Abstract
In French Guiana, leishmaniasis is an essentially cutaneous infection. It constitutes a major public health problem, with a real incidence of 0.2 to 0.3%. Leishmania guyanensis is the causal species most frequently encountered in French Guiana. The treatment of leishmaniasis is essentially drug based, but the therapeutic compounds available have major side effects (e.g., liver damage and diabetes) and must be administered parenterally or are costly. The efficacy of some of these agents has declined due to the emergence of resistance in certain strains of Leishmania. There is currently no vaccine against leishmaniasis, and it is therefore both necessary and urgent to identify new compounds effective against Leishmania. The search for new drugs requires effective tests for evaluations of the leishmanicidal activity of a particular molecule or extract. Microculture tetrazolium assays (MTAs) are colorimetric tests based on the use of tetrazolium salts. We compared the efficacies of three tetrazolium salts—3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT), and 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-8)—for quantification of the promastigotes of various species of Leishmania. We found that the capacity of Leishmania to metabolize a tetrazolium salt depended on the salt used and the species of Leishmania. WST-8 was the tetrazolium salt best metabolized by L. guyanensis and gave the best sensitivity.
INTRODUCTION
Leishmaniasis is a disease caused by a protozoan of the genus Leishmania, belonging to the family Trypanosomatidae. The disease may take several forms—cutaneous, visceral, or mucocutaneous—depending on the species of Leishmania involved and the immune status of the host. Leishmaniasis is a zoonosis affecting many mammals, including humans, and it is transmitted by insect vectors: sandflies (Psychodidae: Phlebotominae). Leishmaniasis is a public health problem in tropical countries, with more than 2 million new cases each year (1). It is treated essentially with parasiticidal drugs. Several therapeutic compounds are available (e.g., antimony derivatives and pentamidine), but they may have major adverse effects (such as liver damage and diabetes) and may require parenteral administration or are too costly to be readily available in certain countries. The oldest of the compounds used are antimony derivatives, which were released onto the market in the 1930s but have since decreased in efficacy due to the emergence of resistance in certain strains of Leishmania infantum and Leishmania donovani (2, 3). The other most widely used compounds are pentamidine and amphotericin B. In the first decade of this century, a new compound, miltefosine, was released onto the market. It can be administered orally, a key advantage, but its use is not authorized in all countries.
There is currently no vaccine against leishmaniasis. It is therefore both necessary and urgent to identify new compounds active against Leishmania. The search for such compounds requires an in vitro test for the reliable evaluation of their effects on the viability and proliferation of Leishmania.
Cell counts can be carried out by simple and readily accessible techniques, such as direct counting under the microscope, or by much more sophisticated and costly techniques, such as flow cytometry. The reliability of a test depends on both the method used and the cell type used, or even the stages or species considered, which must be defined in advance. Leishmania can be cultured in its mobile promastigote form or as amastigotes, either resident within macrophages or axenic. Infected macrophages more closely resemble the situation in humans, but the use of promastigotes is more widespread in tests of the activity of molecules or complex extracts due to the ease with which these cells can be cultured in vitro.
Direct counting under the microscope, which is still used in some laboratories, is a particularly straightforward technique. However, the mobility of the promastigotes and the presence of rosettes may complicate the task, and although the cells can be immobilized with glutaraldehyde, this method is not suitable for the simultaneous counting of cells in multiple samples; the cells counted include dead cells, and the bias resulting from the presence of rosettes remains. This fastidious and time-consuming technique is not very suitable for precise evaluations of leishmanicidal activity. Methods based on the incorporation into DNA of radiolabeled markers, such as tritiated uracil ([5-3H]uracil [4]) or tritiated thymidine ([3H]thymidine [5]) are rapid and simple and facilitate large-scale analyses of samples. The assimilation of this radioactive isotope into the DNA during its synthesis makes it possible to follow cell proliferation, since there is a linear correlation between the radiation emitted and cell multiplication. The major disadvantages of this technique are the need to manipulate radioactive compounds, for which accreditation is required, and the production of radioactive waste, the elimination of which remains problematic. In addition, the incorporation of these radioactive isotopes into cells during division may lead to DNA damage, cell cycle arrest, and cell apoptosis, with erroneous results. The replacement of radioactive isotopes with stable isotopes is possible but requires a mass spectrophotometer (6), an item of equipment not available in all laboratories. Flow cytometry (7, 8) can provide a quantification of the number of cells that is so precise that it is possible to determine the absolute number of cells per unit volume of culture, for cell densities from 100 cells/ml to 1 × 106 cells/ml, in a highly reproducible manner (9). However, the excessively high cost of this technique in terms of materials and reagents constitutes a major barrier to the widespread adoption of this technique. Other similar but less cumbersome devices that are practical and simple to use, such as automatic portable or bench cell counters, are accessible, but costs of consumables remain excessive.
As attested by many publications, the technique of choice remains colorimetric determination, which is cheap, effective, simple, and rapid. A wide selection of reagents is available, including resazurin-3H-phenoxazin-3-one 10-oxide (alamarBlue), a blue molecule that is reduced by the dehydrogenase activity of mitochondria to give rise to a pink molecule, resorufin (7-hydroxy-3H-phenoxazin-3-one), which can be quantified by spectrophotometry. This technique provides results similar to those obtained by tritiated thymidine incorporation (10). However, it has been reported (11) that the reduction of resazurin by the mitochondrial enzymes of Leishmania is much slower than that observed in other cell types (Trypanosoma and mammalian cells). Indeed, Leishmania must be cultured at a temperature of about 27°C, whereas Trypanosoma and mammalian cells can be cultured at 37°C, which is the optimal temperature for resazurin reduction. The reaction rate is thus much lower in Leishmania, resulting in much lower absorbance values for similar incubation times (11).
