Abstract
Anaplasma platys is an obligate intracellular rickettsial pathogen that infects platelets of dogs, forming basophilic intracellular morulae. In the present report, cellular inclusions were documented in bone marrow thrombocyte precursors of two young naturally infected dogs, indicating that A. platys can infect megakaryocytes and promegakaryocytes.
CASE REPORTS
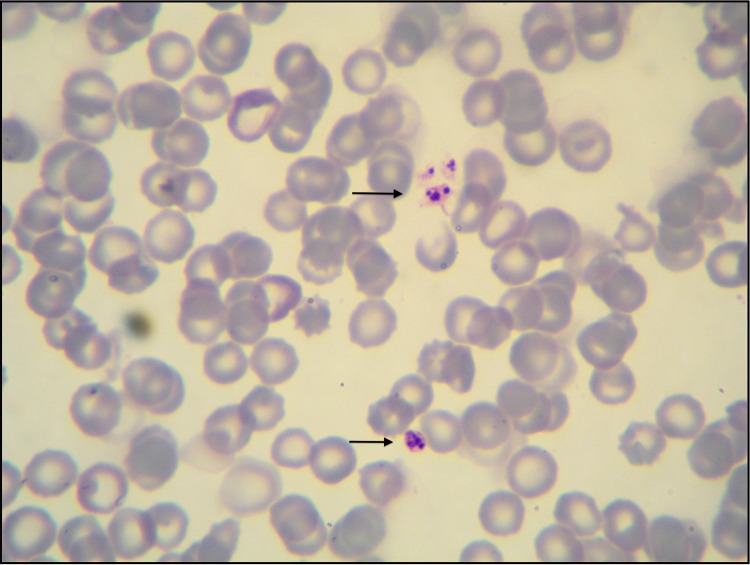
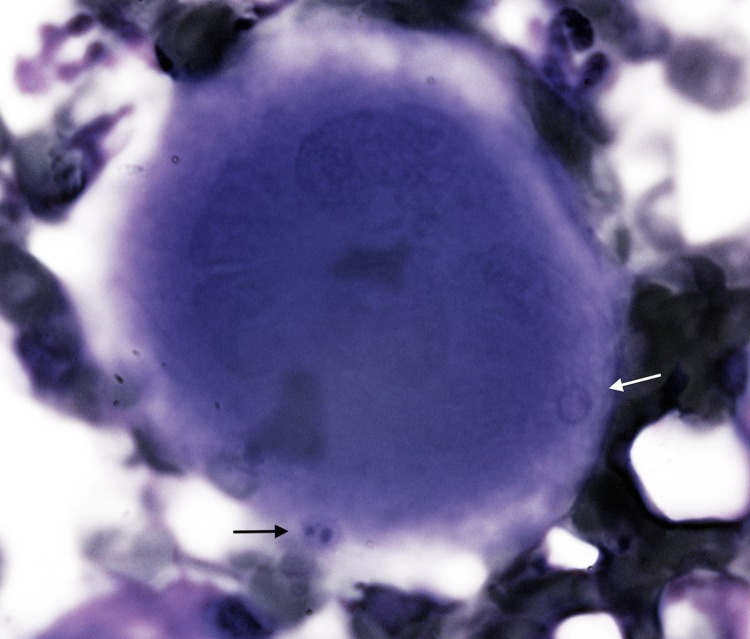
Due to the presence of unique bone marrow examination abnormalities, two 3-month-old, mixed-breed female (case 1) and male (case 2) dogs were selected from 124 dogs described in previous studies involving canine vector-borne diseases (CVBDs) (1, 2). Both dogs originated from a kennel located in Putignano (Bari, Apulia, Italy). At the outset of the study, the dogs were vaccinated by two intramuscular injections of DuramuneH DAPPI+LC (Fort Dodge Animal Health, Italy) against common dog pathogens (canine parvovirus, adenovirus type 2, distemper virus, Leptospira interrogans serovar Canicola, and Leptospira interrogans serovar Icterohaemorrhagiae) and dewormed using a combination of febantel-pyrantel-praziquantel (Drontal plusH; Bayer AG, Germany). At the initial evaluation, each dog had a massive Rhipicephalus sanguineus sensu lato tick infestation and no previous acaricidal treatment. Physical examination abnormalities for both dogs included an underweight (body condition score [BCS] = 2/5) appearance and moderate depressed attitude. Blood and bone marrow samples were collected for testing (Italian Ministry of Health authorization DGSA no. 0001997; authorized on 2 April 2011; as reported in references 1 and 2). Hematological findings for dog 1 included normocytic normochromic nonregenerative anemia (red blood cell [RBC] count of 4.44 × 106/μl, with reference intervals of 5.50 × 106 to 8.0 × 106/μl; hemoglobin [Hb] of 9.5 g/dl, with reference intervals of 14.0 to 19.5 g/dl; hematocrit [Ht] of 28.6%, with reference intervals of 38.0 to 54.0%) and monocytosis (1,224/μl, with reference intervals of 200 to 1,200/μl), and dog 2 had normocytic hypochromic anemia (RBC count of 4.13 × 106/μl, with reference intervals of 5.50 × 106 to 8.0 × 106/μl; Hb of 7.8 g/dl; Ht of 26.4%) and neutropenia (3,024 neutrophils/μl, with reference intervals of 3,500 to 9,300/μl); severe thrombocytopenia was recorded in both cases (platelet counts of 30,000 and 23,000/μl, respectively, with reference intervals of 180,000 to 450,000/μl). Cytological abnormalities on Giemsa-stained blood smears from both dogs included anisocytosis, hypochromasia, and reduced platelet numbers, containing intraplatelet inclusions indicative of Anaplasma platys (Fig. 1). Bone marrow cytology findings included a normal myeloid-to-erythroid ratio (M/E) in dog 1 and a reduced M/E with myeloid hypoplasia in dog 2; monocytic hyperplasia and dysplasia (including vacuolized cytoplasm), macrophagic hyperplasia, erythrophagocytosis, and platelet phagocytosis were evident in both animals. At low magnification, megakaryocytic hyperplasia and dysplasia characterized by nuclei disorganization were evident. In particular, 1 to 3 densely basophilic inclusions, similar to those noted in blood platelets, were observed in promegakaryocytes (Fig. 2), accompanied by emperipolesis. The organisms were morphologically identical to A. platys inclusions in peripheral platelets. PCR confirmation of A. platys infection and the absence of concomitant vector-borne pathogens (i.e., Leishmania infantum, Ehrlichia canis, Hepatozoon canis, Babesia vogeli, and Bartonella spp.) by serological and molecular assays, performed on both blood and bone marrow samples, were previously reported (1, 2).
