Abstract
In allogeneic hematopoietic stem cell transplant recipients with bone marrow (BM) suppression, cytomegalovirus (CMV) pp65 antigenemia and DNA were detectable in peripheral blood leukocytes (PBL) and BM cells. A relationship between CMV infection of PBL and BM cells has been found.
TEXT
The presence of cytomegalovirus (CMV) in bone marrow (BM) cells has been investigated in several in vitro and in vivo studies (1–4). Secondary cytopenias (e.g., neutropenia, anemia, and thrombocytopenia) are frequent complications after allogeneic hematopoietic stem cell transplant (HSCT) and are potentially related to viral infections, septicemia, graft-versus-host disease, and myelotoxic drugs (5, 6). In patients suffering from CMV disease, significant correlations of high levels of DNA and pp65 antigenemia (pp65-AG) in plasma and peripheral blood leukocytes (PBL), respectively, have been found in HSCT patients (7, 8). However, low levels of CMV DNA are frequently detected following an allogeneic HSCT, and CMV disease may still develop in some HSCT recipients and solid organ transplant patients who have negative pp65-AG or low or undetectable levels of CMV DNA in PBL or plasma (9–12). Several studies have compared CMV infection in different blood compartments (plasma, PBL, and whole blood) (7, 10, 13) but, to our knowledge, this is the first prospective in vivo study comparing CMV infection in the BM and PBL compartments of HSCT recipients. (Part of this research has been presented at the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), 9 to 12 September 2012, San Francisco, CA [14].)
In the present study, we analyzed two groups of patients at risk of CMV infection (donor and/or recipient CMV seropositive) who had BM suppression and preceding blood samples negative for CMV and who were not receiving ganciclovir, foscarnet, or cidofovir at the onset of BM suppression. Group I (selected from November 2001 to December 2009) included 24 CMV-seropositive patients who received an allogeneic HSCT and whose preceding blood samples were negative for CMV pp65-AG. Virological monitoring was performed by pp65-AG (CINA pool; Argene, France) detection from PBL (2 × 105 cells) and from total BM cells (2 × 105 cells). The presence of one or more pp65-positive cells/2 × 105 cells in PBL and/or BM was considered a positive result (10).
Group II (selected from January 2010 to May 2012) was a group of 14 allogeneic HSCT patients with preceding blood samples negative for CMV DNA. Virological monitoring was performed by CMV real-time PCR (ELITech Group; Nanogen Advanced Technologies, Italy) from PBL (2 × 105 cells) and from total BM cells (2 × 105 cells). The real-time PCR is specific for a region of the CMV major immediate-early gene (UL123). The DNA lower detection limit was 200 copies/105 cells.
BM aspirates were obtained from the patients at the onset of BM suppression in order to exclude the recurrence of hematological disease, poor bone marrow engraftment, and the presence of CMV infection. BM function was considered to be suppressed (either a delay in engraftment or the development of myelosuppression after an initial engraftment) as previously defined (15). This study was approved by the institutional review board.
In group I, CMV pp65-AG was detected in 24 of the patients examined and was found in PBL (18 patients) and BM (19 patients) sample pairs (see Table S1 in the supplemental material). The clinical characteristics of the 24 patients are illustrated in Table S2 in the supplemental material. There was a good correlation between the CMV pp65 antigen PBL and BM cell numbers (R = 0.514, P = 0.03 [95% confidence interval, 0.04 to 0.79]) (Fig. 1). No difference was found between the number of pp65-AG-positive cells in PBL (median, 11.5 positive cells [range, 1 to 350 positive cells]) and in BM cells (median, 5 positive cells [range, 1 to 60 positive cells]) (Mann-Whitney Test). No patient developed CMV disease.
FIG 1.
