible E. coli contaminants. Three different sera (kindly provided by M. Wittenbrink) from rabbits immunized with E. coli antigens were used to test the levels of antibodies directed to E. coli contaminants of the p15E cloning product. p15E antibody ELISA revealed that OD values were maximally 0.117 (serum 2), which is comparable to the negative control (naive cat serum, OD = 0.106) (Fig. 1). The other two E. coli sera (sera 1 and 3) exhibited even lower OD values (OD < 0.05), which were considered negligible. These results indicated that our p15E cloning product was highly pure and could be used for ELISAs without cross-reactions due to E. coli contaminants.
Antibody levels in experimentally infected cats.
Receiver operating characteristic (ROC) curve analysis showing a plot of the true-positive rate (sensitivity) against the false-positive rate (1-specificity) was used to evaluate the diagnostic utility of the different FeLV antigens and to compare experimentally infected cats with naturally infected cats.
In the first ROC analysis for experimentally infected animals, cutoff points resulting in optimal test specificity and sensitivity were determined to discriminate uninfected, provirus-negative, and specific-pathogen-free (SPF) cats from infected, provirus-positive (seroconverted) cats. To have a defined and comparable sample set of cats of the same age, the old SPF cats (n = 10) were excluded from this first study.
Evaluation of the antibody levels of experimentally infected cats represented a proof-of-principle test. Using provirus as a gold standard, the ROC curve for those antibody levels is represented in Fig. 2a. As a rule, the cutoff was defined as specificity ≥80% and sensitivity × specificity as the maximum (Fig. 2b). Antigen p15E exhibited an excellent fit, following the left-hand border and the top border of the ROC space. An optimal OD cutoff of 0.0495 represented the best trade-off between sensitivity, which was found to be 95.7%, and specificity, which was found to be 100%. The optimal cutoff (−0.039) for EPK211 displayed a sensitivity of 65.7% and a specificity of 81.1%, whereas p45 (cutoff 0.016) revealed a sensitivity of 80% and a specificity of 88.9%. Whole virus displayed a sensitivity of 65.7% and a specificity of 81.1% with a cutoff of −0.007.
FIG 2.
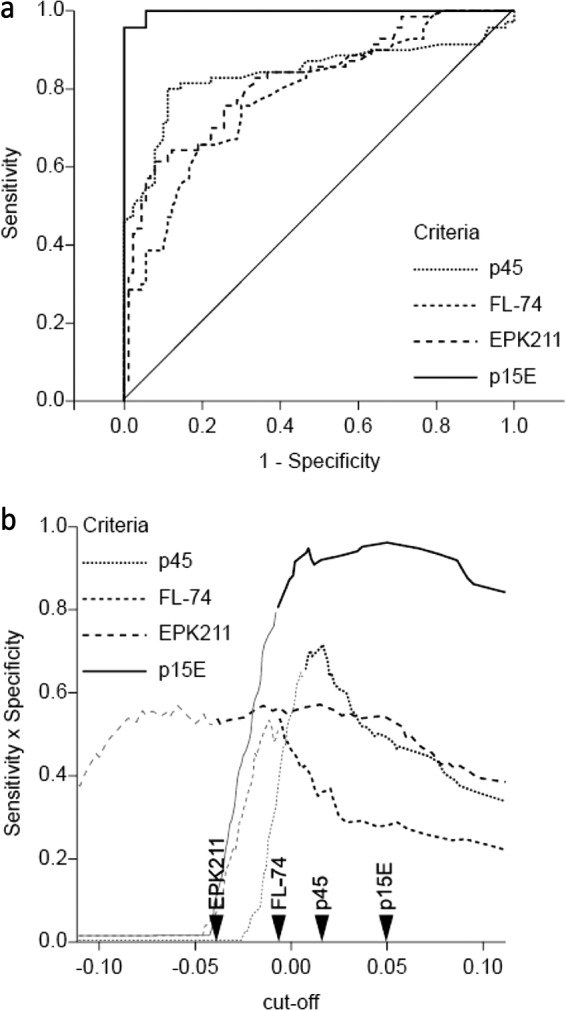
(a) Empirical provirus receiver operating characteristic (ROC) curves for the experimentally infected cats, including specific-pathogen-free (SPF) cats (n = 94) and provirus-positive (p27 positive and p27 negative) cats (n = 70). The true-positive rate (sensitivity, y axis) is plotted against the false-positive rate (1-specificity, x axis) at various cutoff points. (b) Determination of the optimal cutoff for experimentally infected cats. The sensitivity × specificity (y axis) is plotted against the cutoff (x axis). Dark lines represent specificities of ≥80%. The arrows indicate the cutoff points for each antigen.
