Abstract
Wohlfahrtiimonas chitiniclastica is an emerging human pathogen that has been identified as the cause of septicemia in humans in Europe and South America. Here we report the first case of a unique disease manifestation of Wohlfahrtiimonas chitiniclastica-induced bacterial septicemia secondary to wound myiasis in a deer in Michigan in the United States.
CASE REPORT
A 2-year-old, white-tailed deer (Odocoileus virginianus) with a history of pyretic, severe drooling and a hard, swollen tongue with gray areas around the edges was submitted to the Diagnostic Center for Population and Animal Health, in Michigan, for necropsy. Treatments with antibiotics and a steroid were administered, but disease progressed. The animal was found dead on 19 July 2013.
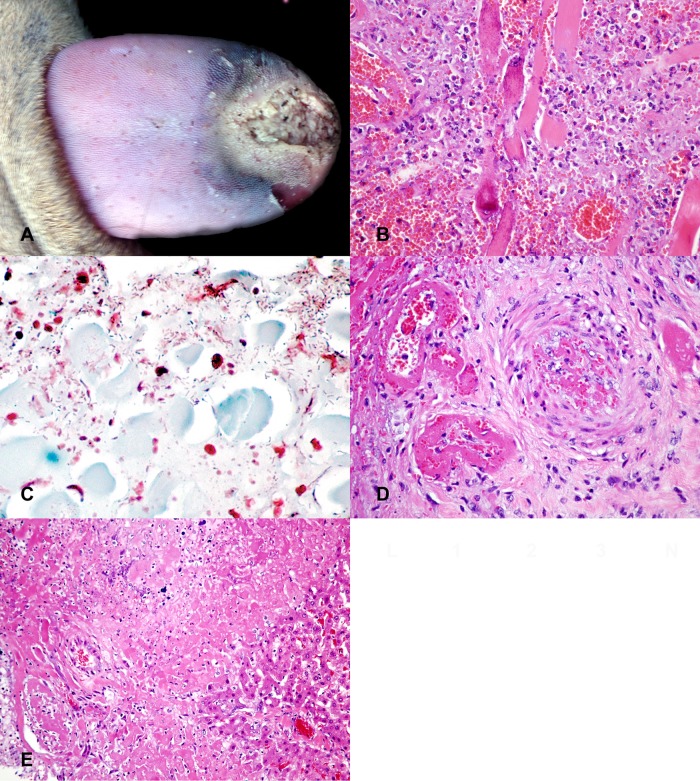
On gross examination, the rostral tip of the tongue was gray, dry, friable, and sharply demarcated by a dark red line from the caudal portion of the tongue (Fig. 1A). The mucosa was focally ulcerated. The affected tissue could easily be separated from the tongue (sequestration of gangrenous tissue). The liver was dark red and swollen with rounded edges. There were multifocal 1- to 2-mm white spots randomly distributed throughout the hepatic parenchyma. The spleen was diffusely enlarged, congested, and oozed blood from cut surface. Based on gross examination, the tongue lesion combined with the suspected multifocal necrotizing hepatitis and congested spleen was most suggestive of bacterial septicemia as the cause of death of this animal.
FIG 1.
Wohlfahrtiimonas chitiniclastica septicemia in a white-tailed deer following wound infection. (A) The rostral tip of the tongue is severely necrotic. (B to D) Microscopically, there is severe necrotizing glossitis with intralesional Gram-negative bacilli (C) and necrotizing vasculitis with thrombosis (D). (E) The liver has multifocal, randomly distributed areas of necrosis and vasculitis with thrombosis.
On microscopic examination, the tongue exhibited severe, locally extensive, necrotizing inflammation, characterized by a large area of devitalized tissue, with the remaining myocytes being hypereosinophilic with loss of cross-striation and mineralization (Fig. 1B). Necrotic myocytes were surrounded by cellular debris and degenerate neutrophils and lesser numbers of macrophages, lymphocytes, plasma cells, and fibroblasts. Within the superficial portion of affected regions, there were abundant intralesional Gram-negative bacilli (Fig. 1C). Medium-size arteries in close proximity had undergone fibrinoid necrosis and were commonly thrombosed (Fig. 1D). The liver had multifocal, randomly distributed areas of hepatocellular coagulative necrosis surrounded by small numbers of neutrophils and macrophages and numerous vessels exhibiting changes similar to those described for the tongue (Fig. 1E). The choroid plexus in the brain had moderate edema, with accumulation of fibrin strands and multiple fibrin thrombi. There was marked, diffuse congestion of the lungs and spleen. All other organs appeared microscopically unremarkable.