FIG 1.
Thrombocytes containing Anaplasma platys inclusions (black arrows). Blood, Diff-Quick stain (×100 magnification).
FIG 2.
Promegakaryocytes with Anaplasma platys inclusions in a developing platelet (black arrow) and a platelet without inclusions (white arrow). Bone marrow, Diff-Quick stain (×100 magnification).
Anaplasma platys, the causative agent of the infectious canine cyclic thrombocytopenia (ICCT), infects platelets of dogs, forming basophilic intracellular morulae (3, 4). Recent publications report A. platys infections in a veterinarian and in cats, highlighting the importance of this organism for cats, as well its zoonotic potential (5). This rickettsial pathogen has a wide geographic distribution, including in the Americas, Africa, Asia, the Middle East, southern Europe, and Australia (6, 7, 8), and is believed to be transmitted by ticks, as A. platys DNA has been amplified from R. sanguineus sensu lato (9, 10); however, the role of this tick species as a competent vector needs to be confirmed (11). In the canine host, A. platys enters the platelets by endocytosis and replicates by binary fission within a vacuole, resulting in the formation of morulae that are evident in the blood 10 to 14 days after intravenous inoculation, which corresponds with the onset of thrombocytopenia (3, 12); the severity of thrombocytopenia and the percentage of infected platelets are highest during the first infection cycle (13), and thrombocytopenia is thought to develop as a consequence of direct injury to platelets by replicating organisms (initial infection) and also due to immune-mediated mechanisms (antiplatelet antibodies) in subsequent thrombocytopenic episodes (3, 14). Dogs infected with different A. platys strains may show minimal clinical disease, although severe disease has been reported, with platelet counts of 20,000/μl or less (15). To the best of our knowledge, A. platys inclusions have been reported only in platelets and have not been previously described in platelet precursors of naturally infected dogs, specifically megakaryocytes and promegakaryocytes (3, 12, 16). Dense basophilic inclusions were observed in bone marrow platelet precursors of both dogs. The inclusions, located within the cell cytoplasm, were round to oval in shape (1 to 2 μm in diameter) and numbered 1 to 3 per cell. Some inclusions were surrounded by a visible membrane, whereas others seemed to be in the cytoplasm of the platelet precursors without a membrane (Fig. 1). The megakaryocytic organisms appeared morphologically identical to A. platys infecting peripheral platelets. The findings herein reported indicate that A. platys may infect developing platelet precursors in the bone marrow rather than, or in addition to, directly infecting platelets (6, 17, 18). Therefore, the cellular invasion mechanisms of megakaryocytic precursors with transfer to proplatelets need to be more thoroughly elucidated. Indeed, a similar phenomenon was described with Anaplasma phagocytophilum experimental infection of a human megakaryocytic cell culture line, where platelet progenitors became infected by this pathogen, which is closely related to A. platys; however, the experimental infection described above did not impair proplatelet formation and platelet production (19). Both A. platys-infected dogs had mild clinical signs, despite hematological abnormalities (i.e., anemia, neutropenia, and severe thrombocytopenia), compatible with a rickettsial infection (20, 21). Evaluation of blood samples identified a reduced platelet count and a medium to high A. platys rickettsemia (≤50% of platelets infected), indicating