Abstract
Three commercial antimicrobial susceptibility testing (AST) methods were compared to broth microdilution for testing of Staphylococcus aureus and enterococci against vancomycin, daptomycin, and linezolid. Despite high levels of categorical agreement and essential agreement, vancomycin MICs determined by MicroScan were often 1 log2 concentration higher and MICs determined by Phoenix 1 log2 concentration lower. Daptomycin MICs were 1 to 2 log2 concentrations higher by all AST methods, except Etest, potentially impacting definitive antimicrobial therapy for bloodstream infections due to these organisms.
TEXT
Despite a recently reported decline in incidence, Staphylococcus aureus is still a major cause of bacteremia and sepsis worldwide, and up to 50% of bacteremic episodes caused by S. aureus have been attributed to methicillin-resistant S. aureus (MRSA) (1–5). Vancomycin is considered a cornerstone for the empirical treatment of bloodstream infections (BSI) due to Gram-positive bacteria and, specifically, for the treatment of bacteremia due to MRSA (6). In 2006, the Clinical and Laboratories Standards Institute (CLSI) adjusted the susceptibility and resistance breakpoints for vancomycin MICs against S. aureus: the breakpoint for susceptible (S) results was lowered from ≤4 μg/ml to ≤2 μg/ml, the breakpoint for intermediate (I) results was changed from 8 to 16 μg/ml to 4 to 8 μg/ml, and the breakpoint for resistant (R) results was changed from ≥32 μg/ml to ≥16 μg/ml (7, 8). However, clinical failures with vancomycin treatment for MRSA bacteremia still occur with isolates having MICs within the susceptible range (9–12). Recognized clinical treatment failure with vancomycin has prompted the use of high-dose vancomycin treatment regimens and the use of alternate antimicrobial agents (12–15). While recent guidelines suggest that changes in treatment for MRSA bacteremia should not be based solely on vancomycin MICs, the use of alternate antimicrobial agents is recommended for certain clinical settings when vancomycin MICs are ≥1 μg/ml (6, 16). Several studies suggested that the determination of the vancomycin MIC is method dependent and that, despite categorical agreement (CA), various commercially available antimicrobial susceptibility testing (AST) methods differ from the broth microdilution method (BMD) in their accuracy and/or agreement in determining MICs for vancomycin (17). If certain AST methods indeed give higher MIC values for vancomycin-susceptible MRSA isolates, some health care providers may be inclined to use an alternate antimicrobial agent in anticipation of vancomycin treatment failure. The purposes of this study were to determine the distribution of MICs for vancomycin and to select alternate antimicrobial agents against S. aureus (MRSA and methicillin-susceptible S. aureus [MSSA]), Enterococcus faecium, and Enterococcus faecalis and, furthermore, to determine the accuracy of various commercially available AST methods compared to the “gold standard” broth microdilution method.
(This work was presented in part at the 112th General Meeting of the American Society for Microbiology, San Francisco, CA [18].)
During a 12-month period, AST was performed using commercially available methods for testing clinical, nonduplicate isolates of S. aureus (n = 150) and enterococci (n = 51) obtained from blood cultures of unique patients hospitalized at our institution. AST was performed with the following commercial methods and systems: Etest (bioMérieux, Durham, NC); the MicroScan WalkAway system; Pos Combo Panel Type 29, using the prompt and the turbidity inoculum preparation methods (Siemens Healthcare Diagnostics, West Sacramento, CA); and the Phoenix system, panel PMIC/ID-105 (Becton, Dickinson and Co., Sparks, MD). All commercial AST panels were inoculated and incubated according to the manufacturers' specifications. For each test run, the following