Abstract
This commentary will introduce lean concepts into the clinical microbiology laboratory. The practice of lean in the clinical microbiology laboratory can remove waste, increase efficiency, and reduce costs. Lean, Six Sigma, and other such management initiatives are useful tools and can provide dividends but must be accompanied by organizational leadership commitment to sustaining the lean culture in the laboratory setting and providing resources and time to work through the process.
TEXT
The intent of this commentary is to present the concept of lean management practices as they can be applied in the clinical microbiology laboratory.
In its simplest form, the Affordable Care Act includes a number of Medicare reforms that will reduce the rate of Medicare escalation over the next decade and beyond. The goal of these reforms is to provide Medicare beneficiaries with an improved quality of care, create new models of care, price services more appropriately, and hopefully rid the health care system of waste, fraud, and abuse. In addition, as a result of reductions in overall levels of Medicare reimbursement, Medicare Advantage cuts, and sequestration, it is hoped that the Affordable Care Act will lead to savings of hundreds of billions of dollars in health care costs.
Organizations across the country have been preparing for these reductions in a number of ways. Creating, maintaining, and improving patient quality, access to care, and affordability have been center stage in many health care organizations. Patient centeredness as well as the development of accountable-care organizations is a part of key integrated-care movements that bring the aforementioned concepts into a sharpened focus. Moreover, creation of greater efficiency of care, removal of waste, and unit cost reduction (reduction in the volume of unnecessary care) are a few areas of effort.
Over the last decade, there has been an upsurge in the consolidation of hospitals, which often results in the consolidation of laboratory services. In addition, as of 2012, there were 48 million uninsured people in the United States, which means that going forward, many institutions are anticipating providing care to a broader patient base (1). Elimination of waste in the U.S. health care system overall would help reduce the massive price tag that the United States is saddled with in delivering quality health care. Several examples of waste reduction, such as appropriate antibiotic stewardship, continuity of care, and implementation of best practices, are highlighted in the literature. The clinical laboratory is an integral part of the overall care delivery system, and 70% of medical decisions are based on results produced by clinical laboratorys (2). A leaner, more efficient laboratory leaves room for growth by removing excess waste and improving processes related to specialized testing services. In addition to the impact of Medicare reimbursement and the other challenges noted above is the impact of fewer medical technology programs producing fewer qualified medical technologists. The number of clinical laboratory scientists graduating from training programs has significantly declined in the last 10 years. Recent data show staffing vacancies within the field of microbiology to have decreased from 2010 to 2012, which is very promising (3). The practice of lean addresses much of what is discussed above and, when adopted in the clinical microbiology laboratory, can remove waste, increase efficiency, and reduce costs. Depending on the organization, the pathways to lean can be varied. This commentary will touch on some of the basic concepts of lean and stress its importance within the laboratory environment. While a comprehensive review of lean is beyond the scope of this commentary, we will discuss some of the key tools and concepts that were crucial to the success of implementing lean in two large clinical microbiology laboratories. Kaiser Permanente Regional Reference Laboratories is a high-volume reference laboratory which supports an integrated health care system of 14 hospitals and over 200 medical office buildings within the southern California area. The Microbiology Division within the Henry Ford Health System (HFHS) consists of a core laboratory facility that serves 5 hospitals and 29 clinics distributed throughout southeast Michigan.
DEFINITION AND CULTURE OF LEAN
Lean is the system of management first pioneered by Toyota Motor Corporation that supports a production system based on continuous process improvement (Kaizen) and worker engagement (4). It involves a long-term philosophy of investing in the development of teams of individuals and equipping them with the tools necessary to identify and reduce waste/defects within a system. Lean eschews the idea of quick-fix solutions to serve short-term goals in favor of a more sustained approach to process improvement. Although the concept of lean originated in the manufacturing industry, lean practices have been employed within many other organizations, including medical laboratories (5–7).
