Abstract
Research to develop and validate novel methods for diagnosis of aspergillosis based on detection of galactomannan requires the use of clinical specimens that have been stored frozen. Data indicating that galactomannan remains stable when frozen are scant. The objective of this study was to determine the stability of galactomannan in clinical specimens stored at −20°C that were positive in the Platelia Aspergillus enzyme immunoassay when initially tested. Prospective real-time testing of serum and bronchoalveolar lavage (BAL) fluid pools from positive and negative patient specimens showed no decline in galactomannan index (GMI) over 11 months at −20°C and no development of positive reactions in the negative-control pool. Retrospective testing of positive specimens that had been stored at −20°C for 5 years showed that 28 of 30 serum (n = 15) or BAL (n = 15) specimens remained positive. These findings support the use of frozen serum or BAL specimens stored for at least 5 years in evaluation of diagnostic tests based on detection of galactomannan.
INTRODUCTION
The detection of galactomannan (GM) antigen in the serum or bronchoalveolar lavage fluid (BALF) using the Platelia Aspergillus enzyme immunoassay (EIA) is an important method for early diagnosis of invasive aspergillosis in immunocompromised patients (1, 2). Recently, several investigators developed lateral flow devices for detection of Aspergillus GM, to allow point-of-care testing (3–7). Others have described monoclonal antibodies that recognize glycoproteins containing GM that may be useful for diagnostic tests based on detection of these antigens (8, 9). The Aspergillus Technology Consortium (AsTeC) is an NIH-contracted consortium that was established to create and maintain a repository of specimens from patients with invasive aspergillosis that could be used in the evaluation of new diagnostic methods (10). Ensuring the integrity of frozen specimens is crucial for the development of clinical assays for the detection of GM. The aim of this project was to assess the stability of Aspergillus GM in serum and BALF stored at −20°C.
MATERIALS AND METHODS
Platelia Aspergillus EIA.
The Platelia Aspergillus EIA was performed according to the manufacturer's recommendations (Bio-Rad Laboratories, Redmond, WA). BAL specimens were handled in a biosafety cabinet. Serum and BAL fluid were treated with EDTA at 104°C for 6 min, followed by centrifugation, prior to testing. Results greater than 0.5 were considered to be positive and were verified by repeat testing, including EDTA-heat treatment the following day. Results were expressed as a GM index (GMI).
Prospective real-time evaluation.
Negative, low-positive, and high-positive serum and BAL fluid pools were created each containing specimens from six different patients. The specimens were selected based on results of Aspergillus GM antigen testing in the Platelia Aspergillus EIA performed at MiraVista Diagnostics, a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory, between 17 May and 10 June 2012. Clinical or other laboratory information was unavailable for these patients. The negative serum pool contained specimens with GMIs between 0.06 and 0.18 (mean, 0.11); the low-positive pool contained specimens with GMIs between 1.05 and 1.73 (mean, 1.35); the high-positive pool contained specimens with GMIs between 2.44 and 4.69 (mean, 3.49). The negative BAL fluid pool contained specimens with GMIs between 0.10 and 0.45 (mean, 0.22); the low-positive pool contained specimens with GMIs between 1.01 and 1.72 (mean, 0.26); and the high-positive pool contained specimens with GMIs between 2.74 and 6.79 (mean, 4.77). Pools were divided into four aliquots for testing at four dates during 11 months of storage at −20°C. All specimens met the requirements of the manufacturer for original testing: storage at 2 to 8°C for up to 48 h and at −20°C if more than 48 h before the initial clinical testing. Testing was performed by a single technologist using a single lot (2J0118) of the Platelia Aspergillus EIA kit. Triplicates of each of the four pools were tested on 6 July 2012 (zero time point), 28 September 2012 (3 months), 21 December 2012 (6 months), and 7 June 2013 (11 months), and each aliquot underwent a single freeze-thaw cycle. The final test date was at 11 months, to comply with the 1-year shelf life of the Platelia Aspergillus EIA kit.
Retrospective long-term evaluation.