Microculture tetrazolium assays (MTAs) are colorimetric tests based on the use of tetrazolium salts, including 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2(4-sulfonyl)-2H-tetrazo-lium (MTS), 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT), (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-1), 2-(4-iodophenyl)-3-(2,4-dinitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-3), and 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-8). These methods are based on the cleavage of a tetrazolium salt by a mitochondrial enzyme, succinate dehydrogenase, leading to the formation of a colored product, formazan (12), which can be quantified by spectrophotometry. In practice, most of these tetrazolium salts have been found to be of variable efficacy for the quantification of Leishmania (13–17).
In this study, we compared the efficacies of three tests based on tetrazolium salts, MTT, XTT, and WST-8, for quantifying promastigotes of different strains of Leishmania. Then, the most suitable salt was used to evaluate the reliability of the test: in the presence of an ethanolic plant extract, Lantana camara, and a drug used as first-line treatment of cutaneous leishmaniasis in French Guiana, pentamidine.
MATERIALS AND METHODS
Parasites and cultures.
Tests were carried out on promastigotes from several strains of Leishmania: eight New-World strains of Leishmania, including two reference strains [Leishmania (Viannia) guyanensis MHOM/GF/97/LBC6 (LG-R) and Leishmania (Leishmania) amazonensis MPRO/BR/72/M1845 (LA-R)] and six strains isolated from patients in French Guiana [L. (V.) guyanensis 12.01.13-2008 {LG-1}, L. (V) guyanensis 04.04.13-2068 {LG-2}, L. (V) guyanensis 01.03.13-2014 {LG-3}, L. (V.) braziliensis 09.10.12-2031 {LB-1}, L. (V.) braziliensis 24.05.12-2068 {LB-2}, and L. (L.) amazonensis 10.10.12-2048 {LA-1}, together with one Old-World strain (Leishmania (L.) donovani MHOM/IN/96/THAK72 {LD-R}]. The reference strains were obtained from the national reference center for leishmaniasis in Montpellier, France, and the other strains were kindly supplied by the parasitology and mycology laboratory of Cayenne University Hospital, French Guiana, with strict respect for patient anonymity.
The reference strains, which were stored in liquid nitrogen, were thawed and then cultured in 3 ml of RPMI 1640 (Gibco) containing l-glutamine, 20 mM HEPES, and phenol red and supplemented with 20% inactivated calf fetal serum (CFS) (Gibco), 50 IU/ml penicillin (Invitrogen), 0.05 mg/ml streptomycin (Invitrogen), and nonessential amino acids (Gibco) at 26°C.
The medium was changed when the cells reached stationary phase: the cultures were centrifuged for 5 min at 514 × g and the parasites were resuspended in 10 ml of RPMI 1640 medium (Gibco), containing glutamine but devoid of phenol red, to limit background noise (18), and supplemented with 10% inactivated CFS (Gibco), 50 IU/ml penicillin (Invitrogen), 0.05 mg/ml streptomycin (Invitrogen), and nonessential amino acids (Gibco). This medium is referred to as RPMIØRP medium. The cells were cultured at 26°C until they reached exponential growth phase.
Parasite quantification.
All tests were carried out in a final volume of 100 μl in microtiter plates.
Exponentially growing Leishmania was counted directly in a hemocytometer. Serial dilutions of the culture were prepared, and 100 μl of each dilution was dispensed, in triplicate, in the wells of a 96-well plate to obtain the following final concentrations: 1 × 106, 0.5 × 106, 0.25 × 106, 0.125 × 106, 0.0625 × 106, 0.0312 × 106, 0.0156 × 106, and 0.0078 × 106 parasites/well, for (i) direct counting in a hemocytometer, (ii) an MTT test, (iii) an XTT test, and (iv) a WST-8 test.
Phenol red increases background noise (18); the presence of other colored compounds (as drugs) might also modify absorbance or lead to interactions with tetrazolium salts (18). We therefore prepared replica plates for the following tests: (i) a test in which the parasites were centrifuged (series C) to allow the replacement of the culture medium and (ii) a test in which the parasites were not centrifuged (series NC).
The plates were incubated for 72 h at 26°C (14). The controls were blanks containing RPMIØRP medium with no parasites.
Tetrazolium salt tests.
The cell viability tests were based on the manufacturer's recommendations and the method described by Dutta (14), with the following modifications: after 72 h of culture, the parasites were quantified either by the direct addition of tetrazolium salts to the parasite cultures for the NC series or after centrifuging the parasites for 5 min at 514 × g and medium replacement for the C series.
We added tetrazolium salts to the following concentrations: 10% for MTT, from a 5-mg/ml solution (Sigma); 20% for XTT, from a 1-mg/ml solution supplemented with 1% 5-methylphenazinium methyl sulfate (PMS) (Sigma); or 10% for WST-8, supplemented with 1-methoxy-PMS (Cell Counting kit 8; Sigma).
The plates were incubated for 3 h at 26°C. We then added 100 μl of dimethyl sulfoxide (DMSO) to the wells containing MTT, and the plates were returned to the 26°C incubator for 1 h.
We determined absorbance at 570 nm for the MTT tests and at 450 nm for the XTT and WST-8 tests, using a Tristar LB941 spectrophotometer (Berthold Technologies). We eliminated the background noise by subtracting the value of the blank (no parasites and no tetrazolium salts) from the values obtained in the tests.
Counting in a hemocytometer.
The cells were counted directly, with or without prior centrifugation, in a hemocytometer. Counts were carried out in parallel for the three tetrazolium salt tests.
Sensitivity tests.
The sensitivity tests were carried out with L. guyanensis isolate LG-2, obtained from a patient. When the parasites reached exponential growth phase, we added 90 μl of culture, containing 106 parasites, to each well. We dispensed 10 μl of various dilutions of pentamidine (Sigma) or of an extract in ethanol (70:30) of Lantana camara leaves into the test wells to obtain final concentrations of 0.125, 0.0625, 0.0312, 0.015, 0.0078, and 0.0039 μg/ml pentamidine, or 2,000, 1,000, 500, 250, 125, and 62.5 μg/ml Lantana camara leaf extract. We added 10 μl of RPMIØRP to the control wells. The tests were carried out in triplicate.