Cohen's kappa values, which represent a chance-corrected measure of agreement between two sets of categorized data, revealed an almost perfect level of agreement (κ = 0.96) between p15E and provirus (Table 1). The agreements between provirus and p45, whole virus (FL-74), and EPK211 showed lower levels ranging between 0.47 and 0.70. Agreement between pairs of antigens showed satisfying to good levels but lower than those for agreement between p15E and provirus.
TABLE 1.
Cross table of Cohen's kappa valuesa
| Antigen | Kappa value for: |
||||
|---|---|---|---|---|---|
| Provirus | p45 | FL-74 | EPK211 | p15E | |
| Provirus | 0.18 | 0.13 | 0.08 | 0.55 | |
| p45 | 0.69b | 0.42 | 0.16 | 0.21 | |
| FL-74 | 0.47 | 0.45 | 0.24 | 0.12 | |
| EPK211 | 0.47 | 0.57 | 0.42 | 0.06 | |
| p15E | 0.96 | 0.70 | 0.51 | 0.48 | |
Shown are the agreement levels of antigen pairs and provirus-antigen pairs. The specificity is >80%.
Values in bold type are for experimentally infected cats (ntotal = 157), and those in regular type indicate naturally infected cats (ntotal = 294).
Longitudinal effects on antibodies from uninfected SPF cats were evaluated, and it was revealed that antibody levels of SPF cats stayed below the cutoff during 22 weeks (data not shown). Moreover, the comparison of young SPF cats and old SPF animals revealed that 2 of the old animals had values slightly exceeding the cutoff (data not shown). These 2 cats were over 10 years old and had lived for many years in a separate room and served as blood donors. It can be imagined that during their handling for many years by persons who also had access to clinics, they had become exposed to low doses of FeLV and as a consequence showed seroconversion. Unfortunately, this postulate could not be verified as the cats still serve as blood donors.
Antibody levels in privately owned, naturally infected cats.
Cats that were naturally infected with FeLV were evaluated with ROC analyses using provirus as the gold standard (Fig. 3a). Results revealed a good course for antigen p15E, representing a striking contrast to that for the other antigens. Because the values of these samples were generally increased, new cutoff points for the data sets had to be determined. To simplify, we excluded from this study 8 cats that had been immunized but were provirus positive, indicating that they might have been immunized after FeLV infection, which rendered their immune state highly undefined. The optimal cutoffs were defined as a specificity of ≥80% and a sensitivity × specificity as the maximum (Fig. 3b), which was calculated to be an OD of 0.163 for p15E, resulting in a sensitivity of 77.1% and a specificity of 85.6% (specificity of ≥80%). FL-74 had a cutoff of 0.647, a sensitivity of 42.9%, and a specificity of 80.1%, p45 had a cutoff of 0.531, a sensitivity of 40%, and a specificity of 81.1%, and EPK211 had a sensitivity of 17.1% and a specificity of 84.6% with a cutoff of 2.411.
FIG 3.
