Abstract
Neisseria meningitidis is an obligate human commensal that commonly colonizes the oropharyngeal mucosa. Carriage is age dependent and very common in young adults. The relationships between carriage and invasive disease are not completely understood. In this work, we performed a longitudinal carrier study in adolescents and young adults (173 subjects). Overall, 32 subjects (18.5%) had results that were positive for meningococcal carriage in at least one visit (average monthly carriage rate, 12.1%). Only five subjects tested positive at all four visits. All meningococcal isolates were characterized by molecular and serological techniques. Multilocus sequence typing, PorA typing, and sequencing of the 4CMenB vaccine antigens were used to assess strain diversity. The majority of positive subjects were colonized by capsule null (34.4%) and capsular group B strains (28.1%), accounting for 23.5% and 29.4% of the total number of isolates, respectively. The fHbp and nhba genes were present in all isolates, while the nadA gene was present in 5% of the isolates. The genetic variability of the 4CMenB vaccine antigens in this collection was relatively high compared with that of other disease-causing strain panels. Indications about the persistence of the carriage state were limited to the time span of the study. All strains isolated from the same subject were identical or cumulated minor changes over time. The expression levels and antigenicities of the 4CMenB vaccine antigens in each strain were analyzed by the meningococcal antigen typing system (MATS), which revealed that expression can change over time in the same individual. Future analysis of antigen variability and expression in carrier strains after the introduction of the MenB vaccine will allow for a definition of its impact on nasopharyngeal/oropharyngeal carriage.
INTRODUCTION
Neisseria meningitidis has a unique survival niche in humans (1). The bacterium has developed sophisticated mechanisms to evade the human immune system (2), and although it can cause serious invasive disease, it can be considered a normal temporary commensal of the upper respiratory tract of healthy carriers, constituting the reservoir of the microorganism (3, 4). The worldwide epidemiology of invasive meningococcal disease (IMD) varies by region and over time. This disease burden mainly affects children <5 years of age, with peaks of incidence in infants <1 year of age and in adolescents (5, 6). The relationship between asymptomatic carriage and the development of invasive disease is not still completely understood. Carriage rates are low in infants and school-age children, but they increase during adolescence and early adulthood (7, 8). Carrier status can be regarded as a natural booster that contributes to the spontaneous acquisition of herd immunity (9). Little knowledge is available on the duration of meningococcal carriage, as few studies have been carried out that follow subjects with repeat throat samples performed over time (10, 11).
Studies performed in some European countries using multilocus sequence typing (MLST) have demonstrated that a limited number of hypervirulent genetic lineages are overrepresented in invasive isolates worldwide (12, 13). Despite extensive genetic exchange among meningococci, the hypervirulent lineages are stable over time and associated with specific antigenic repertoires. In contrast, meningococci isolated from healthy individuals present extensive genetic diversity (14–16). The capsule is the major virulence factor described for N. meningitidis. Indeed, carriage meningococcal isolates are often noncapsulated (i.e., are “nongroupable”) (17, 18). This is due to either genetic downregulation of the capsule expression (phase variation) or the absence of the genes involved in capsule expression. The latter strains are referred to as capsule null locus (cnl) meningococci (19). On the contrary, invasive meningococcal disease is with rare exceptions caused by encapsulated strains. Twelve capsular groups have been identified, five of which (A, B, C, Y, and W) are responsible for the majority of worldwide invasive disease (20). Recently, capsular group X meningococci have also revealed an epidemic potential (21).
Vaccination with polysaccharide conjugate vaccines is highly effective in preventing invasive disease caused by capsular groups A, C, W, and Y (meningococcus A [MenA], MenC, MenW, and MenY, respectively). In Italy, conjugate vaccines against MenC were introduced in 2005 (see http://www.salute.gov.it/imgs/C_17_pubblicazioni_543_allegato.pdf). The vaccination is usually offered free of charge to all infants 12 to 13 months of age. At the moment, two tetravalent-conjugate anti-MenACWY vaccines (see http://www.ema.europa.eu) are available and have been recommended to adolescents in some Italian regions since 2012. In contrast, as the development of group B capsular polysaccharide-based vaccines has not been successful (22), meningococcus B (MenB) remains one of the major causes of invasive disease in the United States and Europe. Recently, 4CMenB (brand name Bexsero), a multicomponent recombinant protein vaccine targeting MenB strains, was licensed in Europe, Australia, and Canada (23–26) (see http://www.hc-sc.gc.ca and http://www.tga.gov.au). The 4CMenB vaccine includes outer membrane vesicles (OMV) from the New Zealand epidemic isolate N. meningitidis NZ98/254 (27) and three major protein antigens: factor H-binding protein main variant 1 subvariant 1 (fHbp-1.1) (28), neisserial heparin-binding antigen subvariant or peptide 2 (NHBA-2) (29), and Neisseria adhesin A variant 3 (NadA-3) (30).
The serum bactericidal assay (SBA) is the only accepted correlate of protection to estimate the effectiveness of a meningococcal vaccine against meningococcal disease (5, 6). An assessment of the potential coverage of a multicomponent MenB vaccine would require performing SBA against many isolates due to both sequence and expression variability of the vaccine antigens. For this reason, a rapid and reproducible meningococcal antigen typing system (MATS) was developed, which allows for an estimation of vaccine coverage by determining for each strain the expression level and cross-reactivity to the 4CMenB vaccine variants of fHbp, NHBA, and NadA (31).
This work is a longitudinal carriage study on Italian adolescents and young adults 14 to 22 years of age aiming at characterizing the carriage of N. meningitidis 5 years after the introduction of the MenC-conjugate vaccine. In order to assess the permanence of the same isolate at different visits by the same individuals, the meningococcal carrier isolates were analyzed for capsular group and sequence type (ST), as well as for the presence and sequence variability of the genes coding for PorA, fHbp, NHBA, and NadA. All strains were also analyzed by MATS in order to monitor the 4CMenB antigen expression level of the same isolate over time.
MATERIALS AND METHODS
The ethics committee of San Martino Hospital (Genoa, Italy) approved the study protocol (no. 117/09) (29 October 2009), which conforms to the ethics guidelines of the 1975 Declaration of Helsinki and good clinical practice.
The study was carried out from January 2011 to October 2012, and the collection of the samples was performed from February to May 2011.
Study population and recruitment.
Subjects 14 to 22 years of age attending two secondary technical schools in Genoa were enrolled in the study. The first 200 subjects who agreed to participate in the study and met the inclusion criteria (consecutive set of subjects) were enrolled. Individuals who had taken antibiotics within 1 week before the collection of pharyngeal swabs were excluded.
A clear complete written informed consent form was signed by each participant; consent was also obtained from the parent(s)/guardian(s) if the subject was <18 years of age. The aims and procedures of the study were explained to each volunteer by the medical staff. Before the first sample collection, the volunteers answered a structured questionnaire (see Table S1 in the supplemental material) that included information on risk factors for N. meningitidis carriage and meningococcal C vaccination. Meningococcal C vaccination status was also checked by referring to the vaccination registers of the local health unit (LHU). Previous vaccination with MenC vaccine was not considered to be an exclusion criterion.
A trained physician took a posterior pharyngeal swab (ESwab 480CE; Copan, Italy) from behind the uvula, the posterior wall of the oropharynx, and both tonsils. As one of the aims of the study was to evaluate the duration of carrier status, three further swabs were taken at 1-month intervals, for a total of 4 visits (February to May 2011).