The blanks for the tests consisted of 90 μl of RPMIØRP medium and 10 μl of the various concentrations of pentamidine or plant extract. The blanks for the controls consisted of 100 μl of RPMIØRP medium.
After incubation for 48 h at 26°C, we added 10% WST-8 supplemented with 1-methoxy-PMS to each well, including the blanks. The plate was incubated for a further 24 h. The results were read by spectrophotometry at 450 nm after 72 h (in total) of incubation in the presence of the drug or plant extract.
The absorbance values for the blanks were subtracted from the corresponding test absorbance values, and the percentage of inhibition was calculated as follows: % inhibition = [(Acontrol − Atest)/Acontrol] × 100.
The 50% inhibitory concentration (IC50) was then calculated using the GraphPad Prism6 software program.
Statistical analysis.
We calculated the standard deviation (SD) for each point using the software program Excel (ECARTYPEP function). The r coefficient of correlation value was determined using Excel to assess correlation between the parasite number and optical density (OD).
RESULTS
The various colorimetric tests based on MTT, XTT, and WST-8 were compared using the reference strain L. guyanensis LG-R.
Impact of centrifugation on quantification tests.
Direct counts of parasites were obtained in parallel, with a hemocytometer, to validate the colorimetric results. Parasite quantification tests were carried out at different cell densities, with and without centrifugation, to evaluate the potential impact of centrifugation on the results obtained (Fig. 1).
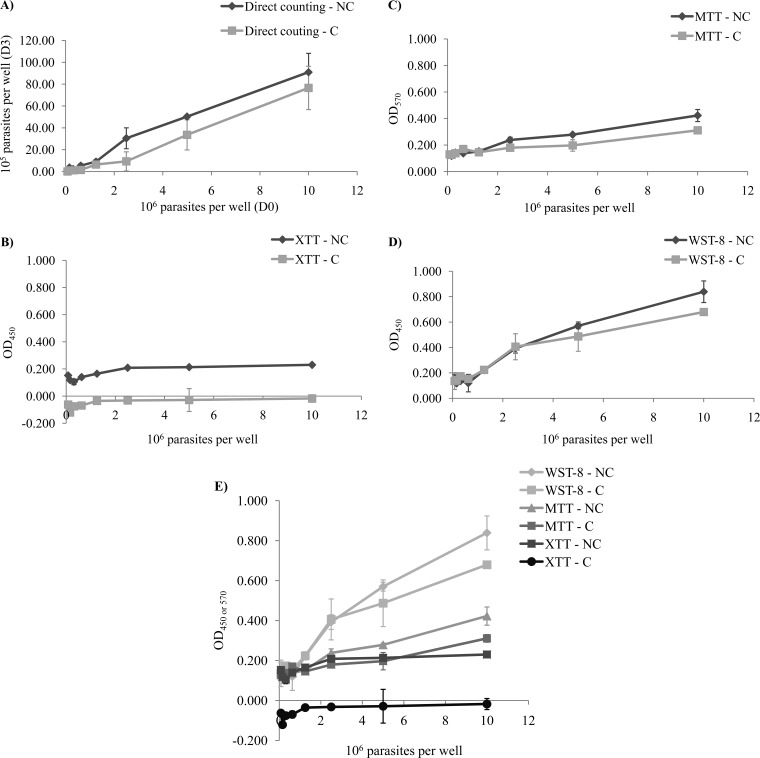
FIG 1.
Quantification of L. guyanensis LEM 3319 parasites seeded at various concentrations (0, 0.0078 × 106, 0.015 × 106, 0.0312 × 106, 0.0625 × 106, 0.125 × 106, 0.25 × 106, 0.5 × 106, and 1 × 106 parasites/well) after 72 h of incubation, by direct counting in a hemocytometer (A) or colorimetric tests with XTT (B), MTT (C), or WST-8 (D). For each test, two series were carried out, one in which the parasites were centrifuged (series C) and another in which the parasites were not centrifuged (series NC). The results of the various colorimetric tests with tetrazolium salts were then compared (E). Absorbance was measured at 570 nm for MTT tests and at 450 nm for XTT and WST-8 tests.
Comparison of the 3 tetrazolium salt tests.
Direct counting of the parasites in a hemocytometer after 72 h of culture generated a linear growth curve (Fig. 1A), with a proportional relationship between the number of parasites used to inoculate the medium on day 0 (D0) and the number of parasites counted on D3. However, this linear relationship was observed at parasite densities exceeding 1.25 × 105 parasites/well. We consider this density to be the threshold of detection for direct counting. Centrifugation did not seem to affect the results obtained. Indeed, the number of parasites was smaller in series C but remained proportional to that in series NC. Centrifugation led to a reproducible loss of cells, with a coefficient of about 2.5, in all wells.
An increase in the population by a factor of 10 after 72 h of culture was noted. We can therefore consider the number of parasites to be 10 times higher on D3 than on D0, and these values can be used to interpret the results of colorimetric tests.
Parasite quantification by colorimetric tests based on XTT (Fig. 1B) gave very low or even negative absorbance values, ranging from −0.121 to −0.015 for series C and from 0.105 to 0.230 for series NC. The values obtained in this test were too low to demonstrate any real differences in the numbers of cells present.
In contrast, parasite quantification with the MTT test (Fig. 1C) gave higher absorbance values, ranging from 0.118 to 0.423 for series NC and from 0.129 to 0.311 for series C. The curves for the C and NC series were almost parallel, and the numbers of parasites before and after centrifugation may therefore be considered proportional.