that the cycle of bacteremic infection was occurring during the time of sampling. The cytological findings in the bone marrow of both dogs were consistent with the hematological abnormalities concurrently found in the blood. Anaplasma platys infection induces platelet phagocytosis as a consequence of direct injury or immune-mediated destruction (3); moreover, the increase in circulating monocytes of dog 1 and bone marrow monocyte and macrophage hyperplasia and platelet phagocytosis observed in both dogs may be related to an overwhelming A. platys infection. Thrombocytopenia in both animals was classified as regenerative on the basis of an appropriate bone marrow response, as observed by cytology. Both dogs had severe megakaryocytic hyperplasia; the mean numbers of megakaryocytes and promegakaryocytes were significantly increased, as reported for experimental A. platys and E. canis infections (22). Dysplasia was also evident and characterized by cytoplasm fragmentation and nuclear abnormalities, including hyperlobulation and disorganized nuclei. Megakaryocytic emperipolesis, the movement of blood cells (erythrocytes, neutrophils, and lymphocytes) within megakaryocytes (23), was observed in the bone marrow of both dogs. However, to the author's knowledge, this phenomenon has not been previously described in dogs naturally infected by A. platys.
In conclusion, the findings herein reported indicate that A. platys can infect megakaryocytes and promegakaryocytes of the bone marrow of naturally infected dogs also associated with dysmegakaryocytopoiesis. Further studies are required to establish if the young age of the animals could represent a predisposing factor for platelet precursor infection.
Footnotes
Published ahead of print 12 March 2014
REFERENCES
- 1.Dantas-Torres F, Capelli G, Giannelli A, Ramos RA, Lia RP, Cantacessi C, de Caprariis D, De Tommasi AS, Latrofa MS, Lacasella V, Tarallo VD, Di Paola G, Qurollo B, Breitschwerdt E, Stanneck D, Otranto D. 2013. Efficacy of an imidacloprid/flumethrin collar against fleas, ticks and tick-borne pathogens in dogs. Parasit. Vectors 6:245. 10.1186/1756-3305-6-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otranto D, Dantas-Torres F, de Caprariis D, Di Paola G, Tarallo VD, Latrofa MS, Lia RP, Annoscia G, Breitshwerdt EB, Cantacessi C, Capelli G, Stanneck D. 2013. Prevention of canine leishmaniosis in a hyper-endemic area using a combination of 10% imidacloprid/4.5% flumethrin. PLoS One 8:e56374. 10.1371/journal.pone.0056374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvey JW, Simpson CF, Gaskin JM. 1978. Cyclic thrombocytopenia induced by a Rickettsia-like agent in dogs. J. Infect. Dis. 137:182–188. 10.1093/infdis/137.2.182 [DOI] [PubMed] [Google Scholar]
- 4.Arraga-Alvarado C, Palmar M, Parra O, Salas P. 2003. Ehrlichia platys (Anaplasma platys) in dogs from Maracaibo, Venezuela: an ultrastructural study of experimental and natural infections. Vet. Pathol. 40:149–156. 10.1354/vp.40-2-149 [DOI] [PubMed] [Google Scholar]
- 5.Qurollo BA, Balakrishnan N, Cannon CZ, Maggi RG, Breitschwerdt EB. 20 January 2014. Co-infection with Anaplasma platys, Bartonella henselae, Bartonella koehlerae and ‘Candidatus Mycoplasma haemominutum' in a cat diagnosed with splenic plasmacytosis and multiple myeloma. J. Feline Med. Surg. 10.1177/1098612X13519632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvey JW. 2012. Anaplasma platys infection (Thrombocytotropic Anaplasmosis), p 256–258 In Greene CE. (ed), Infectious diseases of the dog and cat, 4th ed. Saunders Elsevier, St. Louis, MO [Google Scholar]