It should be noted, however, that lean, Six Sigma, and other such management initiatives oftentimes end up failing to achieve their goals (6). If the culture of lean is not guided and nurtured appropriately, laboratorians quickly learn that most of these initiatives will eventually disappear. Reasons for failure include not obtaining the complete support of leadership, a lack of buy-in from managers, failure to create a blameless environment, fears relating to job security, and an initial focus primarily on cost reduction rather than quality improvements. It is also essential that there be a structure that enables individuals from different units to work collaboratively regardless of barriers created by silos of control and finance. The most difficult part of implementing a successful lean program is creating a culture of lean that is self-sustaining and productive (6). This requires a level of commitment from both the leadership and staff to realize that it will take time and persistence to yield dividends. In addition, successful implementation of lean requires informed decision making with the involvement of bench staff to drive continuous process improvements (8).
DEVELOPMENT OF LEAN PROGRAMS
(i) The HFHS experience.
The HFHS Department of Pathology and Laboratory Medicine began its lean adoption in 2005 by developing the first lean laboratory enterprise modeled after Toyota's process; it was called the Henry Ford Production System. The department created a Quality Systems Division that took advantage of training available from institutions such as the Pittsburgh Regional Healthcare Initiative (8) to jump-start the lean process at HFHS. The Quality Systems Division was in turn charged with developing an internal, laboratory-specific program to train the entire pathology service line, from physician leaders and residents to laboratory assistants, in the concepts and practical application of lean. The training process ranged from a half day to 2 days. Once training was completed, the quality staff members were tasked with guiding the various divisions within the department as they implemented process improvements using lean concepts. This approach avoided the “sink or swim” situation that many laboratories find themselves in once the lean consultants have left. One of the key concepts learned during the implementation process was that lean quality programs will be successful only if the staff is given an appropriate structure with educated and incentivized managers to come to the right solutions. Over a period of 4 years, roughly 800 lean-practice-educated employees across the pathology service line have accomplished over 4,900 process improvements.
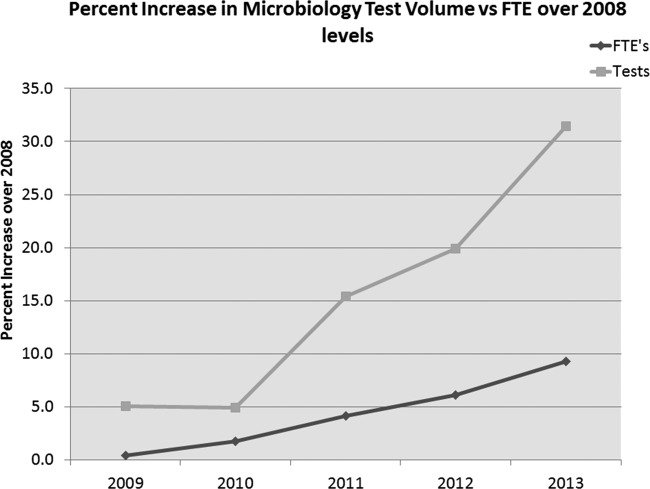
In 2007, the Microbiology Division within HFHS consisted of four microbiology laboratories that were tasked with consolidating into one core laboratory with a projected staff of 55 full-time equivalents (FTEs) who performed 700,000 tests annually. The Department of Pathology and Laboratory Medicine used a lean management approach to accomplish this goal without compromising existing laboratory services but lowered the overall level of staffing by attrition (Fig. 1).
FIG 1.
Lean process improvements allowed the HFHS Core Microbiology Laboratory to take on additional work during the integration process, without a comparable increase in staffing.
(ii) The Kaiser Permanente experience.