The threshold of detection for parasites was 2.5 × 106 parasites/well, the curve increasing after this point. The standard deviations for MTT tests were relatively high (data not shown).
Quantification in the WST-8 test (Fig. 1D) gave absorbance values well above those obtained in the other two colorimetric tests (MTT and XTT), with values ranging from 0.177 to 0.839 for series NC and from 0.143 to 0.679 for series C. The threshold for parasite detection was about 0.625 × 107 parasites/well.
For series NC, each of the coefficients of correlation (r) was calculated. Thus, direct counting yielded an r value of 0.99, the MTT test had an r value of 0.98, the WST-8 test had an r value of 0.98, and the XTT test had an r value of 0.82.
Metabolization of tetrazolium salts by L. guyanensis and L. amazonensis.
We compared the metabolism of the various tetrazolium salts and the efficacies of these tests by omitting the 72 h of incubation after cell inoculation and the centrifugation step. The tetrazolium salts were added to the cultures directly after inoculation.
We evaluated the metabolism of the three tetrazolium salts after 4 h of incubation, using the reference strains L. guyanensis LG-R and L. amazonensis LA-R, at three concentrations: 1 × 106, 5 × 105, and 2.5 × 105 parasites/well. The results are presented as a histogram in Fig. 2. The absorbance values obtained in the MTT tests were very similar for the three densities of L. amazonensis LA-R (r = 0.80) tested, whereas those for L. guyanensis LG-R (r = 0.82) decreased with decreasing cell density.
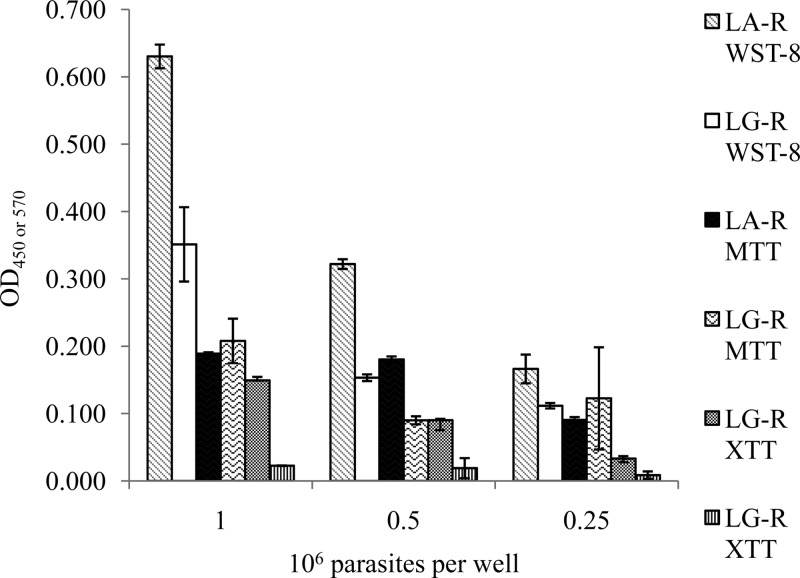
FIG 2.
Comparison of the metabolism of different tetrazolium salts (XTT, MTT, and WST-8) by the LG-R and LA-R strains at densities of 1 × 106, 0.5 × 106, and 0.25 × 106 parasites/well.
For the XTT tests, the absorbance results obtained were consistent with the number of parasites for L. amazonensis LA-R (r = 0.98), whereas for L. guyanensis LG-R (r = 0.89), the absorbance values obtained were very low or zero.
The absorbance values obtained in WST-8 tests were higher than those for the other tests, particularly for L. amazonensis LA-R, which had an absorbance value of 0.630, versus 0.351 for the L. guyanensis LG-R strain, for a density of 1 × 106 parasites/well. The absorbance values decreased proportionally with the number of cells for the L. amazonensis LA-R strain (r = 1) and L. guyanensis LG-R (r = 0.98).
The WST-8 test was the colorimetric test with the best coefficient of correlation (r), the best sensitivity (Fig. 1D), and the best tetrazolium salt metabolism (Fig. 2).
Metabolization of WST-8 by Leishmania spp.
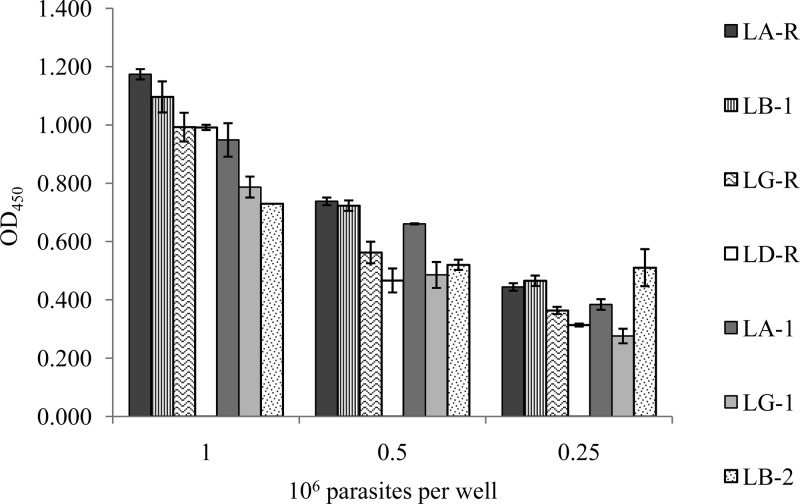
We assessed the efficacy of the WST-8 test for quantifying the various strains of Leishmania, using strains L. guyanensis LG-R, L. guyanensis LG-1, L. amazonensis LA-R, L. amazonensis LA-1, L. braziliensis LB-1, L. braziliensis LB-2, and L. donovani LD-R at densities of 1 × 106, 5 × 105, and 2.5 × 105 parasites/well (Fig. 3). Absorbance depended on the strain tested, with A450 values ranging from 0.606 for L. braziliensis LB-2 to 1.05 for L. amazonensis LA-R for 1 × 106 parasites/well. Some strains from different species gave similar absorbance values: for example, L. amazonensis LA-R (A450 = 0.738) and L. braziliensis LB-1 (A450 = 0.723) for 5 × 105 parasites/well and L. guyanensis LG-R (A450 = 0.993) and L. donovani LD-R (A450 = 0.992) for 1 × 106 parasites/well.