Fifteen BALF and 15 serum specimens with positive results ranging from 0.67 to 7.70 units when originally tested by multiple technologists in 2004 or 2005 were stored at −20°C and then retested by a single technologist on consecutive days in January 2010 using one lot (9B0028) of the Platelia Aspergillus EIA. Specimens were EDTA-heat treated and tested twice for confirmation of positivity, as recommended by the manufacturer. As this was a retrospective study, kit lots and operators were not controlled variables in the original testing in 2004 or 2005.
Statistical analysis.
Comparison of mean GMIs determined from the initial samples and from samples that were stored frozen for 5 years was performed using the paired Student t test. Correlation of GMI results of initial and frozen specimens was performed by linear regression analysis. P values of <0.05 were considered significant.
RESULTS
Real-time evaluation.
The average GM indexes (GMIs) for the negative serum pool were 0.26 unit at time zero, 0.09 unit at 3 months, 0.09 unit at 6 months, and 0.14 unit at 11 months (Table 1). One of the three aliquots of the zero-time-point serum was higher than the other aliquots (0.46 compared to 0.15 and 0.18 unit). The averages for the three aliquots of the low-positive serum pool were 0.98 unit at time zero, 0.89 unit at 3 months, 0.92 unit at 6 months, and 1.19 units at 11 months. The averages for the high-positive pool were 2.45 units at 0 months, 2.24 units at 3 months, 1.98 units at 6 months, and 2.61 units at 11 months. The low number of aliquots at each time point did not support statistical analysis.
TABLE 1.
Real-time stability of pooled specimens at −20°C
| Specimen type and aliquot no. | GMI (% changea) |
||||
|---|---|---|---|---|---|
| 0 mo | 3 mo | 6 mo | 11 mo | Avg | |
| Serum negative | |||||
| 1 | 0.46 | 0.09 | 0.09 | 0.14 | |
| 2 | 0.15 | 0.09 | 0.08 | 0.14 | |
| 3 | 0.18 | 0.09 | 0.10 | 0.13 | |
| Avg | 0.26 | 0.09 | 0.09 | 0.14 | 0.14 |
| Serum low, positive GMI | |||||
| 1 | 0.92 | 0.88 | 0.98 | 1.22 | |
| 2 | 1.05 | 0.94 | 0.86 | 1.10 | |
| 3 | 0.98 | 0.85 | 0.91 | 1.24 | |
| Avg | 0.98 | 0.89 (−9.0) | 0.92 (−6.0) | 1.19 (+21.4) | 0.99 |
| Serum high, positive GMI | |||||
| 1 | 2.50 | 2.22 | 2.09 | 2.94 | |
| 2 | 2.24 | 2.04 | 2.06 | 2.88 | |
| 3 | 2.59 | 2.46 | 1.79 | 2.02 | |
| Avg | 2.45 | 2.24 (−8.6) | 1.98 (−19.2) | 2.61 (+2.0) | 2.32 |
| BAL negative | |||||
| 1 | 0.09 | 0.24 | 0.13 | 0.09 | |
| 2 | 0.13 | 0.29 | 0.3 | 0.11 | |
| 3 | 0.10 | 0.26 | 0.22 | 0.10 | |
| Avg | 0.11 | 0.26 | 0.22 | 0.10 | 0.17 |
| BAL low, positive GMI | |||||
| 1 | 0.95 | 0.77 | 1.04 | 1.16 | |
| 2 | 0.91 | 0.76 | 0.93 | 0.97 | |
| 3 | 0.86 | 0.74 | 0.75 | 0.96 | |
| Avg | 0.91 | 0.76 (−16.5) | 0.91 (0) | 1.03 (+13.2) | 0.9 |
| BAL high, positive GMI | |||||
| 1 | 3.56 | 3.47 | 3.64 | 4.94 | |
| 2 | 3.54 | 3.59 | 3.68 | 4.32 | |
| 3 | 3.38 | 3.43 | 4.29 | 4.24 | |
| Avg | 3.49 | 3.50 (+0.3) | 3.87 (+10.9) | 4.50 (+28.9) | 3.84 |
Relative to the 0-mo GMI.
For BALF specimens, the average GMIs for the negative pool was 0.11 unit at 0 months, 0.26 units at 3 months, 0.22 unit at 6 months, and 0.10 unit at 11 months. The average GMIs for the low-positive BALF pool were 0.91 unit at time zero, 0.76 unit at 3 months, 0.91 unit at 6 months, and 1.03 units at 11 months. The average GMIs for the high-positive BALF pool were 3.49 units at time zero, 3.50 units at 3 months, 3.99 units at 6 months, and 4.50 units at 11 months.