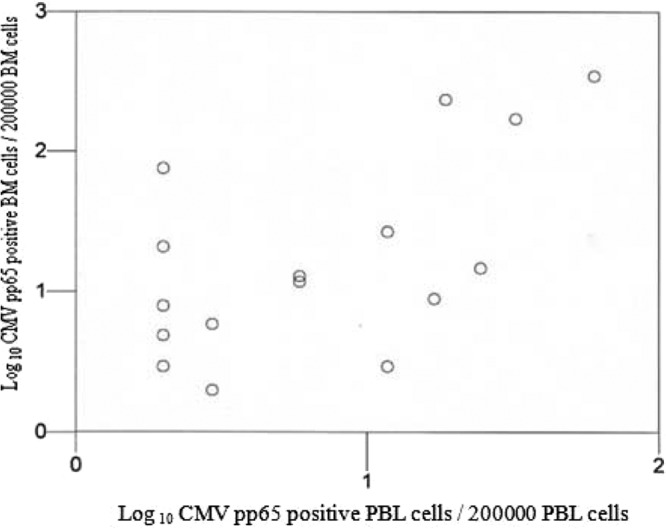
Correlation between the number of pp65-positive cells in BM cells and the number of pp65-positive cells in PBL on the basis of the 17 samples which were positive by pp65-AG in PBL and BM cells. The number of pp65-positive cells in PBL was plotted on a logarithmic graph against the number of pp65-positive cells in BM cells. The correlation was examined by the Spearman rank test and found to be significant, with a correlation coefficient of 0.51 (P = 0.03).
In group II, CMV DNA was detected in 14 of the patients examined and was found in PBL (13 patients) and BM (10 patients) sample pairs (see Table S3 in the supplemental material). The clinical characteristics of the 14 patients are reported in Table S2 in the supplemental material. A good correlation between the positive CMV DNA in PBL and in BM cells was found (R = 0.72, P = 0.02 [95% confidence interval, 0.12 to 0.93]) (Fig. 2). There was no difference between the CMV DNA load in PBL (median, 600 copies/105 cells [range, 114 to 11,890 copies/105 cells]) and in BM cells (median, 882 copies/105 cells [range, 202 to 7,500 copies/105 cells]) (Mann-Whitney Test). No patient developed CMV disease.
FIG 2.
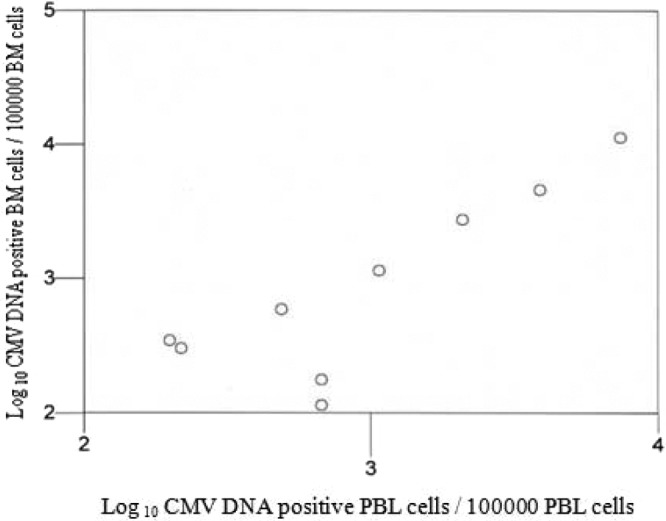
Correlation between CMV DNA copy number in BM cells and the CMV DNA copy number in PBL on the basis of the 9 samples which were positive by CMV DNA in PBL and BM cells. The CMV DNA copy number in PBL was plotted on a logarithmic graph against the CMV DNA copy number in BM cells. The correlation was examined by the Spearman rank test and found to be significant, with a correlation coefficient of 0.72 (P = 0.02).