FIG 3.
Comparison of the metabolism of WST-8 by strains LG-R, LG-1, LA-R, LA-1, LB-1, LB-2, and LD-R at densities of 1 × 106, 0.5 × 106, and 0.25 × 106 parasites/well.
Optimal incubation time for the WST-8 quantification test.
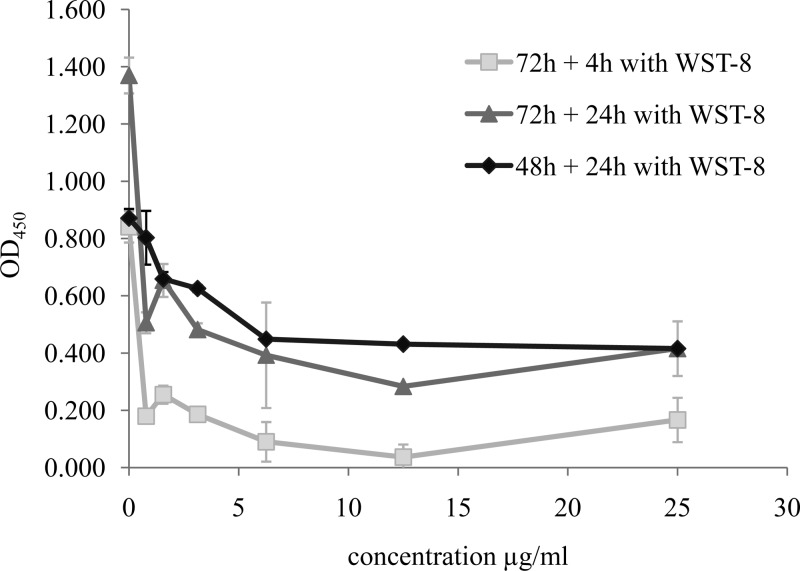
We determined the optimal incubation time for the WST-8 quantification test, with the patient isolate L. guyanensis LG-3, in the presence of various concentrations of amphotericin B (Fig. 4). Incubation for 4 h with WST-8 was not sufficient to obtain consistent results (Fig. 4) and yielded very low absorbance values due to cell death. Incubation with WST-8 for longer periods was therefore required, with 24 h identified as the most functional incubation time. However, incubation for 24 h in the presence of WST-8, after 72 h of cell culture in the presence of amphotericin B, gave a curve similar to that for incubation for 4 h in the presence of WST-8 and resulted in a total incubation time of 96 h rather than 72 h. For the performance of the test over a period of 72 h, we recommend adding the WST-8 after 48 h of incubation with the drug or plant extract and then reading the absorbance 24 h later.
FIG 4.
Optimization of the incubation time of parasites in the presence of WST-8. LG-3 parasites (1 × 106 parasites/well) were placed in contact with various concentrations of amphotericin B for 48 h or 72 h and were then incubated for 4 or 24 h in the presence of WST-8.
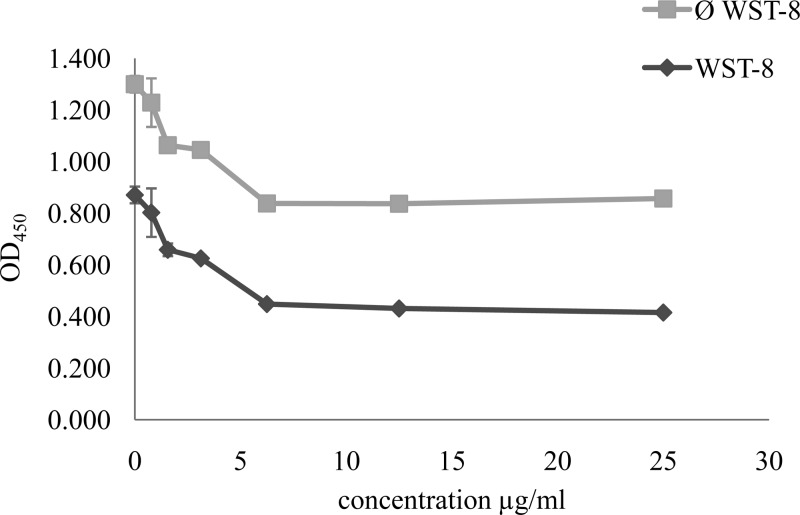
Interaction between amphotericin B and WST-8.
We checked that the compounds tested did not interact with WST-8. Figure 5 shows the results obtained in the presence of amphotericin B. Similar curves were obtained for the absorbance measured in the presence or absence of WST-8.
FIG 5.
Demonstration of the absence of interaction between WST-8 and amphotericin B. We placed 1 × 106 parasites/well of LG-3 in contact with various concentrations of amphotericin B. The test blanks consisted of 90 μl of RPMIØRP and 10 μl of amphotericin B at various concentrations, with (WST-8) or without (Ø WST-8) the addition of WST-8. The absorbance values of the test blanks were subtracted from the test absorbance values.
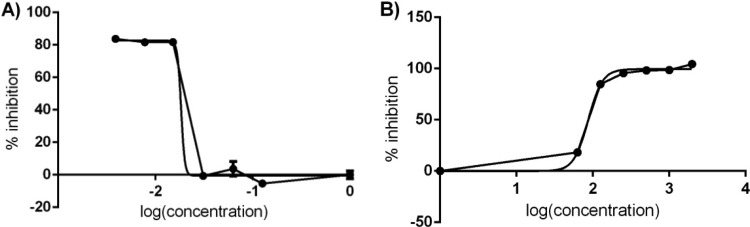
Sensitivity test of plant extracts and pentamidine.