Long-term evaluation.
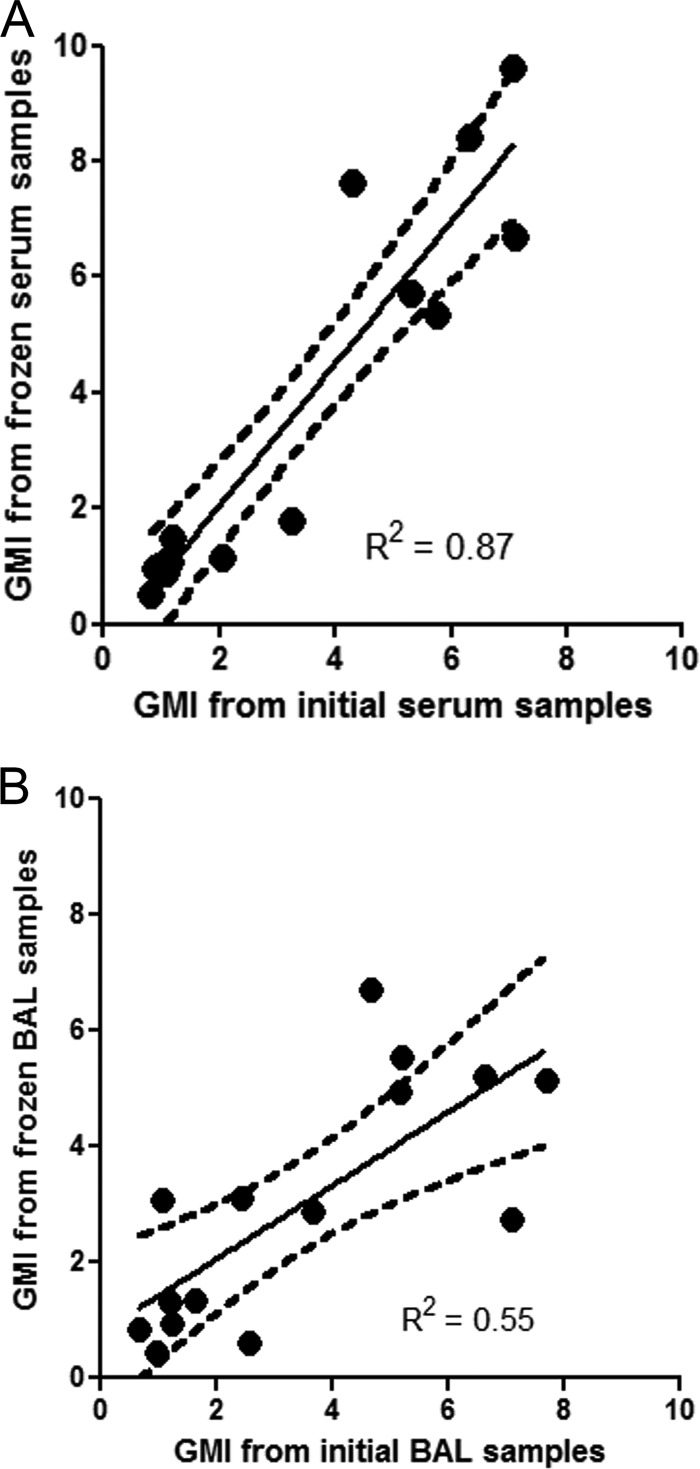
Results of the original and the repeat tests are shown in Table 2. Fourteen of 15 serum specimens remained positive after storage at −20°C for an average of 1,969 days (5.4 years), ranging from 1,804 to 2,035 days (4.9 to 5.6 years). The serum that was not reproducibly positive had a GMI of 0.83 unit in 2004 and 0.54 unit in the first test but 0.48 unit in the confirmatory test in 2010. The goodness of fit between the GMI determined from the original samples and that from the frozen samples were determined using linear regression analysis. The coefficient of determination (R2) between GMI determined from the original serum samples and the frozen samples was 0.87 (P < 0.0001), and 87% (13/15) of the data points were within the boundaries of the 95% confidence interval (Fig. 1). The mean GMIs of the initial serum samples were not statistically different from those of the stored serum samples at −20°C (3.22 versus 3.55, respectively; P = 0.308). There was no correlation between the duration of storage and the percentage changes in GMI in serum samples (P = 0.39; R2 = 0.06).