quality control (QC) strains were tested: S. aureus ATCC 43300 (MRSA), S. aureus ATCC 29213 (MSSA), and E. faecalis ATCC 51299 and ATCC 29212. All QC results were within the expected ranges for each of the QC strains tested. Upon completion of AST by the commercial methods, bacterial isolates were stored frozen (−70°C), and susceptibility testing by the reference method was performed in a batched mode after completion of enrollment. Prior to testing isolates by the BMD reference method, all previously stored frozen isolates were subcultured twice on BBL Trypticase soy agar (TSA II) with 10% sheep blood agar (SBA) (BD Diagnostic Systems, Sparks, MD) to ensure viability and purity before use for antimicrobial susceptibility testing. The CLSI broth microdilution method (BMD) was used as the reference method, following established guidelines (19). Custom-designed, frozen-form AST panels were purchased from Trek Diagnostic Systems (Thermo Fisher Scientific, Cleveland, OH), and CLSI-approved ATCC quality control strains were used for quality control and validation testing prior to panels being used. Bacterial isolates were tested using cation-adjusted Mueller-Hinton broth as the test medium, according to manufacturer's specifications and CLSI guidelines (19). Inoculated AST panels were incubated for 24 h at 35°C in ambient air. Daptomycin-containing wells in MIC panels contained a final concentration of approximately 50 μg of calcium/ml. The dilution ranges (μg/ml) for all antimicrobial agents used in the BMD reference method and all commercial AST methods were as follows: for the MicroScan system Pos Combo panel, type 29, vancomycin, 0.25 to 16, daptomycin, 0.5 to 4, and linezolid, 1 to 4; for the Phoenix system PMIC/ID-105 panel, vancomycin, 0.5 to 32, daptomycin, 0.25 to 4, and linezolid, 0.5 to 4; for Etest, vancomycin, daptomycin, and linezolid, 0.016 to 256 (each); and for BMD, vancomycin, 0.06 to 256, daptomycin, 0.03 to 32, and linezolid, 0.03 to 32. CLSI breakpoints for categorical susceptibility assessment of MICs were used according to the 2012 CLSI guidelines, and essential agreements (EA) (MIC ± 1 log2) were assessed accordingly (7). Using the CLSI method as the gold standard, categorical agreement (CA) and essential agreement (EA) between the test methods was assessed as described previously (20). In the absence of an intermediate/resistant interpretive category for daptomycin, all categorical errors were defined as either major errors (false nonsusceptible [NS]) or very major errors (false susceptible, where nonsusceptible values equal resistance). Similarly, in the absence of an intermediate category for testing linezolid against S. aureus, categorical errors were either major (false resistant) or very major (false susceptible) errors. In addition, simple descriptive statistical analyses, including t test and chi-square test analyses, were performed. The study was approved by the Institutional Review Board of the Johns Hopkins Medical Institutions.
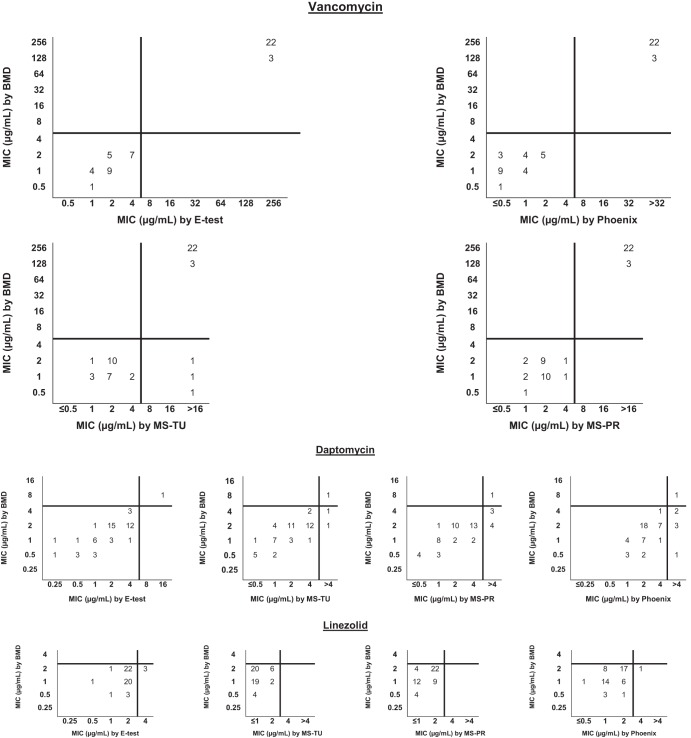
MIC results, interpretative categories, and comparisons between commercial AST methods and the BMD reference method for the 150 isolates of S. aureus are shown in Fig. 1 and for the 51 enterococcal isolates in Fig. 2. The modal MIC value for testing staphylococci against vancomycin by BMD, Etest, and the MicroScan system, irrespective of inoculum preparation method, was 1 μg/ml; for the Phoenix system, the vancomycin modal MIC was 0.5 μg/ml. For testing staphylococci against daptomycin, the modal MIC value for BMD, Etest, and MicroScan was 0.5 μg/ml; for the Phoenix system, the modal MIC was 0.25 μg/ml. The linezolid modal MIC was 1 μg/ml using the BMD, Etest, and Phoenix systems; using the MicroScan system, the linezolid modal MIC was 2 μg/ml, irrespective of the method of inoculum preparation. The CA and EA of the four commercial methods compared with those of