Isolation of N. meningitidis strains.
The swabs were immediately plated on-site on selective medium (Martin-Lewis modified agar [BD], chocolate II agar plus amphotericin B plus vancomycin, and 1% IsoVitaleX). Both the plates and the swabs were transported to the microbiology laboratory of the Department of Health Sciences (University of Genoa) in insulated containers to ensure a constant temperature of about 20 to 25°C. The time from sample collection to arrival at the microbiology laboratory was never >2 h.
The plates were then incubated at 35 to 37°C for a period ranging from 36 to 48 h (with the time depending on the growth of the colonies) in a 5 to 10% CO2 atmosphere. Subsequently, a morphological evaluation of bacterial colonies was performed. All colonies recognized as possible colonies of N. meningitidis were plated on chocolate agar (GC II agar plus IsoVitaleX; BD), and were then incubated at 35 to 37°C for 24 h in a 5 to 10% CO2 atmosphere. If necessary, colony sowing was repeated in order to obtain pure colonies at the end of the procedure.
Genomic DNA extraction.
DNA was extracted from the isolates by emulsifying the colonies in 500 μl of 0.85% saline to obtain a 0.5 McFarland standard suspension. The homogenized suspension was extracted by means of a QIAamp DNA minikit (Qiagen, Valencia, CA), according to the manufacturer's instructions, after a preincubation step of 1 h in 180 μl of prelysis buffer (animal tissue lysis [ATL] buffer supplemented with 20 μl of proteinase K) at 56°C.
crgA and ctrA PCR amplification.
To confirm N. meningitidis species identity, all isolates were assayed by PCR amplification for the presence of the conserved genes crgA and ctrA (32). All confirmed isolated were frozen at −80°C in Mueller-Hinton broth plus 10 to 20% glycerol and sent to the research center of Novartis Vaccines and Diagnostics for typing at a controlled temperature.
Capsular and PorA typing.
Serogroups were assessed by a standard bacterial latex agglutination test (Difco and Oxoid), according to each manufacturer's instructions. In addition, the molecular detection of the capsular groups A, B, C, W, X, Y, Z, and E was accomplished by PCR (33, 34). Detection of the capsule null locus by PCR amplification and sequencing was performed according to the methods described in Claus et al. (19). Amplification of the porA gene was performed according to the protocol in Mölling et al. (35). DNA was sequenced as indicated in Clarke et al. (36), and VR1 and VR2 were assigned according to the Neisseria MLST website (see http://pubmlst.org/neisseria/PorA/).
MLST and 16S rRNA gene typing.
MLST was performed according to the method described in Maiden et al. (13), and sequence types (STs) were assigned according to the information available at the Neisseria MLST website (see http://pubmlst.org/neisseria). 16S rRNA gene typing was carried out as previously described (37).
fHbp, nhba, and nadA sequence typing.
PCR and gene sequencing of fHbp, nhba, and nadA were evaluated as previously described (16, 28, 30, 38, 39). Alleles and the corresponding protein variants were assigned using the meningococcal typing database (see http://pubmlst.org/neisseria).
MATS.
MATS consists of the combination of an enzyme-linked immunosorbent assay (ELISA) performed on bacterial lysates (for NHBA, fHbp, and NadA) and the sequencing of the PorA-VR2 variable region, as described in Donnelly et al. (31). Briefly, the bacteria were grown on chocolate agar plates and then resuspended in Mueller-Hinton broth until an optical density at 600 nm (OD600) of 0.4 was reached. Following the addition of a detergent, the bacterial extracts were serially diluted in ELISA plates coated with an antigen-specific capture antibody. The binding of the antigen was then detected by means of a biotin-labeled secondary antibody and a streptavidin-horseradish peroxidase (HRP) conjugate. The plates were read at 492 nm in an ELISA reader. For each strain, the MATS ELISA reactivity of each of the three tested antigens was obtained by mathematically comparing the serial dilution curve of each N. meningitidis strain to that obtained using a reference MenB strain (relative potency [RP]).
Statistical analysis.
The quantitative variables are expressed as means and standard deviations (SD) and the qualitative variables as frequencies and percentages with 95% confidence intervals (95% CI).
Fisher's exact test was used to evaluate the association between the single risk condition (active or passive smoking and cohabitation with ≥4 persons) and outcome (meningococcal carrier status). The level of significance adopted was a P value of <0.05. Gender and nationality were not considered to be risk conditions.
RESULTS
Carriage rates.
A total of 200 students 14 to 22 years of age were invited to participate to the study, 23 of whom declined (response rate, 88.5% [95% CI, 84.1 to 93.0%]). The reasons for refusal were not further investigated. Overall, 177 subjects were recruited. Four subjects (2 females and 2 males) withdrew their consent before the first sampling. As shown in Table 1, 173 subjects were studied, of whom 66.5% were male (33.5% female).
TABLE 1.
Number of subjects within studied populations, stratified by age and gender
| Age (yr) | No. of males | No. of females | Total no. |
|---|---|---|---|
| 14 | 10 | 3 | 13 |
| 15 | 19 | 11 | 30 |
| 16 | 13 | 9 | 22 |
| 17 | 3 | 13 | 16 |
| 18 | 34 | 13 | 47 |
| 19 | 23 | 7 | 30 |
| ≥20 | 13 | 2 | 15 |
| Total | 115 | 58 | 173 |
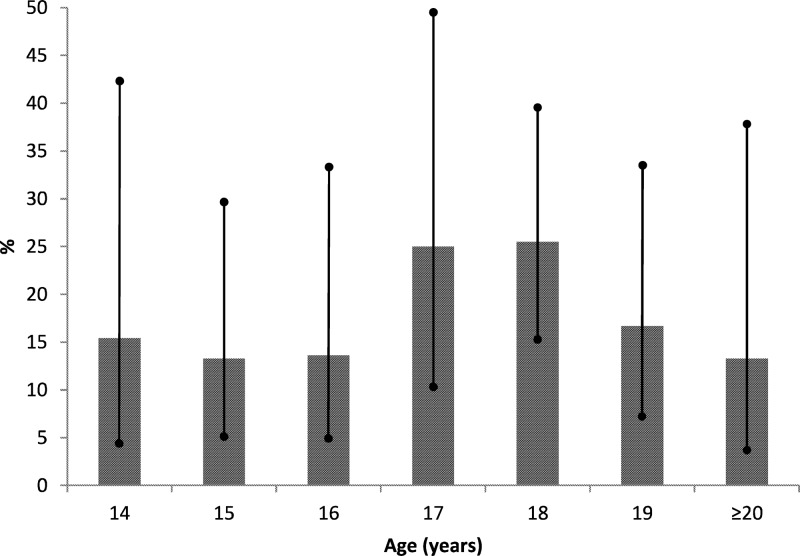
Thirty-two (18.5%) subjects were found to be carriers of N. meningitidis from at least one sample. Figure 1 shows the percentages of carriers at different ages. The highest rate of carriage was observed at 17 and 18 years of age (25.0% and 25.5%, respectively). However, comparing the 95% CI, the differences between the ages were never statistically significant. No statistically significant difference was found considering gender as a variable.
FIG 1.
Distribution of the percentage of carriages, with 95% confidence intervals, by age.