The sensitivities of the patient isolate L. guyanensis LG-2 to various antimicrobial compounds was assessed as a function of the incubation time determined above. The results obtained in the presence of pentamidine or an extract of Lantana camara leaves in ethanol are shown in Fig. 6A and B, respectively. The IC50s obtained were 0.018 μg/ml for pentamidine and 86.81 μg/ml for Lantana camara leaf extract. Ethanol did not affect parasite growth (data not shown).
FIG 6.
Percent inhibition of patient isolate LG-2 in the WST-8 test in the presence of pentamidine at concentrations of 0.125, 0.0625, 0.0312, 0.015, 0.0078, 0.0039, and 0 μg/ml (A) or of Lantana camara leaf extract in ethanol at concentrations of 2,000, 1,000, 500, 250, 125, 62.5, and 0 μg/ml (B) for a parasite density of 1 × 106 cells/well.
DISCUSSION
Parasite quantification is a crucial step in proliferation and cytotoxicity tests. Simple, rapid, and effective tests are required, allowing the analysis of multiple samples.
Many cell quantification techniques are available, including colorimetric tests in particular. In this study, we compared three tetrazolium salt tests: those based on MTT, XTT, and WST-8. We chose to include the MTT test on the basis of its reputation, this test being the most widely used in cell quantification studies (12, 19, 20). XTT is also widely used, since it palliates some of the deficiencies reported for the MTT test (21–23), and WST-8 is one of the most recently synthesized tetrazolium salts (24).
We found that the sensitivity of the MTT test was quite low for Leishmania guyanensis, indicating that this species probably metabolizes this compound poorly, if at all. This test also displayed a lack of reproducibility, as already reported by Wan et al. (25), due to the insolubility of formazan, the product formed. The MTT test therefore requires a solubilization step to dissolve the product in an organic solvent, such as DMSO (26). Unfortunately, this dissolution is not always complete and may generate biases; also, the presence of bubbles generated by this additional step interfere with absorbance readings. In this study, we obtained standard deviations of more than 0.2 in some tests (data not shown). In addition, the quality of the correlation with the results obtained by tritiated thymidine counting remains variable (13, 27).
XTT (21) and MTS (28) tests were developed to overcome these problems of solubility. Indeed, these salts are reduced to soluble derivatives of formazan, and their reduction is accelerated by the addition of an electron-coupling agent, phenazine methosulfate (PMS) (29), thereby increasing test sensitivity. However, although the efficacy of these tests has been demonstrated in several studies (17, 27) for evaluations of the sensitivity of Leishmania to drugs or plant extracts (30), we found that XTT tests displayed a lack of sensitivity with L. guyanensis strains. Furthermore, the presence of PMS may lead to the formation of crystals in the culture medium, modifying the absorbance of the product formed and resulting in erroneous findings (21). Menadione, another electron-coupling agent, also promotes the rapid reduction of XTT (31) in many cell lines, and its use also decreases background noise. However, crystals are also observed in the presence of this electron-coupling agent, albeit in smaller numbers than for PMS (21).
The WST-8 test gave highly satisfactory results in our study, with an acceptable sensitivity and a proportional relationship between the number of parasites and absorbance. WST-8 is, in fact, an improved version of WST-3, which is itself an improved version of WST-1: it combines the high stability of WST-1 with the high sensitivity of WST-3 (24). WST-3 is less stable than WST-1 due to the presence of two nitro (-NO2) groups on the same benzene ring. However, it is a much more sensitive agent than WST-1 for tests of viability. WST-8 was synthesized to improve the stability of this molecule while conserving its sensitivity. It was produced by transferring one of the nitro groups of WST-3 onto another benzene ring (24). The electron-coupling agent used with WST-8, 1-methoxy-PMS, is also an improved form of PMS that is more effective and insensitive to the photochemical deterioration observed with PMS (32).
The results presented in this study are consistent with those obtained by Ishiyama et al. and Tominaga et al., whose work on human and rabbit cells showed the absorbance values obtained to be proportional to the number of cells, highlighting the greater sensitivity of the WST-8 test than of those based on the other tetrazolium salts tested, WST-1, XTT, and MTS (24, 33).
Many studies have highlighted the efficacy of MTA tests, but this efficacy varies as a function of the following: (i) the type of tetrazolium salt used (33) (in our study, XTT was less well metabolized than WST-8), (ii) the species of Leishmania tested (15) (L. amazonensis was, in this study, the species that best metabolized most of the tetrazolium salts tested, whereas L. guyanensis metabolized these salts poorly), and (iii) the strains studied, the mitochondria of which may have different enzymatic activities (15, 21). Indeed, our observations show that for a density of 1 × 106 parasites/well, the absorbance value obtained with L. braziliensis LB-2 was 0.730, whereas that obtained with L. braziliensis LB-1 was 1.096.
The sensitivity tests with patient isolate L. guyanensis LG-2 incubated with pentamidine or extract of Lantana camara leaves, carried out with WST-8, provided satisfactory results, with IC50s of 0.018 μg/ml for pentamidine and 86.81 μg/ml for Lantana camara extract. The results obtained were consistent with microscopy observations carried out after 72 h of culture in the presence of pentamidine.