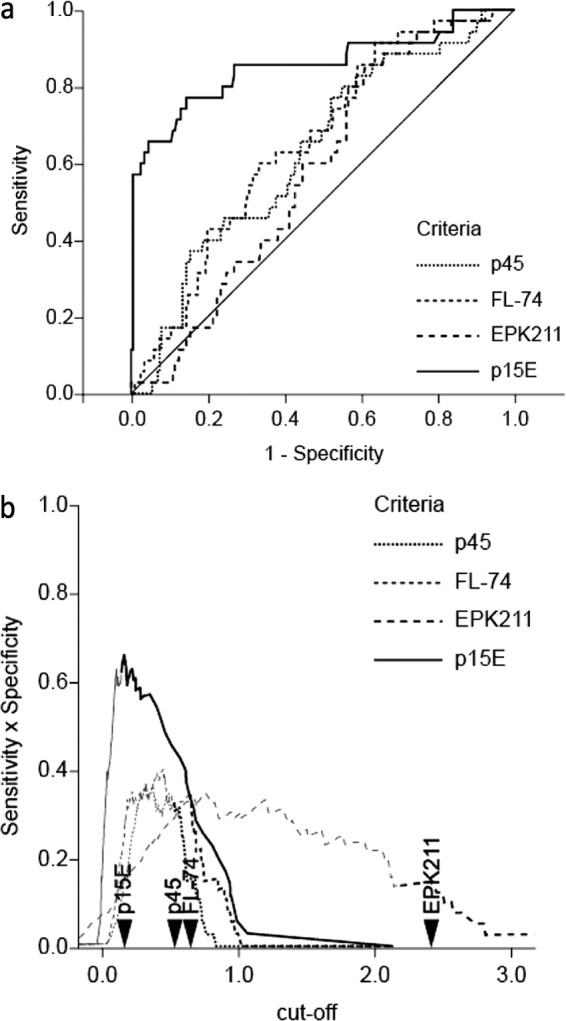
(a) Empirical provirus receiver operating characteristic (ROC) curves for naturally infected study cats, including uninfected cats (n = 201) and provirus-positive (p27 positive and p27 negative) cats (n = 35). The true-positive rate (sensitivity, y axis) is plotted against the false-positive rate (1 − specificity, x axis) at various cutoff points. (b) Determination of the optimal cutoff for naturally infected cats. The sensitivity × specificity (y axis) is plotted against the cutoff (x axis). Dark lines represent specificities of ≥80%. The arrows indicate the cutoff points for each antigen.
Cohen's kappa values (specificity of ≥80%) were lower than those of experimentally infected cats, which was consistent with the differences between the two sets of data evaluated with ROC analyses. The best agreement level was achieved by p15E plotted against the provirus (κ = 0.55) (Table 1). In marked contrast, the other pairs of antigens and pairs of provirus and antigens reached levels of only 0.08 to 0.42 and thus were clearly unsatisfactory. These results indicated that the agreement between p15E and provirus is much better than that for combinations of p15E with the other antigens.
Antibody levels in experimentally vaccinated cats.
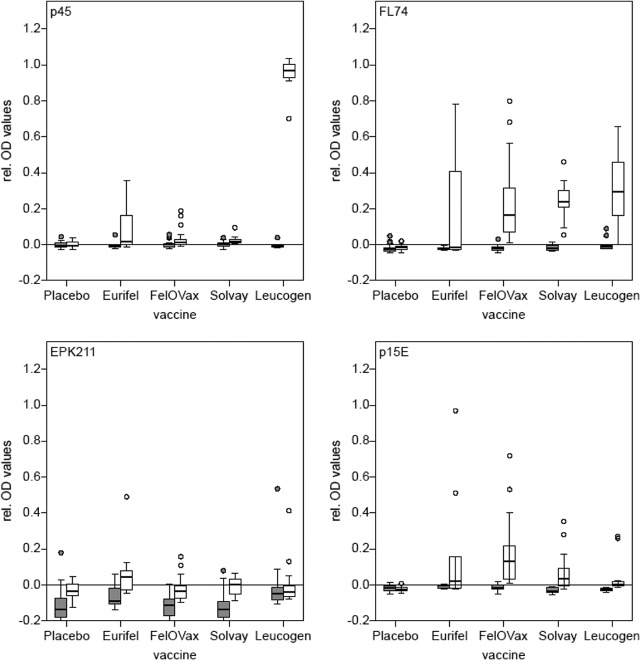
To look also at the antibody levels of vaccinated animals, serum samples from young, 8-week-old SPF cats were tested once before vaccination and again after they had been vaccinated twice but before the challenge infection. Results (Fig. 4) for p45 revealed that of all cats (without placebo), 56.92% exceeded the cutoff, but 100% were over the cutoff when immunized with Leucogen, as expected. Whole FeLV antigen (FL-74) showed an overall increase in antibody responses with the finding that 90.77% exceeded the cutoff, and 100% exceeded the cutoff when vaccinated with Leucogen. In contrast, for EPK211, 58.46% exceeded the cutoff with 40% of the cats vaccinated with Leucogen. For recombinant p15E, 44.62% exceeded the cutoff with 13.3% of Leucogen-vaccinated animals.