Table 2 reports some characteristics and risk conditions of the studied population. In detail, 59.4% of the carriers were exposed to active or passive smoking, while 57.9% of the noncarriers were exposed (P = 0.52). In addition, 71.9% and 55.2% of the carriers and noncarriers, respectively, cohabited with ≥4 persons (P = 0.06).
TABLE 2.
Characteristics and risk conditions of the studied population
| Carrier status | Subject sex (no.)a |
Nationality (no.) |
No. of active smokers | No. exposed to passive smoking | No. of subjects with: |
|||
|---|---|---|---|---|---|---|---|---|
| M | F | Italian | Non-Italian | <4 cohabitants | ≥4 cohabitants | |||
| Carriers | 25 | 7 | 31 | 1 | 16 | 15 | 9 | 23 |
| Noncarriers | 90 | 51 | 126 | 15 | 46b | 56c | 60d | 74 |
M, male; F, female.
Seven subjects did not respond.
Eight subjects did not respond.
Seven subjects did not respond.
A total of 145 pharyngeal swabs were collected at visit 1, 17 of which were positive for N. meningitidis (11.7%). After 1 month (visit 2), 157 pharyngeal swabs were obtained (20 positives [12.7%]). At visit 3, 136 swabs were obtained (15 positives [11.0%]). Finally, at visit 4, 16 positives (13.0%) from 123 total swabs were found (Fig. 2). In total, 68 isolates of N. meningitidis were identified. The average carriage rate was 12.1%. Twenty (62.5%) of the 32 carrier subjects were positive more than once, while 12/32 (37.5%) were positive only once. Figure 2 reports all 68 positive swabs, both consecutive and not consecutive.
FIG 2.
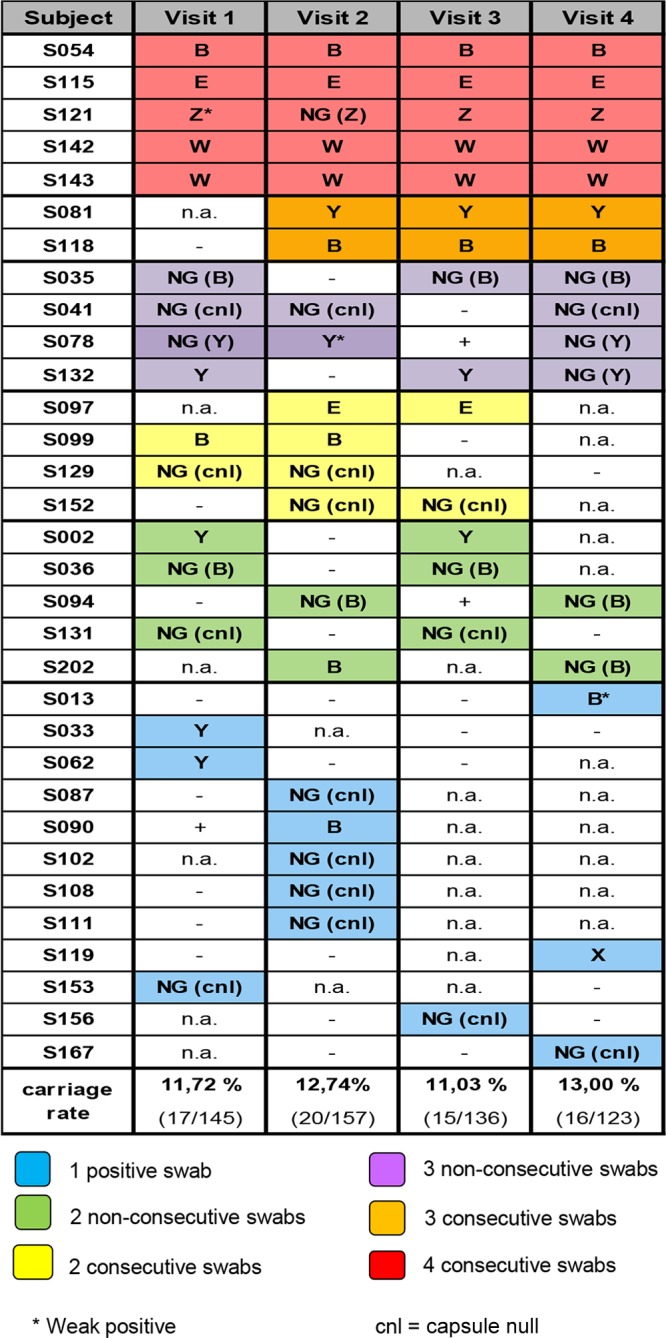
Representation of N. meningitidis carriage positivity across the four visits for the 32 carrier subjects. The subjects are ordered based on the number of positive visits and by numerical order. Boxes corresponding to positive visits are colored. The color coding is indicated in the legend. Serogroups are reported (*, weak expression of capsule). For nongroupable (NG) isolates, capsular groups are reported in parentheses. −, negative isolation; n.a., not assessed (absence of the subject at a given visit); +, PCR positivity of unavailable isolates. The carriage rates are calculated from the number of individual swabs at each visit.
Capsular groups and molecular typing.
Capsular groups were determined by molecular typing, sequencing of group-specific capsule genes to assess the presence and integrity of the capsule operon, and serologically by slide agglutination to evaluate the expression of the capsular polysaccharide (Fig. 2; see also Table S2 in the supplemental material). The isolates that lacked the capsular operon genes were designated capsule null (cnl), while the isolates that did not react with any of the serological reagents were designated serologically nongroupable (NG). Using molecular procedures, we assessed that the majority of NG isolates showed an intact group B capsule operon. Eleven of the 32 positive subjects (34.4%) were colonized by cnl strains, 9/32 (28.1%) by capsular group B strains, and 18.7%, 6.2%, 6.2%, 3.1%, 3.1% were colonized by MenY, MenE, MenW, MenZ, and MenX strains, respectively. No subject was colonized by MenA or MenC strains.
Capsule null and capsular group B isolates accounted for 23.5% and 29.4% of the total isolates (n = 68), respectively. All cnl strains were found to be devoid of the ctrA gene, as described previously (19).
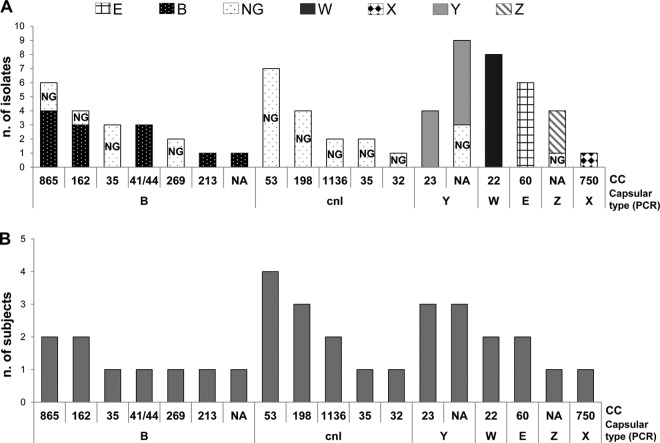
Overall, 79.4% (54/68) of the isolates fell into 14 already known MLST clonal complexes (cc). Twenty-three different sequence types (STs) were identified, 2 of which were new (ST-10178 and ST-10179). Clonal complex 22 (cc22) was the most frequent (11.7%), followed by cc53 (10.3%), cc60 (8.8%), and cc865 (8.8%). The most frequent STs were ST-184 (cc22), ST-5436 (NA), ST-3327 (cc865), and ST-53 (cc53) (see Table S2 in the supplemental material). Figure 3A shows the distribution of the N. meningitidis isolates by capsular group and clonal complex. The majority of the serologically nongroupable (NG) (based on slide agglutination) isolates had intact capsule operons capable of coding for the capsular group B type, as assessed by capsular operon sequencing, and were associated with cc865, cc162, cc35, and cc269. The majority of the serogroupable isolates belonged to serogroups Y (cc23), W (cc22), and E (cc60). Interestingly, as shown in Fig. 3B, the clonal complex associated with the most subjects was cc53 (4 subjects), while the 8 cc22 strains identified belonged to W type and were associated with only two subjects.