Certain substances present in drugs or plant extracts may interfere with tetrazolium salts through chemical effects on cellular respiration processes (34). In such cases, centrifugation can be used to decrease this phenomenon (cell washing step). We therefore evaluated the impact of a centrifugation step on cell quantification. We found that the centrifugation step did not cause a bias (constant cell loss). However, in the presence of a drug or plant extract, the results obtained after centrifugation (data not shown) were not consistent for XTT and MTT. These results may reflect interactions between these tetrazolium salts and the leishmanicidal compounds tested. In contrast, with WST-8, the results obtained were good enough to eliminate the parasite centrifugation step. Indeed, WST-8 did not seem to interact with the drugs or plant extracts, because the results obtained were proportional in both the presence and absence of WST-8, with and without centrifugation. A control would nevertheless be required to check for the absence of interference with all new products tested.
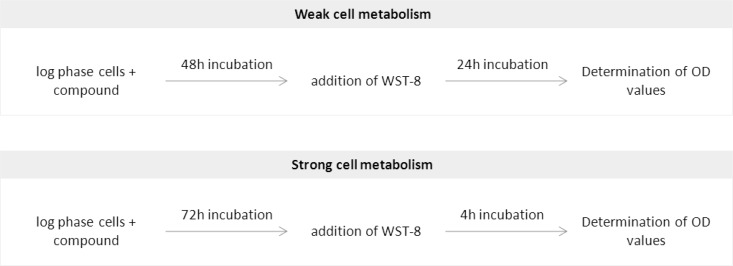
In conclusion, this study confirms that the efficacy of tetrazolium salt reduction depends on the strain of Leishmania tested and the tetrazolium salt used. L. guyanensis accounts for 90% of the strains isolated from patients with cutaneous leishmaniasis in French Guiana. A test suitable for evaluating the level of susceptibility to drugs of the strains of Leishmania responsible for these cases of leishmaniasis is therefore required. We have shown here that the most widely used tests, the XTT and MTT tests, are not suitable for studies of the susceptibility of L. guyanensis. WST-8 is the tetrazolium salt best metabolized by L. guyanensis, resulting in better sensitivity. It also displays satisfactory levels of efficacy in tests of susceptibility to drugs or plant extracts. Thus, this process is ideal for quantification of cells, either to assess cell sensitivity to drugs or to screen new antimicrobial compounds. For this, we suggest performing the tests with WST-8 according to the following protocol: drug or compound is added to cells from exponential phase. The addition of WST-8 is advised after 48 h of incubation, and the absorbance is read in a spectrophotometer at 450 nm after 24 h. However, if the cells metabolize the tetrazolium salt well, addition of WST-8 may be performed after 72 h of incubation and reading after 4 h of incubation (Fig. 7).
FIG 7.
Protocol recommended for quantification of cells by WST-8 test according to their metabolism.
This WST-8 assay is now applied in our lab for preliminary screening of field isolates for resistance to drugs usually used to treat leishmaniasis and for screening of new antileishmanial agents. Thus, the WST-8 assay is a valuable tool to assess viability and proliferation of Leishmania promastigotes or for a variety of other cell types.
ACKNOWLEDGMENTS
This work was supported by the University of the French West Indies and French Guiana and the Ministère Français de l'Enseignement Supérieur et de la Recherche Scientifique. It has benefited from an Investissement d'Avenir grant managed by Agence Nationale de la Recherche (CEBA, reference no. ANR-10-LABX-25-01) and was also supported by the Conseil Régional de la Guyane and the European Union (FEDER-Presage no. 31454).
Footnotes
Published ahead of print 9 April 2014
REFERENCES
- 1.WHO. 2010. Control of the leishmaniases. WHO Technical Report Series 949. WHO, Geneva, Switzerland [Google Scholar]
- 2.Faraut-Gambarelli F, Piarroux R, Deniau M, Giusiano B, Marty P, Michel G, Faugère B, Dumon H. 1997. In vitro and in vivo resistance of Leishmania infantum to meglumine antimoniate: a study of 37 strains collected from patients with visceral leishmaniasis. Antimicrob. Agents Chemother. 41:827–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lira R, Sundar S, Makharia A, Kenney R, Gam A, Saraiva E, Sacks D. 1999. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J. Infect. Dis. 180:564–567. 10.1086/314896 [DOI] [PubMed] [Google Scholar]
- 4.Berman JD, Gallalee JV. 1985. Semiautomated assessment of in vitro activity of potential antileishmanial drugs. Antimicrob. Agents Chemother. 28:723–726. 10.1128/AAC.28.6.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharief AH, Gasim Khalil EA, Theander TG, Kharazmi A, Omer SA, Ibrahim ME. 2006. Leishmania donovani: an in vitro study of antimony-resistant amphotericin B-sensitive isolates. Exp. Parasitol. 114:247–252. 10.1016/j.exppara.2006.03.016 [DOI] [PubMed] [Google Scholar]
- 6.Hu VW, Black GE, Torres-Duarte A, Abramson FP. 2002. 3H-thymidine is a defective tool with which to measure rates of DNA synthesis. FASEB J. 16:1456–1457. 10.1096/fj.02-0142fje [DOI] [PubMed] [Google Scholar]
- 7.Di Giorgio C, Ridoux O, Delmas F, Azas N, Gasquet M, Timon-David P. 2000. Flow cytometric detection of Leishmania parasites in human monocyte-derived macrophages: application to antileishmanial-drug testing. Antimicrob. Agents Chemother. 44:3074–3078. 10.1128/AAC.44.11.3074-3078.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamau SW, Nunez R, Grimm F. 2001. Flow cytometry analysis of the effect of allopurinol and the dinitroaniline compound (Chloralin) on the viability and proliferation of Leishmania infantum promastigotes. BMC Pharmacol. 1:1. 10.1186/1471-2210-1-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross DD, Joneckis CC, Ordóñez JV, Sisk AM, Wu RK, Hamburger AW, Nora RE. 1989. Estimation of cell survival by flow cytometric quantification of fluorescein diacetate/propidium iodide viable cell number. Cancer Res. 49:3776–3782 [PubMed] [Google Scholar]