FIG 4.
Antibody response of experimentally vaccinated cats. The y axes represent the relative OD values; the x axes represent the vaccines used in the study. All four antigens were tested. The gray bars indicate 8-week-old specific-pathogen-free (SPF) cats (n = 63) before immunization; the white bars indicate the same cats (n = 63) after two immunizations and before challenge.
Antibody levels in privately owned, vaccinated cats.
Out of all privately owned provirus-negative cats, 50 animals were vaccinated. Eight cats were excluded from this study, because they were, besides having received immunization, also provirus positive or even viremic. Of all animals, 98% considered for the study received Leucogen, mostly applied in combination with Feligen (against feline panleukopenia and feline rhinotracheitis/cat flu) (Virbac Schweiz AG, Glattbrugg, Switzerland). Results (Fig. 5) revealed that antibody reactions were clearly increased using antigens p45 (70%), whole virus (42%), and EPK211 (24%). Whole TM (p15E) showed a low reactivity in vaccinated cats and in 16% exceeded the cutoff point.
FIG 5.
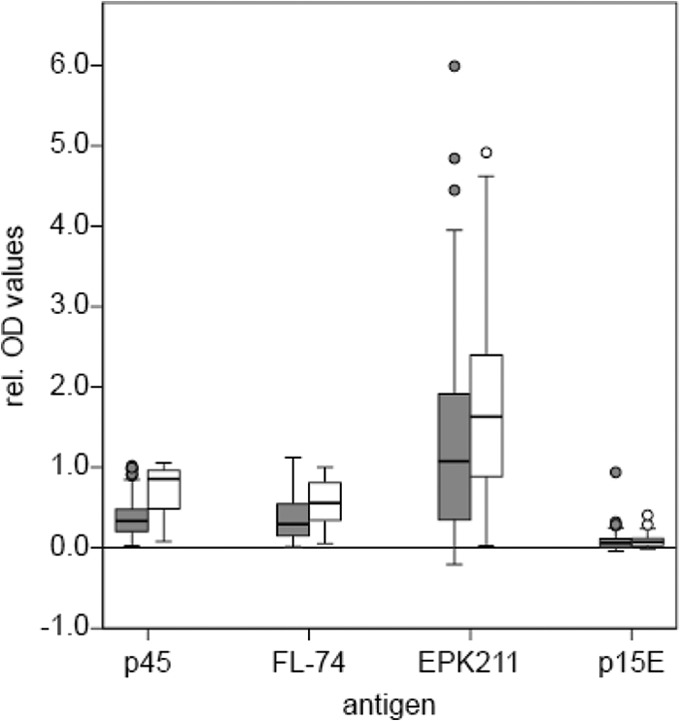
Antibody response of privately owned vaccinated cats. The y axis represents the relative OD values, and the x axis represents the four antigens. The gray bars indicate cats without vaccination (n = 236), and the white bars indicate cats that were vaccinated with Leucogen (98%) (n = 50). The 8 cats found to be p27 and/or provirus positive are not included.
DISCUSSION
This study showed that the FeLV envelope TM protein p15E is a promising candidate for serological diagnosis. The development of a novel diagnostic test based on a specific FeLV antibody needs to define the critical level the antibody is supposed to exceed to reliably predict any contact with FeLV or to fall below to predict naivety against FeLV. Thus, in this study we aimed to screen FeLV-naive, -infected, and -immunized cats for the presence of antibodies to four different FeLV antigens. Based on our results, we defined a threshold to discriminate cats that had contact with FeLV from naive cats. Furthermore, we used a well-established gold standard to compare our ELISA with real-time PCR.