Given the challenges in health care elucidated above, the Kaiser Permanente Regional Reference Laboratories in southern California established a goal of adopting a lean practice philosophy in the laboratory. The laboratory system consists of one regional laboratory which includes a centralized microbiology division that processes a yearly volume of almost 5 million specimens. The Bacteriology Department (110 FTEs) alone processes 1.4 million tests annually. To improve quality of care, increase staff participation in the workplace, and eliminate waste that drives up the health care costs within the organization, a labor management partnership [(L+M)P] that is in place for the whole organization, not just within the laboratory setting, was formed. (L+M)P is a strategy partnering Kaiser Permanente with our coalition of unions that not only examines processes but also engages staff at all levels, empowering everyone to be part of the solution in their respective department. At the core of the (L+M)P are the unit-based teams (UBT). Many departments have already integrated a team-based approach to problem solving in the clinical laboratory setting. Issues are proposed, and both the staff and the managers have equal roles in working toward a solution, with the focus on patient care at the core. The UBT process is similar to the concepts integral to lean as described below. Due to the complexity and the time required for working collectively as a team, departments within Kaiser Permanente are at various stages of working through the UBT process. In addition to adopting (L+M)P, the Kaiser Permanente Regional Reference Laboratories have recently enrolled managerial staff in a formal training program at a local university offering an accredited program in lean. Kaiser Permanente is integrating the UBT process initiated through the (L+M)P and formal training to provide the framework and tools for lean in the workplace.
CONCEPTS OF LEAN
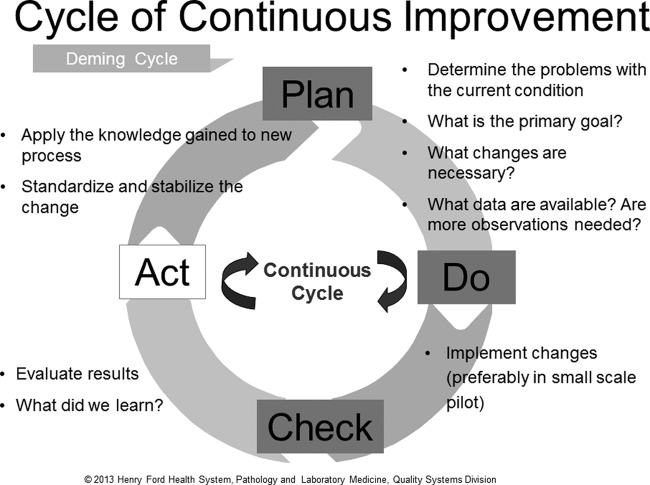
A generally accepted lean principle is that continuous process improvement is best accomplished by small teams of staff who are tasked with making changes using what is called the plan-do-check-act (PDCA) cycle (Fig. 2) (8–10). At Kaiser Permanente, integral to the UBT process described above is a similar approach called plan-do-study-act. Simply stated, each member of the team represents a key stakeholder in the process and has specific responsibilities not just for coming up with a plan of action but also for implementation and follow up. This process ensures that there is a standardized approach to problem solving beginning with a standard questionnaire to define the scope of the problem, followed by use of specific tools to address the issue. Multiple rounds of PDCA may be required before satisfactory resolution of a problem is achieved. Monitoring using selected metrics may be necessary to ensure that the problem does not recur. PDCA cycles are effective in saving time in the long run but require an investment in time and resources to perform root cause analysis using lean tools.
FIG 2.
Plan-do-check-act cycle of continuous improvement. (Reproduced from reference 10 with permission from the publisher. © 2010–2014 American Society for Clinical Pathology. © 2010–2014 American Journal of Clinical Pathology.)
Another integral part of lean is establishing clear lines of communication, which in turn facilitate the resolution of complex problems. There are several examples within lean for continuous communication, with a whiteboard being an effective tool used quite frequently. Whiteboards are excellent yet simple tools for communication that can be placed strategically in the laboratory, enabling workers to document defects or problems within the workplace in real time. Whiteboard issues can be addressed immediately using quick fixes or can be routed through a PDCA cycle if a particular defect occurs with increased frequency. Whiteboards should be monitored each shift throughout the day to maximize the effective use of the tool. This takes commitment and dedication to the process and this communication tool.
Communication among different units within the laboratory and the institution as a whole can be problematic. In lean, is it customary to define each unit within the group as either a customer or a supplier of the other (7). For example, in the laboratory, the specimen-receiving and -processing area supplies the downstream laboratory areas, which, in turn, supply results to health care providers. By putting together teams of individuals that overlap multiple units and holding customer-supplier meetings as needed, problems can be examined and resolved using the PDCA cycle. Feedback using metrics collected at predetermined intervals can be used to determine if problems have actually been solved and to diffuse individual personnel concerns.