FIG 3.
Association of serogroups and MLST clonal complexes identified in this study. (A) A histogram was built, taking into account the total number of N. meningitidis isolates, which are stratified based on the combination of the cc and serogroup, as determined by PCR. The different shading of the bars corresponds to different capsule expression as determined by slide agglutination. (B) A histogram was built showing the correspondence serogroup/cc, assuming that for each subject, identical strains were isolated at different visits (each of the 32 positive subjects corresponds to one strain).
4CMenB sequence typing and MATS analysis.
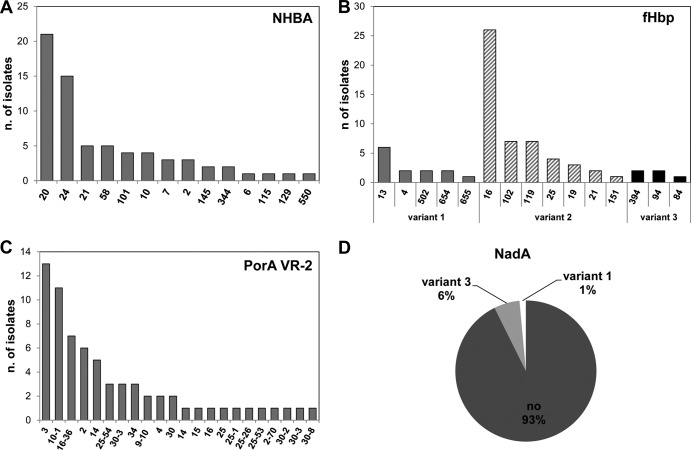
To verify the distribution and variation of the 4CMenB vaccine antigens, all isolates were analyzed for antigen sequence typing and MATS. Figure 4 shows the fHbp, NHBA, PorA-VR2, and NadA genetic characterizations for all isolates. The fHbp gene was present in all isolates. All three fHbp main variants were identified; variant 2 (fHbp-2) was the most prevalent (73.5%), followed by variant 1 (fHbp-1) (19.1%) and variant 3 (fHbp-3) (7.4%). Fifteen fHbp subvariants were found, with the most frequent being fHbp-2.16 (38.2%), mostly associated with cc22 (ST-184), followed by fHbp-2.102 (10.3%) and 2.119 (10.3%). Two fHbp subvariants not previously assigned (fHbp-1.932 and fHbp-1.933) were found. fHbp-1.1, which is present in the 4CMenB vaccine and is predominant among invasive isolates, was not found in the carrier isolates in this study.
FIG 4.
Genetic characterization of the 4CMenB vaccine antigens in the 68 N. meningitidis strains isolated in this study. (A) Prevalence of NHBA peptides; (B) fHbp main variants 1, 2, and 3 and subvariants numeric identifiers; (C) prevalence of PorA VR2 subtype; (D) pie graph of nadA gene presence.
The nhbA gene was found in all the isolates. Fifteen different subvariants or peptides were identified. NHBA peptide 20 (NHBA-20), associated with cc22 (ST-184) and cc162 (ST-162), was the most frequently found (30.9%), followed by NHBA-24 (22.0%, mostly associated with cc865), NHBA-21 (7.3%), and NHBA-58 (7.3%). One peptide, NHBA-550, is described here for the first time. NHBA-2, which is present in the 4CMenB vaccine and is predominant in invasive isolates, was found in 3 isolates (cc41/44) from the same subject (S118).
The nadA gene was present in only 5 isolates (7.3%), 1 belonging to cc32, and 4 isolated from the same subject and not assigned to any cc. NadA variant 3, present in the 4CMenB vaccine, was found in the 4 isolates from the same subject (S121).
As for PorA, we were able to identify 12 different VR1 variants and 20 different VR2 variants, according to the nomenclature available at the Web-accessible database (http://pubmlst.org/neisseria/PorA/). The most common were VR1 variant 18-1 (26.5%) and VR2 variants 3 (19.1%) and 10-1 (16.2%) (see Table S2 in the supplemental material). Furthermore, two new PorA-VR2 variants (25-53 and 25-54) were identified. The VR2 variant 4 present in 4CMenB was found in only 2 consecutively isolated cc162 strains from subject S099.
All MenB isolates were analyzed for the antigenic distance from the 4CMenB antigens and expression level by MATS. The strains carrying fHbp-1 subvariants, isolated from five subjects (15.6%), showed detectable expression levels of the antigen. As expected, the expression of fHbp-2 or fHbp-3 subvariants was not detectable by MATS (see Table S2 in the supplemental material). All the isolates showed detectable expression levels of NHBA (see Table S2). NadA expression was detected by MATS only in the isolates from subject S121, which were molecularly assessed as being positive for the nadA gene (see Table S2).
In subjects with consecutive positive swabs (S054, S081, S115, S118, S121, S142, and S143), the fHbp, NHBA and NadA expression levels of the isolates, detected by MATS, were comparable (Fig. 5). Notably, in the isolates from subject S121, the pattern of NadA expression showed a remarkably higher expression level in the second swab with respect to all the others (Fig. 5C).
FIG 5.
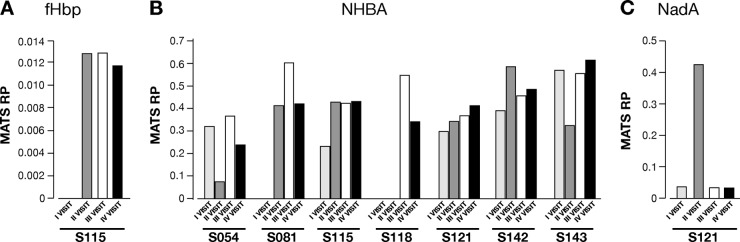
4CMenB antigen expression trends determined by MATS on strains isolated from subjects showing detectable differential expression at different visits. The histograms show the RP trends for fHbp in subject S115 (A), NHBA in subjects S054, S081, S115, S118, S121, S142, and S143 (B), and NadA in subject S121 (C).
Isolate similarities.