TABLE 2.
Long-term stability of individual specimens at −20°C
| Matrix | Initial GMI | No. of days frozen | Frozen GMI |
% differenceb | ||
|---|---|---|---|---|---|---|
| No. 1 | No. 2a | Mean | ||||
| Serum | 7.12 | 1,982 | 6.85 | 6.52 | 6.69 | −6.1 |
| 7.08 | 1,936 | 9.86 | 9.40 | 9.63 | 36.0 | |
| 6.30 | 2,009 | 8.64 | 8.15 | 8.40 | 33.0 | |
| 5.76 | 2,021 | 5.53 | 5.19 | 5.36 | −6.9 | |
| 5.31 | 1,937 | 5.53 | 5.92 | 5.73 | 8.0 | |
| 4.31 | 1,955 | 7.78 | 7.46 | 7.62 | 77.0 | |
| 3.25 | 1,804 | 1.74 | 1.82 | 1.78 | −45.0 | |
| 2.05 | 1,995 | 1.09 | 1.17 | 1.13 | −45.0 | |
| 1.18 | 2,000 | 1.56 | 1.39 | 1.48 | 25.0 | |
| 1.15 | 1,921 | 1.08 | 1.04 | 1.06 | −8.0 | |
| 1.09 | 1,949 | 1.01 | 1.12 | 1.07 | −2.3 | |
| 1.07 | 2,018 | 0.89 | 0.84 | 0.87 | −19.2 | |
| 0.98 | 2,035 | 0.94 | 1.03 | 0.99 | 0.5 | |
| 0.87 | 2,025 | 0.98 | 0.93 | 0.96 | 10.0 | |
| 0.83 | 1,945 | 0.54 | 0.48 | 0.51 | −38.6 | |
| BAL | 7.70 | 1,502 | 5.27 | 5.02 | 5.15 | −33.2 |
| 7.10 | 1,527 | 2.79 | 2.67 | 2.73 | −61.6 | |
| 6.62 | 1,504 | 4.94 | 5.44 | 5.19 | −21.6 | |
| 5.20 | 2,026 | 5.52 | 5.58 | 5.55 | 6.7 | |
| 5.18 | 1,753 | 4.92 | 4.96 | 4.94 | −4.6 | |
| 4.65 | 1,991 | 6.84 | 6.58 | 6.71 | 44.3 | |
| 3.66 | 1,991 | 2.71 | 3.01 | 2.86 | −21.9 | |
| 2.58 | 1,984 | 0.63 | 0.59 | 0.61 | −76.4 | |
| 2.43 | 1,992 | 2.99 | 3.19 | 3.09 | 27.2 | |
| 1.63 | 1,522 | 1.20 | 1.48 | 1.34 | −17.8 | |
| 1.24 | 1,511 | 0.88 | 0.97 | 0.93 | −25.4 | |
| 1.20 | 1,865 | 1.20 | 1.42 | 1.31 | 9.2 | |
| 1.08 | 1,645 | 3.24 | 2.91 | 3.08 | 184.7 | |
| 0.97 | 1,897 | 0.44 | 0.44 | 0.44 | −54.6 | |
| 0.67 | 2,007 | 0.82 | 0.86 | 0.84 | 25.4 | |
A confirmatory test was performed the next day on a second EDTA-heat extracted aliquot of frozen specimen.
Comparison of initial GMI with frozen mean GMI.
FIG 1.
Correlation between the GMI determined from the patient's initial samples and the frozen samples of serum or BAL fluid. The y axis represents the means of two GMIs determined from frozen samples from 2 consecutive days. The dashed lines represent the 95% confidence interval.