EXAMPLES OF LEAN INITIATIVES
The tools used by a team for problem resolution will vary based on the nature of the problem. For example, at HFHS, a backlog of specimens pending processing at the end of the second shift was identified as a major defect. Using collected data having to do with the numbers of specimens received per hour and spaghetti diagrams depicting technologist movement at key times (both concepts of lean), the second-shift technologists were able to significantly reduce the extent of the backlog without the intervention of upper management or the need for additional staffing. They were able to accomplish this by identifying key bottlenecks in specimen processing and peak hours for specimen receipt and by designating specific responsibilities to individual team members to address the shortcomings. In addition, they worked on a first-in, first-out approach to specimen processing and collaborated with other units to reduce batch sizes through more-frequent specimen pickups. A similar approach was taken within Kaiser Permanente. A detailed analysis of when specimens were arriving and being plated in the laboratory was undertaken. Both the shifts of the laboratory assistants and those of technologists were adjusted to more efficiently perform the tasks at hand and improve specimen turnaround time. Spaghetti diagrams were also prepared to show inefficiencies in the processing of specimens once they reached the department. Based on this analysis, simple workflow changes were made with long-term goals to restructure where equipment is located within the workspace.
To convince all participants that change is needed and to document inefficiencies, evidence-based decision making is a core concept of lean. When resources are limited, the natural tendency is to provide a simple fix to a problem. This approach, however, at times does not address the root cause of a problem and may instead serve to exacerbate the situation. Perception of the nature and depth of a problem often does not match reality. Only by collecting data can one determine the true extent and nature of the problem. The team can then make a calculated determination regarding the level of resources that can be applied to resolving the issue at hand. In addition, the collection of preintervention data (predata) allows for comparison with postintervention data, enabling the team to determine the impact of changes. This comparison can also serve as a reminder of the value of lean-based defect resolution by showing administration and staff the return on investment by implementing lean.
The lean approach to problems relating to consistency in the quality of work is standardization not just of the work but often of the physical workspace itself and the test processes (7, 8). A simple example at HFHS was the challenge of standardizing the reading and reporting of Gram stains. The team assigned to address this issue put together an abbreviated flip chart guide (standard work document) for reading and reporting Gram stains for different specimen types. These were then placed at all microscopes used for examining Gram stains. The use of standardized visual aids and work documents that can be displayed in the laboratory can reduce the variation that creeps into laboratory practice over time.
To further facilitate a lean environment, physical redesign of the workplace might be necessary and typically centers on another lean concept known as 5S. In the Toyota Production System (4), the 5 S's are described as sort, set in order, shine, standardize, and sustain. For example, the workstation in many laboratories is often in disarray, with reagents and equipment situated according to individual preferences. In microbiology, the workspaces are shared on a rotating basis, which can be a source for confusion and frustration. 5S was used at HFHS as a systematic approach to organization that involved standardization of the bench workstation, including designated areas of storage and clear labeling of all items. By standardizing each workstation, there was reduced room for error and reduced motion/time in the search for materials/reagents. 5S is meant to be a continuous process with a predetermined frequency of use and occasional audits to determine whether the process is successful. Another tool, called Kanban, can be used for inventory management as a corollary to 5S. Like many laboratories, HFHS faced the challenge of limited space for storage as well as the need to reduce inventory on hand. The Kanban system of just-in-time management of resources allows for reduced need for storage space and waste due to unnecessary inventory by measuring usage patterns for various consumables and modifying the ordering process to fit those patterns.