Experimentally infected and vaccinated cats provided the first results for a proof-of-principle study. We chose laboratory cats, because they have a known infection history and had been living in a defined SPF environment avoiding all external influences; moreover, they are of defined age, gender, and breed. The time points of when to challenge them with FeLV or to apply immunization can be chosen exactly as can the time points of sample collection. The tests revealed that the antigen with the highest diagnostic potential was p15E with a diagnostic sensitivity of 95.7% and a specificity of 100%. The other three antigens (EPK211, FL-74, and p45) were not suitable in this context.
To evaluate the diagnostic efficiency of the antigens in naturally exposed field cats, the cats' viremia (p27) and provirus (PCR) statuses and immunization records were used. In the field cat population, however, many factors were often not controllable or were even completely unknown due to increased interactions of these animals with their environment. Such factors include the cat's age, possible multiple immunizations against viruses other than FeLV, and present or past diseases of any nature. Another unclear point is that we did not have any records of how many times these cats were exposed to FeLV and to different FeLV subtypes, especially if these animals were stray cats or originally from a cat shelter. All of these factors result in a more undefined outcome of the cat's immune status. For example, we had to exclude five animals that were vaccinated but were provirus positive (p27 negative), and three animals that were vaccinated but were p27 positive (viremic). Here, it was not clear whether the five provirus-positive animals were immune due to infection or due to vaccination, and whether the three p27-positive animals became viremic because of vaccine failure, or whether they were vaccinated after they had already established viremia.
As expected, the threshold value for these samples was elevated; the diagnostic sensitivity and specificity were lower than those in the defined laboratory cat populations.
Considering the more complex conditions in field cats, p15E exhibited a good diagnostic sensitivity of 77.1% and a specificity of 85.6%. The relatively low specificity of 85.6% would probably have been much higher if the PCR, which had been used as the gold standard of infection, was more sensitive. Exposure to FeLV can result in infection and seroconversion in the absence of positive p27 and PCR results performed with plasma or serum and whole blood samples (6, 7). In these 2 studies (6, 7), it was shown that, in contrast to experimental infection by needle, nasal or peroral exposure to FeLV can result in seroconversion and infection of several organs with little involvement of the bone marrow. If the PCR results from organs in the privately owned cats in the present study had been available, the specificity would have been much higher. Thus, demonstration of antibodies to p15E appears to be a more sensitive parameter for past contact with FeLV than the most sensitive PCR procedures performed with blood samples. Our results suggest that detection of antibodies to p15E may become relevant as a future diagnostic parameter for exposure to FeLV. As was shown for the privately owned cats, the cutoffs had to be changed from the conditions of the experimentally infected cats in order to reach an optimal trade-off between diagnostic sensitivity and specificity. If an antibody test based on p15E is developed for use in clinical veterinary medicine, larger numbers of privately owned cats would have to be evaluated in order to define an optimal cutoff. Our findings are in line with the results of an earlier publication in which antibodies to FeLV p15E were found to be present at significant levels after FeLV infection (22). On the other hand, the results are in striking contrast to those of Fontenot et al. (27) and Langhammer et al. (25). It is postulated that the discrepancy with the results of Fontenot et al. and Langhammer et al. can be explained by the fact that these authors performed their tests with synthetic peptides which may have shapes different from the three-dimensional shape of the entire protein and therefore may not have been completely recognized by the antibodies induced by the viral protein.
Cohen's kappa values were used to measure agreement between the two antigens and to evaluate the new ELISA compared to a well-established reference test, the real-time PCR, which served as a gold standard. A high kappa value shows that there is strong agreement between the two tests. The low agreement among the four antigens was in striking contrast to the almost perfect agreement between p15E and provirus in experimentally infected cats and the good agreement between p15E and provirus in the field cats. It is possible that these results may represent a step toward a partial replacement of the more expensive PCR test.
We expected that in some cats the presence of antibodies to enFeLV may interfere with the results induced by infection with exogenous FeLV. However, the p15E ELISA did not show elevated antibody values in any of the FeLV-naive SPF cats, indicating that the test is not negatively affected by antibodies to the enFeLV which is present in multiple copies in every cat cell (24). However, the possibility that certain cat breeds might have increased levels of antibodies to enFeLV has to be considered.