Fourteen of the 15 BALF specimens were reproducibly positive following storage at −20°C for an average of 1,781 days (4.9 years), ranging from 1,502 to 2,007 days (4.1 to 5.5 years). The single specimen that was not reproducibly positive had a GMI of 0.97 unit when originally tested in 2004 and 0.44 unit when retested in 2010. R2 between GMIs determined from the initial BAL samples and the frozen samples was 0.55 (P = 0.001), and 67% (10 of 15) of the data points were within the boundaries of the 95% confidence interval (Fig. 1). The mean GMI for the 15 BALF was 3.46 initially and 2.98 when retested (P = 0.262). There was also no correlation between the duration of storage and the percentage changed in GMI in BAL samples (P = 0.76; R2 = 0.08).
The GMIs of the frozen specimens that were retested on consecutive days in 2010 (Table 2) were compared by linear regression analysis (Fig. 2). Results of test 1 and test 2 agreed closely (R2 = 0.99).
FIG 2.
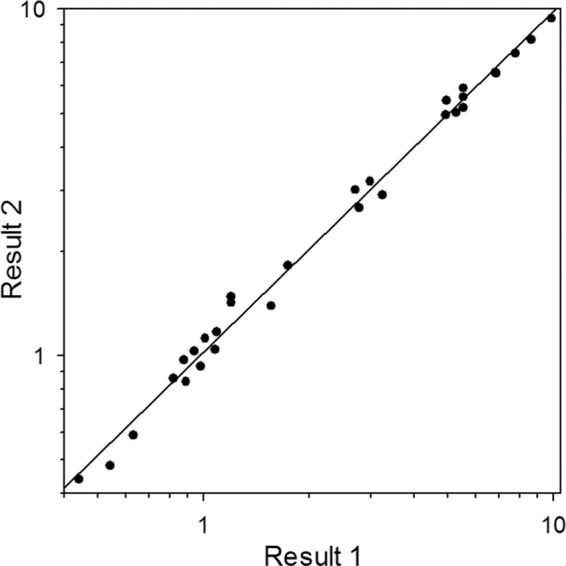
Comparison of EDTA-heat-treated extracts of 15 serum and 15 BAL specimens stored at −20°C for 5 years. Result 1 was from the first test of the frozen specimen, and result 2 was from the confirmatory test, performed the next day following repeat EDTA-heat extraction of the frozen specimen. R2 = 0.99.
DISCUSSION
These findings suggest that Aspergillus GM does not significantly lose reactivity in the Platelia Aspergillus EIA when stored at −20°C. There was minimal change in the GMI for the negative, low-positive, and high-positive serum and BALF pool, and in all cases the GMI increased slightly, ranging from 6.6 to 28.9%, implying that GM remains stable when frozen at −20°C. Assessment of stability beyond 11 months was not possible because of the 1-year shelf life of the Platelia Aspergillus EIA. Greater variability in GMI was seen in the long-term study, but overall results were reproducibly positive in 93% (28/30) of specimens. The single serum specimen that was negative had GMIs of 0.83 unit when originally tested and 0.54 unit when retested after storage for 1,945 days but 0.48 units when confirmatory testing was performed the next day. Similarly, one BALF specimen was negative after long-term storage at −20°C: the GMI was 0.97 unit when the sample was first tested but 0.44 unit after storage at −20°C for 1,897 days. The study of long-term stability was affected by operator-to-operator and kit-to-kit lot variability, reducing its ability to assess variability of individual GMI results. The results of the initial and repeat extraction and testing of the specimens stored for 5 years at −20°C were highly reproducible, supporting the integrity of the specimen.
Despite these encouraging overall findings, we should point out that the correlation between GMIs for BAL samples pre- and poststorage was less than that for serum (R2 of 0.55 versus 0.87). Moreover, 47% (7/15) of BAL specimens and only 20% (3/15) of poststorage serum samples displayed a ≥20% reduction in GMI compared to the initial samples. The reasons for these discrepancies are not clear. GMI measures both fungal-cell-membrane-bound and freely circulating GM. Therefore, one possibility is that the results reflect greater numbers of viable Aspergillus cells in the initial BAL samples than in the other samples. Indeed, significant loss of Aspergillus viability has been noted after 6 months of freezer storage in phosphate-buffered saline (11). Unlike cultures from BAL fluid and other respiratory tract cultures, blood cultures are rarely positive during invasive aspergillosis, indicating that viable organisms are uncommon. Even if injured or dead Aspergillus cells remain after storage, they may have damaged or shortened galactofuran side chains to which the GM monoclonal antibody can no longer adhere (12). An alternative possibility is that polymorphonuclear cells and alveolar macrophages are lysed during the storage process, and spillage of the cell contents renders the BALF more acidic. Since the galactofuran side chain targeted by the GM monoclonal antibody is acid labile, GMI reactivity might consequently be reduced. Lastly, GM may be less stable in BALF over time due to yet-to-be-identified factors.