Comparing the CMV viral loads measured by the pp65-AG assay and by the real-time PCR assay in PBL and BM sample pairs, we found a linear correlation between the viral loads in the two compartments. Discordant results were found using either the pp65 AG assay or the real-time PCR assay in both compartments, and taking into account that preemptive therapy was initiated at the first detection of CMV in the PBL and/or BM samples, viral kinetics in HSCT patients with 2 or more BM examined cannot be evaluated. The possible presence of peripheral blood in the BM aspirates that may have affected the results of the PCR cannot be excluded, since our study was performed on the total, not purified, BM cells. Nonetheless, von Laer et al. (4) provided strong evidence that CD34+ hematopoietic progenitor cells, obtained from bone marrow aspirates of HSCT recipients, were CMV DNA positive.
It is relevant to note that the number of patients who were positive for CMV antigen only in BM samples was slightly higher than the number of the patients positive for CMV DNA only in BM samples (7/24 [29%] versus 1/14 [7%], respectively [P = 0.2, Fisher's exact test]). The reasons for such discrepancies are not clear. Very little is known about the expression of the pp65-AG in BM cells; in two in vitro studies (1, 3) designed to determine whether CMV infects purified CD34+ BM cells, the pp65-AG was detected in hematopoietic progenitor cells. In our study, the higher frequency of CMV DNA observed in PBL than in BM cells could have occurred because, during active CMV infection, all major PBL subpopulations may contain the CMV genome and granulocytes are the cell population most frequently positive for viral DNA (reviewed in reference 2). Since CMV in plasma or in whole blood may originate from the lysis of infected PBL from parenchymal and endothelial cells, it would be useful, using these blood compartments, to perform studies in the setting of low systemic viral load such as observed in our patients.
Very little is known about the clinical relevance of the detection of both pp65-AG and DNA in BM cells of immunocompromised patients. Boeckh et al. (9) observed an unrelated marrow transplant patient who developed marrow suppression with negative CMV blood culture and pp65-AG. CMV pp65-antigen and DNA were found later in cultured marrow stromal cells of the patient. Randolph-Habecker et al. (16) conducted a study on 248 CMV-seropositive HSCT recipients. The presence of CMV in the BM was measured by nested PCR at day 28, day 80, and 1 year following the HSCT; CMV DNA was found in the BM in 87 patients (35%).
In conclusion, the diagnostic evaluation of BM cells, including CMV PCR and/or pp65-AG analysis, may be indicated for HSCT recipients at risk of CMV infection and with BM suppression (either a delay in engraftment or the development of myelosuppression after an initial engraftment) and preceding blood samples negative for CMV. Since the BM suppression may be associated with high mortality or cause severe complications such as bacterial or fungal infections, an antiviral treatment should be initiated early.
Supplementary Material
ACKNOWLEDGMENT
This work was partially supported by Sapienza University of Rome, Italy (Progetto di Ricerca Finalizzato 2004 and 2005).
Footnotes
Published ahead of print 26 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00702-14.