We showed that almost all cats seroconverted after they had contact with FeLV, and we were able to demonstrate with p15E-positive results that the probability of a cat being infected with FeLV is also high in p27-negative cats. However, p15E was not able to discriminate between viremic and immune cats, indicating that p27 is further needed to diagnose viremia. A point-of-care test device in which a p27 antigen test is located on a lateral flow test membrane next to a p15E test field could readily be imagined. Thus, a cat with negative p27 and p15E antibody results is very likely to have never been in contact with FeLV. In contrast, negative p27 and positive p15E results suggest that the cat is not viremic/antigenemic but had been in contact with FeLV and may harbor the virus somewhere in its body. Before introduction of such a cat into a multicat-household situation, the new cat should be further tested for signs of a latent FeLV infection at least by PCR and/or reverse transcriptase PCR (RT-PCR) performed on a blood sample. If the result is negative by PCR or RT-PCR, the latent virus load may indeed be low.
An antibody test may detect not only infected and naive cats but also immunized cats. A cat with a p15E value exceeding the cutoff is considered to have been exposed to FeLV (if the p27 ELISA is negative). As a positive p15E result may not be identical with immunity to FeLV, cats with positive or negative p15E results should be vaccinated where needed.
The goal of this study was to develop a novel serological test that is able to show whether or not a cat had contact with FeLV. To this end, we have evaluated different FeLV antigens. FeLV p15E was found to be useful to differentiate infected from uninfected animals. However, it was found not to be useful to clearly detect vaccination, because most of the vaccinated privately owned cats had antibody values that are lower than the threshold calculated for FeLV-naive cats. Thus, p15E seems to be a sign of infection rather than of vaccination, which is consistent with previous findings (22). Based on our results, we conclude that serological diagnosis based on the p15E antigen may represent valuable support in evaluating the infection state of a cat and partially replace elaborate or expensive diagnostic techniques like PCR.
ACKNOWLEDGMENTS
We thank M. Wittenbrink for providing the E. coli sera, A. Gomes-Keller, C. Geret, Pfizer (Kent, United Kingdom), Merial (Lyon, France), and Virbac (Carros, France) for kindly providing the cat plasma and serum samples, S. Wellnitz (RedBiotec) and O. Sonzogni for technical support, T. Meili Prodan for synthesizing p15E and EspiKem Srl (Florence, Italy) for the synthesis of the peptide EPK211, and the many veterinarians in Switzerland who generously provided information about the vaccination statuses of the privately owned cats.
This work was supported by a grant obtained from the Promedica Foundation, Chur, Switzerland. The study was performed using the logistics of the Centre for Clinical Studies at the Vetsuisse Faculty, University of Zurich, Switzerland.
H.L. initiated the study. E.B. and H.L. conceived and designed the experiments. E.B. carried out the experiments. J.H. designed the experiments for p15E synthesis. S.H. performed the statistical analyses. All authors analyzed the data, wrote the paper, and read and approved the final manuscript.
We declare no conflicts of interest.