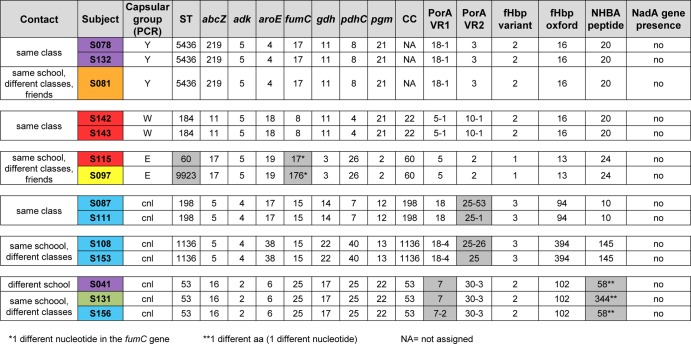
The complete molecular characterization of the 68 isolates allowed for the identification of those that were identical or very similar. In general, when isolated from the same subject, the strains were the same (based on the genetic parameters studied), even though they were sometimes associated with different levels of capsule expression (Fig. 2; see also Table S2 in the supplemental material). For example, 4 isolates from subject S121 differed in their expression of the capsule and in the MLST allelic profile at the adk gene level (visit 4), the three isolates from subject S041 were identical apart from PorA-VR2, and the two isolates from subject S094 differed in PorA-VR2 (see Table S2). Of note, in the few cases for which we evidenced slight differences in the isolates from the same subjects at different time periods, the positive swabs were not consecutive. Interestingly, in 6 cases (Fig. 6), identical or similar strains were isolated in >1 subject. The subjects carrying identical or very similar strains most frequently attended the same class or school or were friends (Fig. 6).
FIG 6.
Relationships among subjects carrying strains with identical or very similar features. Six identical or similar strains were isolated in >1 subject. The different subjects carrying identical or very similar strains most frequently attended the same class or school or were friends. The color coding of the boxes is the same as that shown in Fig. 2. The differences identified through the molecular characterization are shaded in gray.
DISCUSSION
The understanding of N. meningitidis carriage is fundamental to figuring out meningococcal pathogenesis (40). A better understanding of the carriage state might provide key information in the connection between carriage and disease.
For the last 10 years, conjugate vaccines against meningococci of serogroups other than B have been used worldwide (41, 42). Recently, a multicomponent protein-based vaccine against meningococcal group B (4CMenB) was licensed (23). To monitor vaccine-induced changes in meningococcal strains, extensive typing of invasive and carrier isolates will be necessary.
In this study, the overall carriage prevalence (18.5%), with a peak (25.5%) observed in subjects 18 years of age, was comparable to the prevalence rates observed in previous studies. Indeed, Rønne et al. (43) reported a prevalence of carriage of 20.4% in subjects between 16 and 20 years of age in a Danish school in November 1983, and of 19.8% the following March. Moreover, Fraser et al. (44) reported a prevalence of carriage of 25.7% in a cohort of young people 15 to 16 years of age attending a naval school in April 1972 and of 75.8% nine months later.
Few studies have analyzed the duration of meningococcal carriage (10, 11). The duration of carriage may actually depend on the ability of meningococcal strains to establish a long-term commensal relationship with the host and also on the host's immune status and genetic properties. To better understand this point, all carrier isolates were fully characterized to determine their presence in successive swabs from the same individuals and their spread in the community.
Some subjects were carriers for the entire period of our survey, while others were positive at one swab of four. We cannot exclude that negative samples, in particular those from subjects with nonconsecutive positive swabs, might result from the low sensitivity of the swabbing method. It is interesting to note that of 12 carriage episodes at one swab only, 7 were associated with cnl isolates (58.3%). This suggests that cnl strains might be associated with shorter carriage duration. However, in some cases, the same subject was not present at all the four visits. For this reason, while prolonged carrier status was associated with encapsulated strains, a complete association of cnl with sporadic carriage cannot be assumed. Findings related to the persistence of the carriage state were limited to the time span of the study.
MacLennan and colleagues (45) conducted a study on the risk factors that may affect carrier status in British teenagers. They found that the main risk conditions were frequenting pubs/clubs, intimate kissing, and exposure to active and passive smoking. In our study, there was no statistically significant difference between carriers and noncarriers with regard to exposure to active and/or passive smoking. Furthermore, our results showed that the cohabitation with four or more persons potentially conditions the carrier status, even though the association was only indicative and not significant.
Several isolates, although bearing intact capsule operons, were nongroupable by serological methods. Interestingly, the majority of the serologically nongroupable (NG) isolates belonged to capsular group B, as determined by sequence analysis. Besides cnl and MenB strains, MenY and MenW strains were the most frequently found. MenY, which has increased in the United States and United Kingdom during the past decades, has the potential to become important also in Italy (see www.simi.iss.it/meningite_batterica.htm). None of the MenW isolates found in this study were related to the Hajj outbreak (46). Also, one MenX isolate was identified. To date, only one case of invasive disease caused by this capsular group has been reported in Italy (47). We did not detect any MenC isolate, although none of the subjects had been vaccinated with the MenC conjugate vaccine. Provided that capsular group C meningococci have a much lower propensity to be carried by healthy individuals (14), this finding also suggests that the vaccination coverage required to hinder the circulation of MenC strains might be limited. In the region of the study, vaccination coverage in infants is currently about 70%, while it is lower in teenagers (20%). This finding is consistent with the results obtained in Germinario et al. (48) and with British and European data (49, 50). No MenA strains were isolated. This is in line with the fact that capsular group A meningococci have only sporadically caused invasive disease in Europe and are rare in healthy individuals (51).
Few isolates analyzed in this study were associated with meningococcal hypervirulent clonal complexes, as expected for a carrier population. The most frequently identified clonal complexes were cc53 and cc198, which are typically associated with cnl strains (52). Moreover, the isolates belonging to the hypervirulent complexes were often unencapsulated.
The association between certain serogroups and particular clonal complexes (51) was confirmed. cc41/44, cc269, cc162, and cc213 strains were all associated with capsulated or unencapsulated Men B strains. Instead, cc23 was associated with capsular group Y and cc22 with MenW isolates. Unexpectedly, we found two cnl isolates belonging to cc32 and cc35.
We observed that all subjects positive at multiple swabs were carrying the same strains for the entire period of the survey or cumulated very little differences over time. In most cases, close contacts carried identical or very similar strains.
With regard to the 4CMenB antigens, all three variants of fHbp were present in the strains analyzed. fHbp variant 2 clearly prevailed over fHbp-1, as expected from other carriage studies (53, 54). fHbp-1 has indeed been described to be predominant in invasive isolates (16, 31, 55–57). Thirteen of the 15 fHbp subvariants were also detected in invasive isolates, and only two were new. However, the diversity of fHbp in this strain panel was relatively high compared with strain panels composed of disease-causing isolates (58).
Thirteen of the 14 NHBA peptides identified in this study had already been found in invasive isolates, and only one peptide was new. The nadA gene was found in only 5 carrier isolates (corresponding to two carriage episodes). Previous studies have demonstrated that NadA is underrepresented in carrier isolates and is associated with N. meningitidis pathogenicity (59). In this strain panel, the gene was present in one cc32-cnl strain and in one MenZ strain.
Due to the nonrandom association with clonal complexes (16), we found fHbp-1.4, fHbp-3.94, and NHBA-10 to be associated with cnl isolates belonging to cc198, whereas fHbp-2.102 and NHBA-58 were associated with cnl isolates belonging to cc53, as expected from recent studies (60).
The MATS assay was performed in order to evaluate the expression levels and antigen cross-reactivity toward 4CMenB components in all isolates. All data from the phenotypic approach (MATS) were consistent with the genotypic (sequencing) analysis. Moreover, MATS revealed the possibility for a strain to express different amounts of NadA protein at different isolations from the same subject, demonstrating that the level of expression can vary over time. However, it is important to highlight that the positive bactericidal threshold (PBT) has been defined for only MenB strains so far. In the case of unencapsulated strains, the relationship between MATS and SBA might be difficult to define because of the intrinsic susceptibility of unencapsulated strains to complement-mediated killing. Since MenB strains accounted for only 29.4% of the total isolates, any prediction of coverage would hardly have been significant.