- 10.Gogal RM, Jr, Ahmed SA, Larsen CT. 1997. Analysis of avian lymphocyte proliferation by a new, simple, nonradioactive assay (lympho-pro). Avian Dis. 41:714–725. 10.2307/1592166 [DOI] [PubMed] [Google Scholar]
- 11.Mikus J, Steverding D. 2000. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol. Int. 48:265–269. 10.1016/S1383-5769(99)00020-3 [DOI] [PubMed] [Google Scholar]
- 12.Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55–63. 10.1016/0022-1759(83)90303-4 [DOI] [PubMed] [Google Scholar]
- 13.Berg K, Zhai L, Chen M, Kharazmi A, Owen TC. 1994. The use of a water-soluble formazan complex to quantitate the cell number and mitochondrial function of Leishmania major promastigotes. Parasitol. Res. 80:235–239. 10.1007/BF00932680 [DOI] [PubMed] [Google Scholar]
- 14.Dutta A, Bandyopadhyay S, Mandal C, Chatterjee M. 2005. Development of a modified MTT assay for screening antimonial resistant field isolates of Indian visceral leishmaniasis. Parasitol. Int. 54:119–122. 10.1016/j.parint.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 15.Ganguly S, Bandyopadhyay S, Sarkar A, Chatterjee M. 2006. Development of a semi-automated colorimetric assay for screening anti-leishmanial agents. J. Microbiol. Methods 66:79–86. 10.1016/j.mimet.2005.10.011 [DOI] [PubMed] [Google Scholar]
- 16.Sereno D, Lemesre JL. 1997. Use of an enzymatic micromethod to quantify amastigote stage of Leishmania amazonensis in vitro. Parasitol. Res. 83:401–403. 10.1007/s004360050272 [DOI] [PubMed] [Google Scholar]
- 17.Williams C, Espinosa OA, Montenegro H, Cubilla L, Capson TL, Ortega-Barria E, Romero LI. 2003. Hydrosoluble formazan XTT: its application to natural products drug discovery for Leishmania. J. Microbiol. Methods 55:813–816. 10.1016/j.mimet.2003.08.013 [DOI] [PubMed] [Google Scholar]
- 18.Denizot F, Lang R. 1986. Rapid colorimetric assay for cell growth and survival: modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 89:271–277. 10.1016/0022-1759(86)90368-6 [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Peterson DA, Kimura H, Schubert D. 1997. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J. Neurochem. 69:581–593. 10.1046/j.1471-4159.1997.69020581.x [DOI] [PubMed] [Google Scholar]
- 20.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. 1987. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of radiosensitivity. Cancer Res. 47:943–946 [PubMed] [Google Scholar]
- 21.Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. 1988. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 48:4827–4833 [PubMed] [Google Scholar]
- 22.Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. 1991. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J. Immunol. Methods 142:257–265. 10.1016/0022-1759(91)90114-U [DOI] [PubMed] [Google Scholar]
- 23.Meshulam T, Levitz SM, Christin L, Diamond RD. 1995. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanilide (XTT). J. Infect. Dis. 172:1153–1156. 10.1093/infdis/172.4.1153 [DOI] [PubMed] [Google Scholar]
- 24.Ishiyama M, Miyazono Y, Sasamoto K, Ohkura Y, Ueno K. 1997. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta 44:1299–1305. 10.1016/S0039-9140(97)00017-9 [DOI] [PubMed] [Google Scholar]
- 25.Wan H, Williams R, Doherty P, Williams DF. 1994. A study of the reproducibility of the MTT test. J. Mater. Sci. Mater. Med. 5:154–159. 10.1007/BF00053336 [DOI] [Google Scholar]
- 26.Marshall N, Goodwin C, Holt S. 1995. A critical assessment of the use of microculture tetrazolium assays to measure cell growth and function. Growth Regul. 5:69–84 [PubMed] [Google Scholar]
- 27.Wang L, Sun J, Horvat M, Koutalistras N, Johnston B, Ross Sheil AG. 1996. Evaluation of MTS, XTT, MTT and 3HTdR incorporation for assessing hepatocyte density, viability and proliferation. Methods Cell Sci. 18:249–255. 10.1007/BF00132890 [DOI] [Google Scholar]
- 28.Cory AH, Owen TC, Barltrop JA, Cory JG. 1991. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 3:207–212 [DOI] [PubMed] [Google Scholar]
- 29.Buttke TM, McCubrey JA, Owen TC. 1993. Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphokine-dependent cell lines. J. Immunol. Methods 157:233–240. 10.1016/0022-1759(93)90092-L [DOI] [PubMed] [Google Scholar]
- 30.Cole SPC. 1986. Rapid chemosensitivity testing of human lung tumor cells using the MTT assay. Cancer Chemother. Pharmacol. 17:259–263. 10.1007/BF00256695 [DOI] [PubMed] [Google Scholar]
- 31.Singh U, Akhtar S, Mishra A, Sarkar D. 2011. A novel screening method based on menadione mediated rapid reduction of tetrazolium salt for testing of anti-mycobacterial agents. J. Microbiol. Methods 84:202–207. 10.1016/j.mimet.2010.11.013 [DOI] [PubMed] [Google Scholar]
- 32.Hisada R, Yagi T. 1977. 1-Methoxy-5-methylphenazinium methyl sulfate. A photochemically stable electron mediator between NADH and various electron acceptors. J. Biochem. 82:1469–1473 [DOI] [PubMed] [Google Scholar]
- 33.Tominaga H, Ishiyama M, Ohseto F, Sasamoto K, Hamamoto T, Suzuki K, Watanabe M. 1999. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal. Commun. 36:47–50. 10.1039/A809656B [DOI] [Google Scholar]
- 34.Pearse AGE. 1972. Principles of oxidoreductase histochemistry, p 880–920 In Histochemistry, theoretical and applied, 3rd ed. Churchill Livingston, Edinburgh, United Kingdom [Google Scholar]