Pereira et al. reported a reduction in serum GMI in 19% (8/42) of positive (GMI, 1.5 or above) or undetermined (GMI, 1.0 to 1.5) serum specimens that had been stored at −20°C, but the decline was not statistically significant (13). Those authors did not report specific data, and determination of the number of specimens that would have been considered negative using the 0.5 cutoff for positivity was not possible.
Johnson et al. observed a significant decline of median GMI in serum specimens stored at −80°C for 2 years (14). The GMI fell from a median of 0.56 to 0.10, and two-thirds of specimens were negative when retested. Several potential differences between the study by Johnson et al. and the current study may explain the discrepant findings. First, it is possible that well-recognized lot-to-lot variation in the properties of the monoclonal antibody impacted the study by Johnson et al. Second, the experience of the operators who performed the Platelia Aspergillus EIA may have contributed to these differences. A few technologists that specialized in this assay and had tested thousands of specimens performed the testing at MiraVista Diagnostics. Third, the study by Johnson et al. included specimens that were negative for GM, while we evaluated only specimens that were positive. A decline in GMI in negative specimens is less likely to be informative or accurate. In fact, there is no evidence that the degree or dynamics of GMI negativity are useful in patient management. Fourth, our samples were frozen at −20°C, rather than −80°C as in the study by Johnson et al. We cannot exclude the possibility that this difference may have impacted the results, but physiological reasons for such an occurrence are unknown. Lastly, we do not think that the length of storage accounted for the differences in results, since our samples were stored longer (average length of 5 years versus 2 years). The duration of storage of our BAL and serum samples did not correlate with the percentage changes in GM. A more relevant concern would be the development of false positivity with storage of negative specimens, which we assessed and excluded in our real-time study. We did not assess negative specimens in the long-term study, however.
A few limitations of our study merit consideration. First, the number of patients' samples tested was small, and patients' clinical information was not available. Hence, whether the patients had aspergillosis is unknown. False-positive results occur in samples from patients with other fungal infections, caused by the presence of cross-reactive antigens, and from patients receiving piperacillin-tazobactam, ampicillin-sulbactam, and Plasmalyte, due to contamination with a cross-reactive GM. Second, too few specimens with GMIs close to the cutoff were evaluated to establish stability at −20°C for such specimens. Third, this study does not address other biomarkers, such as (1→3)-β-d-glucan, nucleic acids, and genomic products, all of which are attractive targets for new diagnostic tests for aspergillosis not based on GM detection.
These findings support a conclusion that Aspergillus GM does not degrade and that false-positive results do not develop during storage at −20°C and support the use of stored specimens for evaluation of new diagnostic tests for Aspergillus GM.
ACKNOWLEDGMENTS
Special thanks go to Rory Duncan and Alec Richey from the National Institutes of Allergy and Infectious Diseases for their advice and support.
This work was supported by the NIH-NIAID, N01-AI70023/HHSN266200700023C (AsTeC). (L.J.W., M.H.N., B.D.A., D.D., A.M.C., G.M.L., L.R.B., F.M.M., C.C., M.S., and J.R.W. are the members of AsTeC.)
L.J.W. directs MiraVista Diagnostics, the laboratory performing the testing for this study. L.J.W. also has served as a speaker for BioRad Inc., the manufacturer of the Platelia Aspergillus EIA.