the BMD method for vancomycin, daptomycin, and linezolid are shown in Table 1. Considering that the Phoenix and MicroScan systems utilize breakpoint panels, we considered the presence of an MIC above the breakpoint for the intermediate or resistant category to represent agreement should the CLSI reference method provide a more specific, higher MIC indicating an intermediate and/or resistant result for the isolate. For AST of S. aureus isolates against vancomycin, 100% CA was observed for the Etest and Phoenix methods. However, for the MicroScan system, two MRSA isolates and one MSSA isolate that were vancomycin susceptible by the BMD method were vancomycin intermediate by MicroScan, two by the prompt method and one by the turbidity inoculation method (minor errors). Testing S. aureus isolates against daptomycin, 100% CA was observed among all AST methods, except for the MicroScan system using the prompt inoculum preparation method. One MSSA isolate was daptomycin nonsusceptible by this method, whereas the results determined by BMD indicated the isolate was daptomycin susceptible. Despite the high level of CA, differences in vancomycin MIC distributions for staphylococci among all commercial AST methods, using the CLSI BMD method as the gold standard, were statistically significant (P < 0.001), except for MicroScan using the turbidity method (P = 0.29). MICs determined by the Etest method and MicroScan prompt inoculum preparation method were frequently 1 log2 concentration higher than MICs determined by CLSI BMD, and MICs determined by the Phoenix were frequently 1 log2 concentration lower. In contrast, the few differences in the daptomycin MIC distributions among all commercial AST methods were not statistically significant, except for the MicroScan system using the prompt method for inoculation preparation (P < 0.001); MICs determined by this method were frequently 1 log2 concentration higher than BMD MICs. A slightly higher degree of variability in essential agreement between all AST methods was observed for the enterococci tested in this study. The CA for testing enterococci against vancomycin was 100% for Etest, Phoenix, and the MicroScan prompt method and was 94% for the MicroScan turbidity method. One isolate of E. faecium with an MIC of 0.5 μg/ml [S] by BMD had an MIC of >16 μg/ml [R] by the MicroScan turbidity method. Two isolates of E. faecalis had MICs of 1 μg/ml [S] and 2 μg/ml [S] by BMD but had corresponding MICs of >16 μg/ml [R] and 16 μg/ml [I] by the MicroScan turbidity method, respectively. All three instances were considered to be major errors. Using the BMD method as the gold standard, there were no statistically significant differences in the vancomycin MIC distributions among the 51 enterococci tested by all commercial AST methods (Phoenix, P = 0.13; Etest, P = 0.78; MicroScan prompt, P = 0.99; MicroScan turbidity, P = 0.89). We observed 100% CA among all three commercial AST methods compared to BMD for all 29 isolates of E. faecalis against daptomycin. One E. faecium isolate (1/22) was identified as daptomycin nonsusceptible (NS) by BMD. While the one daptomycin-NS isolate was correctly identified by the Phoenix method, this method identified an additional five isolates as NS (major errors). MicroScan using the turbidity inoculation method identified two additional isolates as daptomycin-NS, and using the prompt inoculation method, seven isolates were identified as NS (major errors). For all 51 enterococci, the three commercial methods more commonly resulted in daptomycin MICs that were 1 to 2 log2 concentrations higher than the MICs obtained by the BMD method, and these differences were statistically significant (MicroScan, prompt method, P < 0.001; MicroScan, turbidity method, P = 0.026; Phoenix, P < 0.001); there was no statistically significant difference in daptomycin MIC distribution in the comparisons of Etest to BMD (P = 0.0513).