REFERENCES
- 1.Minton EJ, Tysoe C, Sinclair JH, Sissons JG. 1994. Human cytomegalovirus infection of the monocyte/macrophage lineage in bone marrow. J. Virol. 68:4017–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer-König U, Hufert FT, von Laer DM. 1997. Infection of blood and bone marrow cells with the human cytomegalovirus in vivo. Leuk. Lymphoma 25:445–454 [DOI] [PubMed] [Google Scholar]
- 3.Movassagh M, Gozlan J, Senechal B, Baillou C, Petit JC, Lemoine FM. 1996. Direct infection of CD34+ progenitor cells by human cytomegalovirus: evidence for inhibition of hematopoiesis and viral replication. Blood 88:1277–1283 [PubMed] [Google Scholar]
- 4.von Laer D, Meyer-Koenig U, Serr A, Finke J, Kanz L, Fauser AA, Neumann-Haefelin D, Brugger W, Hufert FT. 1995. Detection of cytomegalovirus DNA in CD34+ cells from blood and bone marrow. Blood 86:4086–4090 [PubMed] [Google Scholar]
- 5.Nakamae H, Storer B, Sandmaier BM, Maloney DG, Davis C, Corey L, Storb R, Boeckh M. 2011. Cytopenias after day 28 in allogeneic hematopoietic cell transplantation: impact of recipient/donor factors, transplant conditions and myelotoxic drugs. Haematologica 96:1838–1845. 10.3324/haematol.2011.044966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torok-Storb B, Boeckh M, Hoy C, Leisenring W, Myerson D, Gooley T. 1997. Association of specific cytomegalovirus genotypes with death from myelosuppression after marrow transplantation. Blood 90:2097–2102 [PubMed] [Google Scholar]
- 7.Razonable RR, Brown RA, Wilson J, Groettum C, Kremers W, Espy M, Smith TF, Paya CV. 2002. The clinical use of various blood compartments for cytomegalovirus (CMV) DNA quantitation in transplant recipients with CMV disease. Transplantation 73:968–973 [DOI] [PubMed] [Google Scholar]
- 8.Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. 2000. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet 355:2032–2036. 10.1016/S0140-6736(00)02350-3 [DOI] [PubMed] [Google Scholar]
- 9.Boeckh M, Hoy C, Torok-Storb B. 1998. Occult cytomegalovirus infection of marrow stroma. Clin. Infect. Dis. 26:209–210. 10.1086/517022 [DOI] [PubMed] [Google Scholar]
- 10.Gentile G, Picardi A, Capobianchi A, Spagnoli A, Cudillo L, Dentamaro T, Tendas A, Cupelli L, Ciotti M, Volpi A, Amadori S, Martino P, de Fabritiis P. 2006. A prospective study comparing quantitative cytomegalovirus (CMV) polymerase chain reaction in plasma and pp65 antigenemia assay in monitoring patients after allogeneic stem cell transplantation. BMC Infect. Dis. 6:167–177. 10.1186/1471-2334-6-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green ML, Leisenring W, Stachel D, Pergam SA, Sandmaier BM, Wald A, Corey L, Boeckh M. 2012. Efficacy of a viral load-based, risk adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 18:1687–1699. 10.1016/j.bbmt.2012.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho BS, Yahng SA, Kim JH, Yoon JH, Shin SH, Lee SE, Choi SM, Lee DG, Eom KS, Park G, Kim YJ, Kim HJ, Lee S, Min CK, Cho SG, Kim DW, Lee JW, Min WS, Park CW. 2013. Impact of cytomegalovirus gastrointestinal disease on the clinical outcomes in patients with gastrointestinal graft-versus-host disease in the era of preemptive therapy. Ann. Hematol. 92:497–504. 10.1007/s00277-012-1632-x [DOI] [PubMed] [Google Scholar]
- 13.Lisboa LF, Asberg A, Kumar D, Pang X, Hartmann A, Preiksaitis JK, Pescovitz MD, Rollag H, Jardine AG, Humar A. 2011. The clinical utility of whole blood versus plasma cytomegalovirus viral load assays monitoring therapeutic response. Transplantation 91:231–236. 10.1097/TP.0b013e3181ff8719 [DOI] [PubMed] [Google Scholar]
- 14.Capobianchi A, Iori AP, Micozzi A, Torelli GF, Girmenia C, Santilli S, Antonelli G, Foà R, Gentile G. 2012. Abstract V-393 Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC), San Francisco, CA [Google Scholar]
- 15.Carrigan DR, Knox KK. 1994. Human herpesvirus 6 (HHV-6) isolation from bone marrow: HHV-6 associated bone marrow suppression in bone marrow transplant patients. Blood 84:3307–3310 [PubMed] [Google Scholar]
- 16.Randolph-Habecker J, Iwata M, Torok-Storb B. 2002. Cytomegalovirus mediated myelosuppression. J. Clin. Virol. 25(Suppl 2):S51–S56 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