In conclusion, two main groups of carrier isolates were distinguished in our study. On one side, we found isolates genetically unable to express a functional capsule because they were missing the capsule operon (60). These isolates are defined as cnl and are typically found to be associated with harmless meningococcal carriage. On the other side, we also found encapsulated isolates and/or isolates capable of expressing the capsule, with the same molecular features of meningococci recovered from patients, that is to say those that are potentially capable of causing meningococcal disease. Anyway, all carrier isolates in this study showed a higher genetic variability with respect to invasive isolates, as already reported (40).
The purpose of this study was to give an overview of the overall serological and molecular diversity of carrier strains isolated from individuals sharing a defined age group and living spaces, and to compare those results with those of previous analogous studies. The study was based on the characterization of strain heterogeneity by standard molecular and serological methods, which were applied to the antigens included in the new vaccine 4CMenB. A future assessment of antigen variability and expression, possibly performed after the introduction of the vaccine and using different age groups, will be of great help for monitoring the ability of 4CMenB vaccine to impact nasopharyngeal/oropharyngeal carriage.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by project PRIN 2007 grant 20074B5ZBC from the Italian Ministry of University and Research and by a grant from Novartis Vaccines and Diagnostics, Siena, Italy.
Roberto Gasparini, Daniela Amicizia, Filippo Ansaldi, Paola Canepa, Andrea Orsi, Giancarlo Icardi, Emanuela Rizzitelli, and Donatella Panatto declare no conflicts of interest. Gabriella De Angelis, Stefania Bambini, Monica Moschioni, Sara Comandi, Isabella Simmini, Giueseppe Boccadifuoco, Brunella Brunelli, Marzia Monica Giuliani, and Mariagrazia Pizza are employees of Novartis Vaccines and Diagnostics, Siena, Italy, and Maurizio Comanducci was an employee of Novartis Vaccines and Diagnostics, Siena, Italy, at the time the work was performed.
We thank the two secondary technical schools in Genoa, Italy (Istituto di Istruzione Secondaria Superiore Duchessa di Galliera and Istituto di Istruzione Secondaria Superiore Majorana-Giorgi), for enrolling the participants in the study.
Footnotes
Published ahead of print 19 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.03584-13.
REFERENCES
- 1.Hill DJ, Griffiths NJ, Borodina E, Virji M. 2010. Cellular and molecular biology of Neisseria meningitidis colonization and invasive disease. Clin. Sci. (Lond.) 118:547–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gasparini R, Amicizia D, Lai PL, Panatto D. 2012. Neisseria meningitidis, pathogenetic mechanisms to overcome the human immune defences. J. Prev. Med. Hyg. 53:50–55 [PubMed] [Google Scholar]
- 3.Muzzi A, Mora M, Pizza M, Rappuoli R, Donati C. 2013. Conservation of meningococcal antigens in the genus Neisseria. mBio 4(3):e00163–13. 10.1128/mBio.00163-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yazdankhah SP, Caugant DA. 2004. Neisseria meningitidis: an overview of the carriage state. J. Med. Microbiol. 53:821–832. 10.1099/jmm.0.45529-0 [DOI] [PubMed] [Google Scholar]
- 5.Goldschneider I, Gotschlich EC, Artenstein MS. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldschneider I, Gotschlich EC, Artenstein MS. 1969. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 129:1327–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen H, May M, Bowen L, Hickman M, Trotter CL. 2010. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect. Dis. 10:853–861. 10.1016/S1473-3099(10)70251-6 [DOI] [PubMed] [Google Scholar]
- 8.Maiden MC, Ibarz-Pavòn AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, Walker AM, Evans MR, Kroll JS, Neal KR, Ala'aldeen DA, Crook DW, Cann K, Harrison S, Cunningham R, Baxter D, Kaczmarski E, Maclennan J, Cameron JC, Stuart JM. 2008. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J. Infect. Dis. 197:737–743. 10.1086/527401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzzetta G, Manfredi P, Gasparini R, Panatto D, Edmunds WJ. 2009. On the relationship between meningococcal transmission dynamics and disease: remarks on humoral immunity. Vaccine 27:3429–3434. 10.1016/j.vaccine.2009.01.092 [DOI] [PubMed] [Google Scholar]
- 10.Glitza IC, Ehrhard I, Müller-Pebody B, Reintjes R, Breuer T, Ammon A, Sonntag HG. 2008. Longitudinal study of meningococcal carrier rates in teenagers. Int. J. Hyg. Environ. Health 211:263–272. 10.1016/j.ijheh.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 11.Caugant DA, Fogg C, Bajunirwe F, Piola P, Twesigye R, Mutebi F, Frøholm LO, Rosenqvist E, Batwala V, Aaberge IS, Rottingen JA, Guerin PJ. 2006. Pharyngeal carriage of Neisseria meningitidis in 2-19-year-old individuals in Uganda. Trans. R. Soc. Trop. Med. Hyg. 100:1159–1163. 10.1016/j.trstmh.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 12.Yazdankhah SP, Kriz P, Tzanakaki G, Kremastinou J, Kalmusova J, Musilek M, Alvestad T, Jolley KA, Wilson DJ, McCarthy ND, Caugant DA, Maiden MC. 2010. Distribution of serogroups and genotypes among disease-associated and carried isolates of Neisseria meningitidis from the Czech Republic, Greece, and Norway. J. Clin. Microbiol. 42:5146–5153. 10.1128/JCM.42.11.5146-5153.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant DA, Feavers IM, Achtman M, Spratt BG. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U. S. A. 95:3140–3145. 10.1073/pnas.95.6.3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caugant DA, Tzanakaki G, Kriz P. 2007. Lessons from meningococcal carriage studies. FEMS Microbiol. Rev. 31:52–63. 10.1111/j.1574-6976.2006.00052.x [DOI] [PubMed] [Google Scholar]
- 15.Urwin R, Russell JE, Thompson EA, Holmes EC, Feavers IM, Maiden MC. 2004. Distribution of surface protein variants among hyperinvasive meningococci: implications for vaccine design. Infect. Immun. 72:5955–5962. 10.1128/IAI.72.10.5955-5962.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bambini S, Muzzi A, Olcen P, Rappuoli R, Pizza M, Comanducci M. 2009. Distribution and genetic variability of three vaccine components in a panel of strains representative of the diversity of serogroup B meningococcus. Vaccine 27:2794–2803. 10.1016/j.vaccine.2009.02.098 [DOI] [PubMed] [Google Scholar]