FIG 1.
Scattergram of MIC results (μg/ml) and MIC interpretation by CLSI reference method and 4 commercial test methods for Staphylococcus aureus (n = 150) (MRSA, n = 100; MSSA, n = 50). Abbreviations: BMD, broth microdilution method (CLSI reference method used in this study); MS-TU, MicroScan system, using the turbidity inoculum preparation method; MS-PR, MicroScan system, using the prompt inoculum preparation method. The CLSI breakpoints for the susceptible category of the respective antimicrobial agents are indicated by vertical and horizontal lines. All 150 isolates of S. aureus were susceptible to linezolid; the CLSI-approved breakpoint for the susceptible category is ≤4 μg/ml.
FIG 2.
Scattergrams of MIC results (μg/ml) and MIC interpretation by CLSI reference method and 4 commercial test methods for Enterococcus faecalis (n = 29) and Enterococcus faecium (n = 22). Abbreviations: BMD, broth microdilution method (CLSI reference method used in this study); MS-TU, MicroScan system, using the turbidity inoculum preparation method; MS-PR, MicroScan system, using the prompt inoculum preparation method. The CLSI breakpoints for the susceptible category of the respective antimicrobial agents are indicated by vertical and horizontal lines.
TABLE 1.
Essential and categorical agreements for 4 commercial AST methods compared to the broth microdilution CLSI reference method for 150 S. aureus and 51 enterococcal isolates tested against vancomycin, daptomycin, and linezolid
| Microorganism(s) | ASTa method or system | Essential agreement (%) |
Categorical agreement (%) |
||||
|---|---|---|---|---|---|---|---|
| Vancomycin | Daptomycin | Linezolid | Vancomycin | Daptomycin | Linezolid | ||
| S. aureus (n = 150) | Etest | 96 | 100 | 99 | 100 | 100 | 100 |
| Phoenix | 99 | 99.3 | 100 | 100 | 100 | 100 | |
| MS—turbidity | 99 | 100 | 98 | 100 | 99.3 | 100 | |
| MS—prompt | 99 | 99.3 | 92 | 100 | 98.7 | 100 | |
| Enterococci (n = 51) | Etest | 100 | 96 | 94 | 100 | 100 | 94b |
| Phoenix | 94 | 84 | 98 | 100 | 88c | 95 | |
| MS—turbidity | 98 | 90 | 100 | 94 | 96d | 100 | |
| MS—prompt | 90 | 96 | 100 | 94 | 86e | 100 | |
Abbreviations: AST, antimicrobial susceptibility testing; MS, MicroScan system.
Categorical agreement of 100% was observed for E. faecalis (29/29 isolates) against linezolid, whereas 86% categorical agreement was observed for E. faecium (19/22 isolates) against linezolid.
Categorical agreement of 97% was observed for E. faecalis (28/29 isolates) against daptomycin, whereas 77% categorical agreement was observed for E. faecium (17/22 isolates) against daptomycin.
Categorical agreement of 100% was observed for E. faecalis (29/29 isolates) against daptomycin, whereas 91% categorical agreement was observed for E. faecium (20/22 isolates) against daptomycin.
Categorical agreement of 100% was observed for E. faecalis (29/29 isolates) against daptomycin, whereas 68% categorical agreement was observed for E. faecium (15/22 isolates) against daptomycin.