- 7.Aktas M, Altay K, Dumanli N, Kalkan A. 2009. Molecular detection and identification of Ehrlichia and Anaplasma species in ixodid ticks. Parasitol. Res. 104:1243–1248. 10.1007/s00436-009-1377-1 [DOI] [PubMed] [Google Scholar]
- 8.Rojas A, Rojas D, Montenegro V, Gutiérrez R, Yasur-Landau D, Baneth G. 2014. Vector-borne pathogens in dogs from Costa Rica: first molecular description of Babesia vogeli and Hepatozoon canis infections with a high prevalence of monocytic ehrlichiosis and the manifestations of co-infection. Vet. Parasitol. 199:121–128. 10.1016/j.vetpar.2013.10.027 [DOI] [PubMed] [Google Scholar]
- 9.Inokuma H, Raoult D, Brouqui P. 2000. Detection of Ehrlichia platys DNA in brown dog ticks (Rhipicephalus sanguineus) in Okinawa Island, Japan. J. Clin. Microbiol. 38:4219–4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanogo YO, Davoust B, Inokuma H, Camicas JL, Parola P, Brouqui P. 2003. First evidence of Anaplasma platys in Rhipicephalus sanguineus (Acari: Ixodida) collected from dogs in Africa. Onderstepoort J. Vet. Res. 70:205–212 [PubMed] [Google Scholar]
- 11.Dantas-Torres F. 2008. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet. Parasitol. 152:173–185. 10.1016/j.vetpar.2007.12.030 [DOI] [PubMed] [Google Scholar]
- 12.Gaunt SD, Baker DC, Babin SS. 1990. Platelet aggregation studies in dogs with acute Ehrlichia platys infection. Am. J. Vet. Res. 51:290–293 [PubMed] [Google Scholar]
- 13.Baker DC, Simpson M, Gaunt SD, Corstevet RE. 1987. Acute Ehrlichia platys infection in the dog. Vet. Pathol. 24:449–453 [DOI] [PubMed] [Google Scholar]
- 14.French TW, Harvey JW. 1993. Canine infectious cyclic thrombocytopenia (Ehrlichia platys infection in dogs), p 195–208 In Woldehiwet Z, Ristic M. (ed), Rickettsial and chlamydial diseases of domestic animals. Pergamon Press, New York, NY [Google Scholar]
- 15.Harrus S, Aroch I, Lavy E, Bark H. 1997. Clinical manifestations of infectious canine cyclic thrombocytopenia. Vet. Rec. 141:247–250. 10.1136/vr.141.10.247 [DOI] [PubMed] [Google Scholar]
- 16.Little SE. 2010. Ehrlichiosis and anaplasmosis in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 40:1121–1140. 10.1016/j.cvsm.2010.07.004 [DOI] [PubMed] [Google Scholar]
- 17.Breitschwerdt EB. 2005. Obligate intracellular bacterial pathogens, p 634–635 In Ettinger SJ, Feldman EC. (ed), Textbook of veterinary internal medicine, 6th ed. Elsevier Saunders, St. Louis, MO [Google Scholar]
- 18.Lappin MR. 2009. Infectious diseases, p 1324–1325 In Nelson WR, Couto CG. (ed), Small animal internal medicine, 4th ed. Elsevier Saunders, St. Louis, MO [Google Scholar]
- 19.Granick JL, Reneer DV, Carlyon JA, Borjesson DL. 2008. Anaplasma phagocytophilum infects cells of the megakaryocytic lineage through sialylated ligands but fails to alter platelet production. J. Med. Microbiol. 57:416–423. 10.1099/jmm.0.47551-0 [DOI] [PubMed] [Google Scholar]
- 20.Harrus S, Waner T, Neer TM. 2012. Ehrlichia canis infection, p 227–238 In Greene CE. (ed), Infectious diseases of the dog and cat, 4th ed. Elsevier Saunders, St. Louis, MO [Google Scholar]
- 21.Dyachenko V, Pantchev N, Balzer H-J, Meyersen A, Straubinger RK. 2012. First case of Anaplasma platys infection in a dog from Croatia. Parasit. Vectors 5:49. 10.1186/1756-3305-5-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuehn NF, Gaunt SD. 1985. Clinical and hematologic findings in canine ehrlichiosis. J. Am. Vet. Med. Assoc. 186:355–358 [PubMed] [Google Scholar]
- 23.Thiele J, Krech R, Choritz H, Georgii A. 1984. Emperipolesis—a peculiar feature of megakaryocytes as evaluated in chronic myeloproliferative diseases by morphometry and ultrastructure. Virchows Arch. B Cell Pathol. 46:253–263. 10.1007/BF02890314 [DOI] [PubMed] [Google Scholar]