Footnotes
Published ahead of print 2 April 2014
REFERENCES
- 1.Jarrett WF, Crawford EM, Martin WB, Davie F. 1964. A virus-like particle associated with leukemia (lymphosarcoma). Nature 202:567–569. 10.1038/202567a0 [DOI] [PubMed] [Google Scholar]
- 2.Hoover EA, Mullins JI. 1991. Feline leukemia virus infection and diseases. J. Am. Vet. Med. Assoc. 199:1287–1297 [PubMed] [Google Scholar]
- 3.Hardy WD, Jr, McClelland AJ. 1977. Feline leukemia virus. Its related diseases and control. Vet. Clin. North Am. 7:93–103 [DOI] [PubMed] [Google Scholar]
- 4.Levy JK, Scott HM, Lachtara JL, Crawford PC. 2006. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in North America and risk factors for seropositivity. J. Am. Vet. Med. Assoc. 228:371–376. 10.2460/javma.228.3.371 [DOI] [PubMed] [Google Scholar]
- 5.Lutz H, Lehmann R, Winkler G, Kottwitz B, Dittmer A, Wolfensberger C, Arnold P. 1990. Feline immunodeficiency virus in Switzerland: clinical aspects and epidemiology in comparison with feline leukemia virus and coronaviruses (in German). Schweiz. Arch. Tierheilkd. 132:217–225 [PubMed] [Google Scholar]
- 6.Gomes-Keller MA, Gonczi E, Grenacher B, Tandon R, Hofman-Lehmann R, Lutz H. 2009. Fecal shedding of infectious feline leukemia virus and its nucleic acids: a transmission potential. Vet. Microbiol. 134:208–217. 10.1016/j.vetmic.2008.08.011 [DOI] [PubMed] [Google Scholar]
- 7.Major A, Cattori V, Boenzli E, Riond B, Ossent P, Meli ML, Hofmann-Lehmann R, Lutz H. 2010. Exposure of cats to low doses of FeLV: seroconversion as the sole parameter of infection. Vet. Res. 41:17. 10.1051/vetres/2009065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann-Lehmann R, Tandon R, Boretti FS, Meli ML, Willi B, Cattori V, Gomes-Keller MA, Ossent P, Golder MC, Flynn JN, Lutz H. 2006. Reassessment of feline leukaemia virus (FeLV) vaccines with novel sensitive molecular assays. Vaccine 24:1087–1094. 10.1016/j.vaccine.2005.09.010 [DOI] [PubMed] [Google Scholar]
- 9.Anderson MM, Lauring AS, Burns CC, Overbaugh J. 2000. Identification of a cellular cofactor required for infection by feline leukemia virus. Science 287:1828–1830. 10.1126/science.287.5459.1828 [DOI] [PubMed] [Google Scholar]
- 10.Grant CK, Essex M, Gardner MB, Hardy WD., Jr 1980. Natural feline leukemia virus infection and the immune response of cats of different ages. Cancer Res. 40:823–829 [PubMed] [Google Scholar]
- 11.Lutz H, Pedersen NC, Theilen GH. 1983. Course of feline leukemia virus infection and its detection by enzyme-linked immunosorbent assay and monoclonal antibodies. Am. J. Vet. Res. 44:2054–2059 [PubMed] [Google Scholar]
- 12.Hardy WD, Zuckerman EE. 1991. Development of the immunofluorescent antibody-test for detection of feline leukemia-virus infection in cats. J. Am. Vet. Med. Assoc. 199:1327–1335 [PubMed] [Google Scholar]
- 13.Hardy WD, Zuckerman EE. 1991. 10-Year study comparing enzyme-linked-immunosorbent-assay with the immunofluorescent antibody-test for detection of feline leukemia-virus infection in cats. J. Am. Vet. Med. Assoc. 199:1365–1373 [PubMed] [Google Scholar]
- 14.Jarrett O, Ganiere JP. 1996. Comparative studies of the efficacy of a recombinant feline leukaemia virus vaccine. Vet. Rec. 138:7–11. 10.1136/vr.138.1.7 [DOI] [PubMed] [Google Scholar]
- 15.Hofmann-Lehmann R, Huder JB, Gruber S, Boretti F, Sigrist B, Lutz H. 2001. Feline leukaemia provirus load during the course of experimental infection and in naturally infected cats. J. Gen. Virol. 82:1589–1596 [DOI] [PubMed] [Google Scholar]
- 16.Herring IP, Troy GC, Toth TE, Champagne ES, Pickett JP, Haines DM. 2001. Feline leukemia virus detection in corneal tissues of cats by polymerase chain reaction and immunohistochemistry. Vet. Ophthalmol. 4:119–126. 10.1046/j.1463-5224.2001.00187.x [DOI] [PubMed] [Google Scholar]