Although the breakpoints for the vancomycin-susceptible category were lowered by CLSI in 2006, some studies suggested that these breakpoints should perhaps be lowered even further, considering the increasing number of vancomycin treatment failures (12, 13, 17). In this regard, the accuracy of the AST results obtained by the various AST methods is of critical importance. Despite the almost 100% CA and EA between all commercial, FDA-approved AST methods and the BMD reference method in our study, we found significant 1 to 2 log2 variations in the MIC values for S. aureus and enterococci tested against vancomycin, daptomycin, and linezolid. Specifically, vancomycin MICs determined with the Phoenix system were frequently 1 log2 concentration lower for staphylococci, whereas vancomycin MICs determined with the MicroScan system using the prompt method for inoculation preparation were frequently 1 log2 concentration higher. Similarly, the daptomycin and linezolid MICs of S. aureus isolates tested by the MicroScan system, specifically using the prompt inoculation method, were frequently 1 log2 concentration higher than the MICs obtained by BMD (P < 0.001). Our findings were consistent with those from other studies (17, 21). One study evaluated the performance of Etest, MicroScan, and the Trek Sensititre system compared to that of the CLSI BMD method for testing of staphylococci, including 20 vancomycin-intermediate S. aureus (VISA) isolates, against vancomycin (21). Those authors found that vancomycin MICs determined by the CLSI BMD method were frequently 1 log2 concentration lower than those determined by Etest or MicroScan. Interestingly, those authors suggested that the CLSI BMD method is more likely to misclassify VISA as being vancomycin susceptible and therefore suggested verifying the identification of VISA by either MicroScan or Etest. In their study, which used a national repository, classification of VISA was based on individual institutions' AST methods, and apparently no further testing to confirm the initial vancomycin-intermediate MIC result of these isolates was performed. Specifically, no gene-sequencing analysis and/or population analysis of the MRSA isolates was reported. The authors of that study furthermore indicated that 2 isolates with initial MICs of 4 to 8 μg/ml had MICs of <2 μg/ml when retested by BMD at the national organism repository site (21). An interesting observation in that study was, however, the significant difference between the vancomycin MICs obtained by various methods; several commercial AST methods, and specifically Etest and MicroScan, frequently produce vancomycin MICs that are 1 log2 concentration higher than the corresponding MICs obtained by the CLSI BMD reference method (17, 21–23). Similarly, other studies demonstrated that various commercial AST methods report MIC values for daptomycin that are lower than those reported by the CLSI BMD reference method (22, 24, 25). However, Jevitt et al. described in their study that despite the overall tendency to underestimate the daptomycin MIC by Etest, a subset of staphylococcal isolates biased toward daptomycin-nonsusceptible isolates presented with MICs determined by Etest that were higher than those MICs determined by the BMD method (24). Additional studies demonstrated not only lot-related differences in daptomycin MICs for the testing of staphylococci but also differences in categorical and essential agreements (73% to 100%) together with various rates of very major errors (3% to 9%) and major errors (6% to 35%) (25). In similarity to our results for linezolid MICs, other studies described AST method-dependent log2 variations of MICs as well as low percentages of CA and EA for testing staphylococci against linezolid (26). With respect to antimicrobial susceptibility testing of enterococci, few studies have reported on the differences in the performance of various commercial AST systems (26–29). One study described 100% categorical agreement and 98% essential agreement for AST of enterococci against vancomycin using the Phoenix system (27). The findings of that study are concordant with the results of our investigation. In accordance with the findings of a study investigating susceptibility of enterococci to daptomycin (28), the results from our investigation further illustrate the fact that the MicroScan system (prompt inoculum preparation method) has a tendency to overestimate nonsusceptibility to daptomycin. This “discrepancy” between the categorical agreement and the essential agreement of results for enterococcal isolates tested by the MicroScan system using the prompt inoculum preparation method was particularly observed in enterococci with MICs at the breakpoint of susceptible to nonsusceptible; these isolates were considered susceptible by the BMD method but nonsusceptible by the MicroScan system.
Information regarding the MICs of vancomycin is of particular relevance to clinicians when choosing appropriate antimicrobial therapy in cases of staphylococcal and/or enterococcal bacteremia/sepsis in the setting of perceived or real decreased clinical effectiveness of vancomycin (30–35). Soriano et al. demonstrated in their study (31) that calculation of the area under the concentration-time curve over 24 h in the steady state divided by the MIC (the AUC/MIC ratio) is the best approach to predict vancomycin efficacy in patients with serious S. aureus infections. It is therefore intuitive that even a 1 log2 difference in the MICs determined not only has an impact on the AUC/MIC ratio but also has the potential to significantly impact the ability to optimize treatment (31, 36). Given the results from our study, it is important for both laboratories and clinicians to know what type of AST system is used for the analyses before considering changes in antimicrobial therapy for serious, systemic MRSA infections.