The use of metrics is another powerful tool in the lean process. Difficulties related to the collection of metrics often represent a significant hurdle in the application of lean principles. When monitoring metrics routinely, the first step should be to determine how to streamline data collection so as to ensure the sustainability of the process. At HFHS, daily review of corrected reports followed by real-time feedback to the technologists involved resulted in a steady decline in the number of corrected reports despite a simultaneous increase in test volume (Fig. 3). At Kaiser Permanente, reporting functionality had changed due do the implementation of a new laboratory information system (LIS), which resulted in a large increase in the number of amended reports. Analysis on a daily basis over time allowed the department to adjust the timing of negative-preliminary-result reporting to reduce the number of amended reports.
FIG 3.
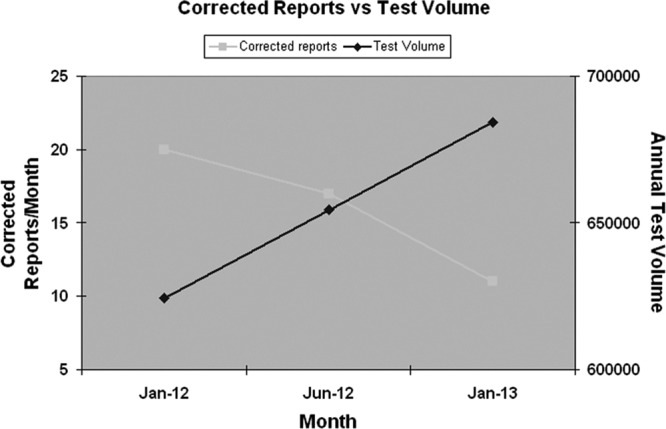
Change in number of corrected reports based on daily report review.
At HFHS, despite multiple rounds of PDCA cycles to improve reporting times for positive blood cultures, no improvement was noted. The team responsible decided to post weekly statistics in the laboratory on turnaround times (TATs) for reporting positive blood cultures by individual technologists. This approach almost immediately led to shortened TATs for blood culture reports, without changes to either the process or staffing levels. Simply raising awareness of the current status of any metric can have an impact of its own on the process. At HFHS, 4 or 5 selected metrics are reviewed each morning. Examples include turnaround times for specific tests, instrument downtime, test statistics, and corrected reports. As part of the process of continuous improvement, once a metric has reached its target goal consistently, it is replaced in the daily review process by another metric.
At Kaiser Permanente, we were faced with moving two molecular assays from one department to another, and staff members were going between two locations to provide the service. The multidepartmental project was driven by the UBT process and engaged staffing in both the Immunology and Virology Departments. Staff participated in time motion studies to adequately determine the exact times necessary to perform both assays. This allowed the group to develop productivity standards and allow the appropriate staffing for that specific function using a team-based approach. Not only did the group determine that the overall needs of FTEs were less than before, but in addition equipment redundancy was eliminated along with savings in service contracts. An online tracking system for the project was created to set the goal, monitor progress, and log results and things learned after successful implementation. Within the Bacteriology Department, a team was formed to address increased calls to the department from physicians needing access to direct susceptibility results for blood culture isolates. By assessing the situation and workload, staff and management were able to add this task to the blood culture bench and enter these results directly into the LIS without an increase in staffing. This has reduced the number of calls coming into the department from the physicians and provided real-time results through electronic medical records to the physicians for better patient care.
One of the challenges faced by many clinical microbiology laboratories is staff motivation, especially given the paucity of opportunities for advancement in the laboratory setting. An unexpected bonus of implementing the lean management system within the HFHS laboratory was that it allowed us to grow the next generation of leaders within our technical staff by giving individuals opportunities to demonstrate their organizational skills, which might otherwise have gone unnoticed. Of note, younger technologists were often particularly eager to be involved in the process, but even veteran technologists were engaged when they realized that they had the opportunity to be involved in the decision-making process. They were given the opportunity to lead teams on their own, thus positively impacting their job. On a monthly basis, short meetings that allowed the technologists to present data generated from process improvements were held.
SUMMARY
Clearly, lean management practices have brought standardization, savings, and quality to many different industries (5, 11, 12). In today's health care world, the laboratory is a place of immense change; new diseases, new health care delivery paradigms, new technologies, a changing workforce, economic pressures, and evolving reimbursement combine to challenge the laboratory in ways that were unimaginable even a decade ago. Lean management practices within the clinical microbiology laboratory can help to address these challenges both today and into the future.