- 17.Hammerschmidt S, Müller A, Sillmann H, Mühlenhoff M, Borrow R, Fox A, van Putten J, Zollinger WD, Gerardy-Schahn R, Frosch M. 1996. Capsule phase variation in Neisseria meningitidis serogroup B by slipped-strand mispairing in the polysialyltransferase gene (siaD): correlation with bacterial invasion and the outbreak of meningococcal disease. Mol. Microbiol. 20:1211–1220. 10.1111/j.1365-2958.1996.tb02641.x [DOI] [PubMed] [Google Scholar]
- 18.Dolan-Livengood JM, Miller YK, Martin LE, Urwin R, Stephens DS. 2003. Genetic basis for nongroupable Neisseria meningitidis. J. Infect. Dis. 187:1616–1628. 10.1086/374740 [DOI] [PubMed] [Google Scholar]
- 19.Claus H, Maiden MC, Maag R, Frosch M, Vogel U. 2002. Many carried meningococci lack the genes required for capsule synthesis and transport. Microbiology 148:1813–1819 [DOI] [PubMed] [Google Scholar]
- 20.Harrison LH, Trotter CL, Ramsay ME. 2009. Global epidemiology of meningococcal disease. Vaccine 27:B51–63. 10.1016/j.vaccine.2009.04.063 [DOI] [PubMed] [Google Scholar]
- 21.Boisier P, Nicolas P, Djibo S, Taha MK, Jeanne I, Maïnassara HB, Tenebray B, Kairo KK, Giorgini D, Chanteau S. 2007. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin. Infect. Dis. 44:657–663. 10.1086/511646 [DOI] [PubMed] [Google Scholar]
- 22.Finne J, Leinonen M, Makela PH. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet 2:355–357 [DOI] [PubMed] [Google Scholar]
- 23.Martin NG, Snape MD. 2013. A multicomponent serogroup B meningococcal vaccine is licensed for use in Europe: what do we know, and what are yet to learn? Expert Rev. Vaccines 12:837–858. 10.1586/14760584.2013.814862 [DOI] [PubMed] [Google Scholar]
- 24.Toneatto D, Ismaili S, Ypma E, Vienken K, Oster P, Dull P. 2011. The first use of an investigational multicomponent meningococcal serogroup B vaccine (4CMenB) in human. Hum. Vaccines 7:646–653. 10.4161/hv.7.6.15482 [DOI] [PubMed] [Google Scholar]
- 25.Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, Evans A, Telford KL, Ypma E, Toneatto D, Oster P, Miller E, Pollard AJ. 2010. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infant. Clin. Infect. Dis. 51:1127–1137. 10.1086/656741 [DOI] [PubMed] [Google Scholar]
- 26.Dull PM, McIntosh ED. 2012. Meningococcal vaccine development–from glycoconjugates against MenACWY to proteins against MenB–potential for broad protection against meningococcal disease. Vaccine 30S:B18–B25. 10.1016/j.vaccine.2012.01.062 [DOI] [PubMed] [Google Scholar]
- 27.Martin DR, Ruijne N, McCallum L, O'Hallahan J, Oster P. 2006. The VR2 epitope on the PorA P1.7-2,4 protein is the major target for the immune response elicited by the strain-specific group B meningococcal vaccine MeNZB. Clin. Vaccine Immunol. 13:486–491. 10.1128/CVI.13.4.486-491.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, Brunelli B, Pieri A, Santini L, Savino S, Serruto D, Litt D, Kroll S, Welsch JA, Granoff DM, Rappuoli R, Pizza M. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197:789–799. 10.1084/jem.20021911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serruto D, Spadafina T, Ciucchi L, Lewis LA, Ram S, Tontini M, Santini L, Biolchi A, Seib KL, Giuliani MM, Donnelly JJ, Berti F, Savino S, Scarselli M, Costantino P, Kroll JS, O'Dwyer C, Qiu J, Plaut AG, Moxon R, Rappuoli R, Pizza M, Aricò B. 2010. Neisseria meningitidis GNA2132, a heparin-binding protein that induces protective immunity in humans. Proc. Natl. Acad. Sci. U. S. A. 107:3770–3775. 10.1073/pnas.0915162107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Comanducci M, Bambini S, Brunelli B, Adu-Bobie J, Aricò B, Capecchi B, Giuliani MM, Masignani V, Santini L, Savino S, Granoff DM, Caugant DA, Pizza M, Rappuoli R, Mora M. 2002. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med. 195:1445–1454. 10.1084/jem.20020407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, Moxon ER, Stella M, Comanducci M, Bambini S, Muzzi A, Andrews W, Chen J, Santos G, Santini L, Boucher P, Serruto D, Pizza M, Rappuoli R, Giuliani MM. 2010. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc. Natl. Acad. Sci. U. S. A. 107:19490–19495. 10.1073/pnas.1013758107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taha MK. 2000. Simultaneous approach for nonculture PCR-based identification and serogroup prediction of Neisseria meningitidis. J. Clin. Microbiol. 38:855–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis C, Clarke SC. 2003. Identification of Neisseria meningitidis serogroups Y and W135 by siaD nucleotide sequence analysis. J. Clin. Microbiol. 41:2697–2699. 10.1128/JCM.41.6.2697-2699.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett DE, Mulhall RM, Cafferkey MT. 2004. PCR-based assay for detection of Neisseria meningitidis capsular serogroups 29E, X, and Z. J. Clin. Microbiol. 42:1764–1765. 10.1128/JCM.42.4.1764-1765.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mölling P, Unemo M, Bäckman A, Olcén P. 2000. Genosubtyping by sequencing group A, B and C meningococci; a tool for epidemiological studies of epidemics, clusters and sporadic cases. APMIS 108:509–516. 10.1034/j.1600-0463.2000.d01-90.x [DOI] [PubMed] [Google Scholar]
- 36.Clarke SC, Diggle MA, Mölling P, Unemo M, Olcén P. 2003. Analysis of PorA variable region 3 in meningococci: implications for vaccine policy? Vaccine 21:2468–2473. 10.1016/S0264-410X(03)00033-1 [DOI] [PubMed] [Google Scholar]
- 37.Sacchi CT, Whitney AM, Reeves MW, Mayer LW, Popovic T. 2002. Sequence diversity of Neisseria meningitidis 16S rRNA genes and use of 16S rRNA gene sequencing as a molecular subtyping tool. J. Clin. Microbiol. 40:4520–4527. 10.1128/JCM.40.12.4520-4527.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucidarme J, Comanducci M, Findlow J, Gray SJ, Kaczmarski EB, Guiver M, Kugelberg E, Vallely PJ, Oster P, Pizza M, Bambini S, Muzzi A, Tang CM, Borrow R. 2009. Characterization of fHbp, nhba (gna2132), nadA, porA, sequence type (ST), and genomic presence of IS1301 in group B meningococcal ST269 clonal complex isolates from England and Wales. J. Clin. Microbiol. 47:3577–3585. 10.1128/JCM.00936-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucidarme J, Newbold LS, Findlow J, Gilchrist S, Gray SJ, Carr AD, Hewitt J, Kaczmarski EB, Borrow R. 2011. Molecular targets in meningococci: efficient routine characterization and optimal outbreak investigation in conjunction with routine surveillance of the meningococcal group B vaccine candidate, fHBP. Clin. Vaccine Immunol. 18:194–202. 10.1128/CVI.00401-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yazdankhah SP, Caugant DA. 2004. Neisseria meningitidis: an overview of the carriage state. J. Med. Microbiol. 53:821–832. 10.1099/jmm.0.45529-0 [DOI] [PubMed] [Google Scholar]