There are some limitations to our study. The fact that all isolates were obtained from a single institution may limit the generalizability of our findings, although other institutions and investigators have previously published similar observations. Furthermore, the sample size was relatively small, particularly for the enterococci. Limited enrollment of isolates may have resulted in a bias toward E. faecalis and an underrepresentation of linezolid-nonsusceptible isolates of S. aureus. In addition, we did not specifically test bacterial isolates with MICs at the CLSI-defined susceptibility breakpoints for specific antimicrobial agents in order to further challenge the commercial AST systems; we instead used clinical isolates in a real-time, clinical setting for this performance evaluation of AST methods. Lastly, the performance of the BMD reference method in a time-delayed, batched mode could have affected AST results, albeit the time between storage and AST of isolated did not in general exceed 9 months. At the time when BMD was performed, repeat AST of a convenience sample with commercial systems was not performed.
In summary, we determined that three commercial AST systems/methods commonly used in clinical microbiology laboratories frequently produce 1 to 2 log2 differences in vancomycin and daptomycin MICs compared to the gold standard BMD method for susceptibility testing of staphylococci and enterococci, despite an otherwise nearly 100% categorical agreement. In our study, the MicroScan prompt inoculation method was less reliable in its performance than the MicroScan turbidity inoculum preparation method with respect providing accurate MIC values. Considering that vancomycin is still considered the first choice for treatment of serious, systemic MRSA infections, such MIC differences determined by the AST method may have implications for the choice of recommended treatment in light of recently reported treatment failures with vancomycin. Additional prospective clinical studies will be necessary to determine the true impact of AST methods and observed differences in MIC values with respect to perceived or real vancomycin treatment failure.
ACKNOWLEDGMENTS
This study was supported in part by funds from Cubist Pharmaceuticals, Lexington, MA.
S.R. received research funding from Cubist Pharmaceuticals.
K.M.N., S.W.E., L.M.D., T.T., and K.C.C. declare that they have no conflicts of interest.
The use of trade names is for identification purposes only and does not constitute endorsement by The Johns Hopkins University and/or The Johns Hopkins Medical Institutions.
Footnotes
Published ahead of print 9 April 2014
REFERENCES
- 1.Khatib R, Sharma M, Iyer S, Fakih MG, Obeid KM, Venugopal A, Fishbain J, Johnson LB, Segireddy M, Jose J, Riederer K. 2013. Decreasing incidence of Staphylococcus aureus bacteremia over 9 years: greatest decline in community-associated methicillin-susceptible and hospital-acquired methicillin-resistant isolates. Am. J. Infect. Control 41:210–213. 10.1016/j.ajic.2012.03.038 [DOI] [PubMed] [Google Scholar]
- 2.Klein EY, Sun L, Smith DL, Laxminarayan R. 2013. The changing epidemiology of methicillin-resistant Staphylococcus aureus in the United States: a national observational study. Am. J. Epidemiol. 177:666–674. 10.1093/aje/kws273 [DOI] [PubMed] [Google Scholar]
- 3.Kullar R, Rybak MJ, Kaye KS. 2013. Comparative epidemiology of bacteremia due to methicillin-resistant Staphylococcus aureus between older and younger adults: a prospective score analysis. Infect. Control Hosp. Epidemiol. 34:400–406. 10.1086/669868 [DOI] [PubMed] [Google Scholar]
- 4.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, Active Bacterial Core surveillance (ABCs) MRSA Investigators 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. 10.1001/jama.298.15.1763 [DOI] [PubMed] [Google Scholar]
- 5.Diekema DJ, Richter SS, Heilmann KP, Dohrn CL, Riahi F, Tendolkar S, McDanel JS, Doern GV. 2014. Continued emergence of USA300 methicillin-resistant Staphylococcus aureus in the United States: results from a nationwide surveillance study. Infect. Control Hosp. Epidemiol. 35:285–292. 10.1086/675283 [DOI] [PubMed] [Google Scholar]