- 17.Jackson ML, Haines DM, Taylor SM, Misra V. 1996. Feline leukemia virus detection by ELISA and PCR in peripheral blood from 68 cats with high, moderate, or low suspicion of having FeLV-related disease. J. Vet. Diagn. Invest. 8:25–30. 10.1177/104063879600800105 [DOI] [PubMed] [Google Scholar]
- 18.Essex M. 1977. Immunity to leukemia, lymphoma, and fibrosarcoma in cats: a case for immunosurveillance. Contemp. Top. Immunobiol. 6:71–106 [DOI] [PubMed] [Google Scholar]
- 19.Stephenson JR, Khan AS, Sliski AH, Essex M. 1977. Feline oncornavirus-associated cell-membrane antigen -evidence for an immunologically cross-reactive feline sarcoma virus-coded protein. Proc. Natl. Acad. Sci. U. S. A. 74:5608–5612. 10.1073/pnas.74.12.5608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Noronha F, Schafer W, Essex M, Bolognesi DP. 1978. Influence of antisera to oncornavirus glycoprotein (Gp71) on infections of cats with feline leukemia-virus. Virology 85:617–621. 10.1016/0042-6822(78)90467-1 [DOI] [PubMed] [Google Scholar]
- 21.Jacquemin PC, Saxinger C, Gallo RC, Hardy WD, Essex M. 1978. Antibody-response in cats to feline leukemia-virus reverse-transcriptase under natural conditions of exposure to the virus. Virology 91:472–476. 10.1016/0042-6822(78)90393-8 [DOI] [PubMed] [Google Scholar]
- 22.Lutz H, Pedersen N, Higgins J, Hubscher U, Troy FA, Theilen GH. 1980. Humoral immune reactivity to feline leukemia-virus and associated antigens in cats naturally infected with feline leukemia-virus. Cancer Res. 40:3642–3651 [PubMed] [Google Scholar]
- 23.Tandon R, Cattori V, Willi B, Lutz H, Hofmann-Lehmann R. 2008. Quantification of endogenous and exogenous feline leukemia virus sequences by real-time PCR assays. Vet. Immunol. Immunopathol. 123:129–133. 10.1016/j.vetimm.2008.01.027 [DOI] [PubMed] [Google Scholar]
- 24.Tandon R, Cattori V, Willi B, Meli ML, Gomes-Keller MA, Lutz H, Hofmann-Lehmann R. 2007. Copy number polymorphism of endogenous feline leukemia virus-like sequences. Mol. Cell. Probes 21:257–266. 10.1016/j.mcp.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 25.Langhammer S, Hubner J, Kurth R, Denner J. 2006. Antibodies neutralizing feline leukaemia virus (FeLV) in cats immunized with the transmembrane envelope protein p15E. Immunology 117:229–237. 10.1111/j.1365-2567.2005.02291.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tandon R, Cattori V, Willi B, Lutz H, Hofmann-Lehmann R. 2008. Quantification of endogenous and exogenous feline leukemia virus sequences by real-time PCR assays. Vet. Immunol. Immunopathol. 123:129–133. 10.1016/j.vetimm.2008.01.027 [DOI] [PubMed] [Google Scholar]
- 27.Fontenot JD, Hoover EA, Elder JH, Montelaro RC. 1992. Evaluation of feline immunodeficiency virus and feline leukemia-virus transmembrane peptides for serological diagnosis. J. Clin. Microbiol. 30:1885–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theilen GH, Kawakami TG, Rush JD, Munn RJ. 1969. Replication of cat leukemia virus in cell suspension cultures. Nature 222:589–590 [DOI] [PubMed] [Google Scholar]
- 29.Jarrett O, Laird HM, Hay D. 1973. Determinants of host range of feline leukemia viruses. J. Gen. Virol. 20:169–175. 10.1099/0022-1317-20-2-169 [DOI] [PubMed] [Google Scholar]
- 30.Engvall E, Perlmann P. 1972. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J. Immunol. 109:129–135 [PubMed] [Google Scholar]
- 31.Voller A, Bidwell DE, Bartlett A. 1976. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull. World Health Organ. 53:55–65 [PMC free article] [PubMed] [Google Scholar]
- 32.Lehmann R, Franchini M, Aubert A, Wolfensberger C, Cronier J, Lutz H. 1991. Vaccination of cats experimentally infected with feline immunodeficiency virus, using a recombinant feline leukemia virus vaccine. J. Am. Vet. Med. Assoc. 1991:1446–1452 [PubMed] [Google Scholar]