Footnotes
Published ahead of print 9 April 2014
REFERENCES
- 1.Fisher BT. 2013. The role of biomarkers for diagnosis of and therapeutic decisions related to invasive aspergillosis in children. Curr. Fungal. Infect. Rep. 7:7–14. 10.1007/s12281-012-0127-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrosky-Zeichner L. 2012. Invasive mycoses: diagnostic challenges. Am. J. Med. 125:S14–S24. 10.1016/j.amjmed.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 3.Dufresne SF, Datta K, Li X, Dadachova E, Staab JF, Patterson TF, Feldmesser M, Marr KA. 2012. Detection of urinary excreted fungal GM-like antigens for diagnosis of invasive aspergillosis. PLoS One 7:e42736. 10.1371/journal.pone.0042736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoenigl M, Koidl C, Duettmann W, Seeber K, Wagner J, Buzina W, Wolfler A, Raggam RB, Thornton CR, Krause R. 2012. Bronchoalveolar lavage lateral-flow device test for invasive pulmonary aspergillosis diagnosis in haematological malignancy and solid organ transplant patients. J. Infect. 65:588–591. 10.1016/j.jinf.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 5.Thornton C, Johnson G, Agrawal S. 2012. Detection of invasive pulmonary aspergillosis in haematological malignancy patients by using lateral-flow technology. J. Vis. Exp. 2012:3721. 10.3791/3721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White PL, Parr C, Thornton C, Barnes RA. 2013. Evaluation of real-time PCR, GM enzyme-linked immunosorbent assay (ELISA), and a novel lateral-flow device for diagnosis of invasive aspergillosis. J. Clin. Microbiol. 51:1510–1516. 10.1128/JCM.03189-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiederhold NP, Najvar LK, Bocanegra R, Kirkpatrick WR, Patterson TF, Thornton CR. 2013. Interlaboratory and interstudy reproducibility of a novel lateral-flow device and influence of antifungal therapy on detection of invasive pulmonary aspergillosis. J. Clin. Microbiol. 51:459–465. 10.1128/JCM.02142-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao W, Pan YX, Ding YQ, Xiao S, Yin K, Wang YD, Qiu LW, Zhang QL, Woo PC, Lau SK, Yuen KY, Che XY. 2008. Well-characterized monoclonal antibodies against cell wall antigen of Aspergillus species improve immunoassay specificity and sensitivity. Clin. Vaccine Immunol. 15:194–202. 10.1128/CVI.00362-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang ZY, Cai JP, Qiu LW, Hao W, Pan YX, Tung ET, Lau CC, Woo PC, Lau SK, Yuen KY, Che XY. 2012. Development of monoclonal antibody-based galactomannoprotein antigen-capture ELISAs to detect Aspergillus fumigatus infection in the invasive aspergillosis rabbit models. Eur. J. Clin. Microbiol. Infect. Dis. 31:2943–2950. 10.1007/s10096-012-1645-3 [DOI] [PubMed] [Google Scholar]
- 10.Lyon GM, Abdul-Ali D, Loeffler J, White PL, Wickes B, Herrera ML, Alexander BD, Baden LR, Clancy C, Denning D, Nguyen MH, Sugrue M, Wheat LJ, Wingard JR, Donnelly JP, Barnes R, Patterson TF, Caliendo AM. 2013. Development and evaluation of a calibrator material for nucleic acid-based assays for diagnosing aspergillosis. J. Clin. Microbiol. 51:2403–2405. 10.1128/JCM.00744-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denning DW, Clemons KV, Stevens DA. 1992. Quantitative preservation of viability of Aspergillus fumigatus. J. Med. Vet. Mycol. 30:485–488. 10.1080/02681219280000661 [DOI] [PubMed] [Google Scholar]
- 12.Mennink-Kersten MA, Donnelly JP, Verweij PE. 2004. Detection of circulating GM for the diagnosis and management of invasive aspergillosis. Lancet Infect. Dis. 4:349–357. 10.1016/S1473-3099(04)01045-X [DOI] [PubMed] [Google Scholar]
- 13.Pereira CN, Del Nero G, Lacaz CS, Machado CM. 2005. The contribution of GM detection in the diagnosis of invasive aspergillosis in bone marrow transplant recipients. Mycopathologia 159:487–493. 10.1007/s11046-005-4996-9 [DOI] [PubMed] [Google Scholar]
- 14.Johnson GL, Sarker SJ, Hill K, Tsitsikas DA, Morin A, Bustin SA, Agrawal SG. 2013. Significant decline in GM signal during storage of clinical serum samples. Int. J. Mol. Sci. 14:12970–12977. 10.3390/ijms140712970 [DOI] [PMC free article] [PubMed] [Google Scholar]