- 6.Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 66:82–98. 10.2146/ajhp080434 [DOI] [PubMed] [Google Scholar]
- 7.CLSI. 2012. Performance standards for antimicrobial susceptibility testing, twenty-second informational supplement. CLSI document M100–S22. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8.Tenover FC, Moellering RC., Jr 2007. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin. Infect. Dis. 44:1208–1215. 10.1086/513203 [DOI] [PubMed] [Google Scholar]
- 9.Hawser SP, Bouchillon SK, Hoban DJ, Dowzicky M, Babinchak T. 2011. Rising incidence of Staphylococcus aureus with reduced susceptibility to vancomycin and susceptibility to antibiotics: a global analysis 2004–2009. Int. J. Antimicrob. Agents 37:219–224. 10.1016/j.ijantimicag.2010.10.029 [DOI] [PubMed] [Google Scholar]
- 10.van Hal SJ, Lodise TP, Paterson DL. 2012. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin. Infect. Dis. 54:755–771. 10.1093/cid/cir935 [DOI] [PubMed] [Google Scholar]
- 11.Holmes RL, Jorgensen JH. 2008. Inhibitory activities of 11 antimicrobial agents and bactericidal activities of vancomycin and daptomycin against invasive methicillin-resistant Staphylococcus aureus isolates obtained from 1999 through 2006. Antimicrob. Agents Chemother. 52:757–760. 10.1128/AAC.00945-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, Stallrecht K. 2008. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob. Agents Chemother. 52:3315–3320. 10.1128/AAC.00113-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. 2006. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections. Arch. Intern. Med. 166:2138–2144. 10.1001/archinte.166.19.2138 [DOI] [PubMed] [Google Scholar]
- 14.Moore CL, Osaki-Kiyan P, Haque NZ, Perri MB, Donabedian S, Zervos MJ. 2012. Daptomycin versus vancomycin for bloodstream infections due to methicillin-resistant Staphylococcus aureus with high vancomycin minimum inhibitory concentration: a case-control study. Clin. Infect. Dis. 54:51–58. 10.1093/cid/cir764 [DOI] [PubMed] [Google Scholar]
- 15.Murray KP, Zhao JJ, Davis SL, Kullar R, Kaye KS, Lephart P, Rybak MJ. 2013. Early use of daptomycin versus vancomycin for methicillin-resistant Staphylococcus aureus bacteremia with vancomycin minimum inhibitory concentration > 1 mg/L: a matched cohort study. Clin. Infect. Dis. 56:1562–1569. 10.1093/cid/cit112 [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:e18–e55. 10.1093/cid/ciq146 [DOI] [PubMed] [Google Scholar]
- 17.Toyokawa M, Francisco M, Nishi I, Sunada A, Ueda A, Sakata T, Kimura K, Inoue Y, Asari S, Tomono K. 2011. Accuracy of commercial susceptibility testing method for measuring vancomycin MIC against methicillin-resistant Staphylococcus aureus (MRSA). Lab. Med. 42:473–477. 10.1309/LM9HB2LB2AORIATX [DOI] [Google Scholar]
- 18.Riedel S, Eisinger SW, Schwartz M, Dam LM, Tekle T, Carroll KC. 2012. Comparison of various commercially available antimicrobial susceptibility test methods for assessment of MIC values for vancomycin, daptomycin, linezolid, and quinupristin-dalfopristin against Staphylococcus aureus and enterococci. Abstr. 112th Gen. Meet. Am. Soc. Microbiol., abstr C 4386 [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7–A8. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 20.Turnidge J, Paterson DL. 2007. Setting and revising antibacterial susceptibility breakpoints. Clin. Microbiol. Rev. 20:391–408. 10.1128/CMR.00047-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadarajah R, Post LR, Lui C, Miller SA, Sahm DF, Brooks GF. 2010. Detection of vancomycin-intermediate Staphylococcus aureus with the updated Trek-Sensititre system and the MicroScan system. Am. J. Clin. Pathol. 133:844–848. 10.1309/AJCPMV1P0VKUAZRD [DOI] [PubMed] [Google Scholar]