Biography

Susan Novak-Weekley is currently the director of Microbiology, Molecular Infectious Disease, and Serology at the Southern California Permanente Medical Group Regional Reference Laboratories in North Hollywood, CA. Dr. Novak received her B.S. in microbiology at Colorado State University and her Ph.D. in microbiology at the University of Arizona. After graduate school, she completed a postdoctoral fellowship in clinical microbiology at the UCLA Medical Center and Wadsworth VA Medical Center in Los Angeles, CA. Dr. Novak has been at the Kaiser Permanente laboratory for ∼20 years. Dr. Novak is currently a member of the editorial board of the Journal of Clinical Microbiology and is conducting various clinical trials in her laboratory which cover human papillomavirus, molecular gastrointestinal pathogen testing, matrix-assisted laser desorption ionization–time of flight (MALDI-TOF), and urine-screening analyzers. Dr. Novak is active as both the fundraising chair and the annual meeting planner chair for the American Society for Microbiology (ASM) in southern California and participates on other committees within ASM nationally.
Footnotes
Published ahead of print 26 February 2014
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.DeNavas-Walt C, Proctor BD, Smith JC. 2013. Income, poverty, and health insurance coverage in the United States: 2012. U.S. Government Printing Office, Washington, DC [Google Scholar]
- 2.Silverstein MD. 2003. An approach to medical errors and patient safety in laboratory services. A white paper prepared for the Quality Institute Meeting. Making the laboratory a partner in patient safety, Atlanta, April 2003, Suppl 2. Division of Laboratory Systems, Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 3.Garcia E, Ali A, Choudhry S. 2013. The American Society for Clinical Pathology 2012 vacancy survey of clinical laboratories in the United States. Lab Med. 44:1–18 [Google Scholar]
- 4.Ohno T. 1988. Toyota Production System: beyond large scale production. Productivity Press, Portland, OR [Google Scholar]
- 5.Serrano L, Hegge P, Sato B, Richmond B, Stahnke L. 2010. Using LEAN principles to improve quality, patient safety, and workflow in histology and anatomic pathology. Adv. Anat. Pathol. 17:215–221. 10.1097/PAP.0b013e3181d98c81 [DOI] [PubMed] [Google Scholar]
- 6.Zarbo RJ. 2012. Creating and sustaining a Lean culture of continuous process improvement. Am. J. Clin. Pathol. 138:321–326. 10.1309/AJCP2QY1XGKTSNQF [DOI] [PubMed] [Google Scholar]
- 7.Spear S, Bowen HK. 1999. Decoding the DNA of the Toyota Production System. Harvard Business Review, Boston, MA [Google Scholar]
- 8.Liker JK, Franz JK. 2011. The Toyota way to continuous improvement: linking strategy and operational excellence to achieve superior performance. McGraw-Hill, New York, NY [Google Scholar]
- 9.Deming WE. 2000. Out of the crisis. MIT Press, Cambridge, MA [Google Scholar]
- 10.Zarbo R. 2010. Leaders wanted: a call to change the status quo in approaching health care quality, once again. Am. J. Clin. Pathol. 134:361–362. 10.1309/AJCPMH2AD5PCWLUX [DOI] [PubMed] [Google Scholar]
- 11.Zarbo RJ, D'Angelo R. 2006. Transforming to a quality culture. The Henry Ford production system. Am. J. Clin. Pathol. 126(Suppl 1):S21–S29. 10.1309/KVT7NWVPJR73T4K6 [DOI] [Google Scholar]
- 12.D'Angelo R, Zarbo RJ. 2007. The Henry Ford production system: measures of process defects and waste in surgical pathology as a basis for quality improvement initiatives. Am. J. Clin. Pathol. 128:423–429. 10.1309/X6N1Y3V2CB9HUL8G [DOI] [PubMed] [Google Scholar]