- 41.Halperin SA, Bettinger JA, Greenwood B, Harrison LH, Jelfs J, Ladhani SN, McIntyre P, Ramsay ME, Sáfadi MA. 2012. The changing and dynamic epidemiology of meningococcal disease. Vaccine 30:B26–B36. 10.1016/j.vaccine.2011.12.032 [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention (CDC). 2013. Infant meningococcal vaccination: Advisory Committee on Immunization Practices (ACIP) recommendations and rationale. MMWR Morb. Mortal. Wkly. Rep. 62:52–54 [PMC free article] [PubMed] [Google Scholar]
- 43.Rønne T, Berthelsen L, Buhl LH, Lind I. 1993. Comparative studies on pharyngeal carriage of Neisseria meningitidis during a localized outbreak of serogroup C meningococcal disease. Scand. J. Infect. Dis. 25:331–339. 10.3109/00365549309008507 [DOI] [PubMed] [Google Scholar]
- 44.Fraser PK, Bailey GK, Abbott JD, Gill JB, Walker DJC. 1973. The meningococcal carrier-rate. Lancet 301:1235–1237. 10.1016/S0140-6736(73)90541-2 [DOI] [PubMed] [Google Scholar]
- 45.MacLennan J, Kafatos G, Neal K, Andrews N, Cameron JC, Roberts R, Evans MR, Cann K, Baxter DN, Maiden MC, Stuart JM, United Kingdom Meningococcal Carriage Group 2006. Social behavior and meningococcal carriage in British teenagers. Emerg. Infect. Dis. 12:950–957. 10.3201/eid1206.051297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aguilera JF, Perrocheau A, Meffre C, Hahné S, W135 Working Group 2002. Outbreak of serogroup W135 meningococcal disease after the Hajj pilgrimage, Europe, 2000. Emerg. Infect. Dis. 8:761–767. 10.3201/eid0808.010422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fazio C, Starnino S, Dal Soldà M, Sofia T, Neri A, Mastrantonio P, Stefanelli P. 2010. Neisseria meningitidis serogroup X sequence type 2888, Italy. Emerg. Infect. Dis. 16:359–360. 10.3201/eid1602.091553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Germinario C, Tafuri S, Napoli C, Montagna MT, Balducci MT, Fortunato F, Martinelli D, Prato R. 2010. Young-adult carriers of Neisseria meningitidis in Puglia (Italy): will the pattern of circulating meningococci change following the introduction of meningococcal serogroup C conjugate vaccines? Hum. Vaccin. 6:1025–1027. 10.4161/hv.6.12.13145 [DOI] [PubMed] [Google Scholar]
- 49.Trotter CL, Maiden MC. 2009. Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert Rev. Vaccines 8:851–861. 10.1586/erv.09.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soriano-Gabarrò M, Wolter J, Hogea C, Vyse A. 2011. Carriage of Neisseria meningitidis in Europe: a review of studies undertaken in the region. Expert Rev. Anti Infect. Ther. 9:761–774. 10.1586/eri.11.89 [DOI] [PubMed] [Google Scholar]
- 51.Trotter CL, Chandra M, Cano R, Larrauri A, Ramsay ME, Brehony C, Jolley KA, Maiden MC, Heuberger S, Frosch M. 2007. A surveillance network for meningococcal disease in Europe. FEMS Microbiol. Rev. 31:27–36. 10.1111/j.1574-6976.2006.00060.x [DOI] [PubMed] [Google Scholar]
- 52.Jacobsson S, Hedberg ST, Mölling P, Unemo M, Comanducci M, Rappuoli R, Olcén P. 2009. Prevalence and sequence variations of the genes encoding the five antigens included in the novel 5CVMB vaccine covering group B meningococcal disease. Vaccine 27:1579–1584. 10.1016/j.vaccine.2008.12.052 [DOI] [PubMed] [Google Scholar]
- 53.Marsh JW, Shutt KA, Pajon R, Tulenko MM, Liu S, Hollick RA, Kiehlbauch JA, Clark TA, Stephens DS, Arnold KE, Myers RA, Mayer LW, Harrison LH. 2011. Diversity of factor H-binding protein in Neisseria meningitidis carriage isolates. Vaccine 29:6049–6058. 10.1016/j.vaccine.2011.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson AS, Hao L, Jiang Q, Harris SL, Jones TR, Perez JL, York L, Eiden J, Jansen KU. 2012. Potential impact of the bivalent rLP2806 vaccine on Neisseria meningitidis carriage and invasive serogroup B disease. Hum. Vaccin. Immunother. 9:471–479. 10.4161/hv.23222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, Cohn A, Comanducci M, Andrew L, Zhao X, MacNeil JR, Schmink S, Muzzi A, Bambini S, Rappuoli R, Pizza M, Murphy E, Hoiseth SK, Jansen KU, Anderson AS, Harrison LH, Clark TA, Messonnier NE, Mayer LW. 2011. Prevalence and genetic diversity of candidate vaccine antigens among invasive Neisseria meningitidis isolates in the United States. Vaccine 29:4739–4744. 10.1016/j.vaccine.2011.04.092 [DOI] [PubMed] [Google Scholar]
- 56.Lucidarme J, Comanducci M, Findlow J, Gray SJ, Kaczmarski EB, Guiver M, Vallely PJ, Oster P, Pizza M, Bambini S, Muzzi A, Borrow R. 2010. Characterization of fHbp, nhba (gna2132), nadA, porA, and sequence type in group B meningococcal case isolates collected in England and Wales during January 2008 and potential coverage of an investigational group B meningococcal vaccine. Clin. Vaccine Immunol. 17:919–929. 10.1128/CVI.00027-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plikaytis BD, Stella M, Boccadifuoco G, DeTora LM, Agnusdei M, Santini L, Brunelli B, Orlandi L, Simmini I, Giuliani M, Ledroit M, Hong E, Taha MK, Ellie K, Rajam G, Carlone GM, Claus H, Vogel U, Borrow R, Findlow J, Gilchrist S, Stefanelli P, Fazio C, Carannante A, Oksnes J, Fritzsønn E, Klem AM, Caugant DA, Abad R, Vázquez JA, Rappuoli R, Pizza M, Donnelly JJ, Medini D. 2012. Interlaboratory standardization of the sandwich enzyme-linked immunosorbent assay designed for MATS, a rapid, reproducible method for estimating the strain coverage of investigational vaccines. Clin. Vaccine Immunol. 19:1609–1617. 10.1128/CVI.00202-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogel U, Taha MK, Vazquez JA, Findlow J, Claus H, Stefanelli P, Caugant DA, Kriz P, Abad R, Bambini S, Carannante A, Deghmane AE, Fazio C, Frosch M, Frosi G, Gilchrist S, Giuliani MM, Hong E, Ledroit M, Lovaglio PG, Lucidarme J, Musilek M, Muzzi A, Oksnes J, Rigat F, Orlandi L, Stella M, Thompson D, Pizza M, Rappuoli R, Serruto D, Comanducci M, Boccadifuoco G, Donnelly JJ, Medini D, Borrow R. 2013. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect. Dis. 13:416–425. 10.1016/S1473-3099(13)70006-9 [DOI] [PubMed] [Google Scholar]
- 59.Comanducci M, Bambini S, Caugant DA, Mora M, Brunelli B, Capecchi B, Ciucchi L, Rappuoli R, Pizza M. 2004. NadA diversity and carriage in Neisseria meningitidis. Infect. Immun. 72:4217–4223. 10.1128/IAI.72.7.4217-4223.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Claus H, Jördens MS, Kriz P, Musilek M, Jarva H, Pawlik MC, Meri S, Vogel U. 2012. Capsule null locus meningococci: typing of antigens used in an investigational multicomponent meningococcus serogroup B vaccine. Vaccine 30:155–160. 10.1016/j.vaccine.2011.11.050 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.