- 22.Sader HS, Romberg PR, Jones RN. 2009. Nine-hospital study comparing broth microdilution and E-test method results for vancomycin and daptomycin against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:3162–3165. 10.1128/AAC.00093-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swenson JM, Anderson KF, Lonsway DR, Thompson A, McAllister SK, Limbago BM, Carey RB, Tenover FC, Patel JB. 2009. Accuracy of commercial and reference susceptibility testing methods for detecting vancomycin-intermediate Staphylococcus aureus. J. Clin. Microbiol. 47:2013–2017. 10.1128/JCM.00221-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jevitt LA, Thorne GM, Traczewski MM, Jones RN, McGowan JE, Tenover FC, Brown SD. 2006. Multicenter evaluation of the Etest and disk diffusion methods for differentiating daptomycin-susceptible from non-daptomycin-susceptible Staphylococcus aureus isolates. J. Clin. Microbiol. 44:3098–3104. 10.1128/JCM.00665-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedrich L, Thorne G, Steenbergen JN, Anastasiou D, Koeth L. 2009. Evidence for daptomycin Etest lot-related MIC elevations for Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 65:306–311. 10.1016/j.diagmicrobio.2009.07.012 [DOI] [PubMed] [Google Scholar]
- 26.Tenover FC, Williams PP, Stocker S, Thompson A, Clark LA, Limbago B, Carey RB, Poppe SM, Shinabarger D, McGowan JE., Jr 2007. Accuracy of six antimicrobial susceptibility methods for testing linezolid against staphylococci and enterococci. J. Clin. Microbiol. 45:2917–2922. 10.1128/JCM.00913-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carroll KC, Borek AP, Burger C, Glanz B, Bhally H, Henciak S, Flayhart DC. 2006. Evaluation of the BD Phoenix automated microbiology system for identification and antimicrobial susceptibility testing of staphylococci and enterococci. J. Clin. Microbiol. 44:2072–2077. 10.1128/JCM.02636-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryant KA, Roberts AL, Rupp ME, Anderson JA, Lyden ER, Fey PD, van Schooneveld TC. 2013. Susceptibility of enterococci to daptomycin is dependent upon testing methodology. Diagn. Microbiol. Infect. Dis. 76:497–501. 10.1016/j.diagmicrobio.2013.04.019 [DOI] [PubMed] [Google Scholar]
- 29.Bobenchik AM, Hindler JA, Giltner CL, Saeki S, Humphries RM. 2014. Performance of the Vitek2 for antimicrobial susceptibility testing of Staphylococcus spp. and Enterococcus spp. J. Clin. Microbiol. 52:392–397. 10.1128/JCM.02432-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moise PA, Sakoulas G, Forrest A, Schentag JJ. 2007. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 51:2582–2586. 10.1128/AAC.00939-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soriano A, Marco F, Martinez JA, Pisos E, Almela M, Dimova VP, Alamo D, Ortega M, Lopez J, Mensa J. 2008. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46:193–200. 10.1086/524667 [DOI] [PubMed] [Google Scholar]
- 32.Hsu DI, Hidayat LK, Quist R, Hindler J, Karlsson A, Yusof A, Wong-Beringer A. 2008. Comparison of method-specific vancomycin minimum inhibitory concentration values and their predictability for treatment outcome of methicillin-resistant Staphylococcus aureus (MRSA) infection. Int. J. Antimicrob. Agents 32:378–385. 10.1016/j.ijantimicag.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 33.Han JH, Mascitti KB, Edelstein PH, Bilker WB, Lautenbach E. 2012. Effect of reduced vancomycin susceptibility on clinical and economic outcomes in Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 56:5164–5170. 10.1128/AAC.00757-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King EA, McCoy D, Desani S, Nyirenda T, Bicking K. 2011. Vancomycin-resistant enterococcal bacteremia and daptomycin: are higher doses necessary? J. Antimicrob. Chemother. 66:2112–2118. 10.1093/jac/dkr255 [DOI] [PubMed] [Google Scholar]
- 35.Forstner C, Dungl C, Tobudic S, Mitteregger D, Lagler H, Burgmann H. 2013. Predictors of clinical and microbiological treatment failure in patients with methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia: a retrospective cohort study in a region with low MRSA prevalence. Clin. Microbiol. Infect. 19:E291–E297. 10.1111/1469-0691.12169 [DOI] [PubMed] [Google Scholar]
- 36.Maclayton DO, Suda KJ, Coval KA, York CB, Garey KW. 2006. Case-control study of the relationship between MRSA bacteremia with a vancomycin MIC of 2 microg/mL and risk factors, costs, and outcomes in inpatients undergoing hemodialysis. Clin. Ther. 28:1208–1216. 10.1016/j.clinthera.2006.08.003 [DOI] [PubMed] [Google Scholar]