Liver and tongue specimens were submitted for routine culture. For each specimen, tissue was plated onto 5% enriched sheep blood agar, Columbia colistin-nalidixic acid agar with 5% sheep blood for selective isolation of Gram-positive cocci and inhibition of Gram-negative bacilli, and MacConkey agar medium selective for Gram-negative bacilli and differential based on lactose fermentation. Inoculated culture medium was incubated at 35 to 37°C in 5% CO2. Bacteria isolated from both the tongue and the liver yielded numerous Gram-negative rod-shaped bacteria, which were initially identified as Wohlfahrtiimonas chitiniclastica by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). PCR amplification using previously described primers of the partial 16S rRNA gene (1) and nucleic acid sequencing was performed for confirmation. The nucleic acid sequence was compared to known sequences in the National Center for Biotechnology Information (NCBI) database, and the isolate was consistent with Wohlfahrtiimonas chitiniclastica (GenBank accession numbers HQ407275, HQ407260, and EU484335) (2). We also designed new primers to identify the partial sequence of DNA gyrase subunit B (gyrB), which has been shown to have a relatively higher discrimination ability than the 16S rRNA gene for the identification of diverse bacterial genera (3). PCR using the newly designed primers was performed, and the amplicon of the targeted gyrB gene was sequenced, confirming Wohlfahrtiimonas chitiniclastica (GenBank accession number FJ966122). A finding of epizootic hemorrhagic disease was negative by PCR (4). Based on the gross, microscopic, microbiological, and molecular results, a final diagnosis of Wohlfahrtiimonas chitiniclastica septicemia was made.
This case highlights a unique disease manifestation of an emerging human pathogen, Wohlfahrtiimonas chitiniclastica, a Gram-negative, short rod-shaped gammaproteobacterium with strong chitinase activity. Septicemia due to Wohlfahrtiimonas chitiniclastica has been described in adult humans as originating from suspected wound infections (5, 6). Reported cases occurred in homeless patients with poor hygiene and multiple skin lesions. Although the pathogenesis of Wohlfahrtiimonas chitiniclastica-associated disease has not been investigated, the fly Wohlfahrtia magnifica is believed to play an important role as carrier of Wohlfahrtiimonas chitiniclastica.
Successful isolation of Wohlfahrtiimonas chitiniclastica from homogenates of the third-stage larvae of the obligate parasitic fly Wohlfahrtia magnifica has been performed (7). Although the pathogenesis of Wohlfahrtiimonas chitiniclastica-associated disease has not been researched, the fly Wohlfahrtia magnifica is believed to play an important role as carrier of Wohlfahrtiimonas chitiniclastica and wound contamination is believed to be the origin of bacterial septicemia in previously described cases. Wohlfahrtia magnifica is an obligate parasitic fly and the most important cause of wound myiasis in warm-blooded vertebrates in southeastern Europe, southern and Asiatic Russia, the Near East, and North Africa. Larvae are deposited near wounds or body openings of humans and animals such as sheep, goats, cattle, horses, donkeys, pigs, dogs, camels, and geese (8). However, identification of ectoparasites or an attempt to isolate bacteria from observed parasites has not been done in previously reported cases of septicemia in humans.
More importantly, Wohlfahrtia magnifica flies or Wohlfahrtiimonas chitiniclastica has rarely been found in areas of continental climate and has not been reported in the United States. However, the distribution of Wohlfahrtia magnifica flies is progressively expanding because of their broad adaptation capacities and as a result of climatic changes (6). In addition, Wohlfahrtiimonas chitiniclastica may be able to colonize other types of Wohlfahrtia flies that are geographically distributed in North America, such as Wohlfahrtia vigil and Wohlfahrtia opaca, whose larvae tend to penetrate livestock skin. Interestingly, there is a possibility that other obligatory parasitic flies may be responsible for transmission of this bacterium, since recently, Wohlfahrtiimonas chitiniclastica has been isolated from the larval gut of Hermetia illucens, the black soldier fly, in South Korea (9).
In conclusion, this case highlights the importance of proper pathology and microbiology diagnostic testing of cases of naturally occurring death, which in this case allowed the detection of an emerging pathogen, Wohlfahrtiimonas chitiniclastica, as the cause of an arthropod-borne bacterial septicemia.
ACKNOWLEDGMENT
We declare that there were no conflicts of interest for this article. No financial support was received for this article.
Footnotes
Published ahead of print 26 March 2014
REFERENCES
- 1.Kiupel M, Desjardins DR, Lim A, Bolin C, Johnson-Delaney CA, Resau JH, Garner MM, Bolin SR. 2012. Mycoplasmosis in ferrets. Emerg. Infect. Dis. 18:1763–1770. 10.3201/eid1811.120072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta AK, Nayduch D, Verma P, Shah B, Ghate HV, Patole MS, Shouche YS. 2012. Phylogenetic characterization of bacteria in the gut of house flies (Musca domestica L.). FEMS Microbiol. Ecol. 79:581–593. 10.1111/j.1574-6941.2011.01248.x [DOI] [PubMed] [Google Scholar]
- 3.Huang WM. 1996. Bacterial diversity based on type II DNA topoisomerase genes. Annu. Rev. Genet. 30:79–107. 10.1146/annurev.genet.30.1.79 [DOI] [PubMed] [Google Scholar]
- 4.Aradaib IE, Smith WL, Osburn BI, Cullor JS. 2003. A multiplex PCR for simultaneous detection and differentiation of North American serotypes of bluetongue and epizootic hemorrhagic disease viruses. Comp. Immunol. Microbiol. Infect. Dis. 26:77–87. 10.1016/S0147-9571(02)00035-8 [DOI] [PubMed] [Google Scholar]
- 5.Almuzara MN, Palombarani S, Tuduri A, Figueroa S, Gianecini A, Sabater L, Ramirez MS, Vay CA. 2011. First case of fulminant sepsis due to Wohlfahrtiimonas chitiniclastica. J. Clin. Microbiol. 49:2333–2335. 10.1128/JCM.00001-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebaudet S, Genot S, Renvoise A, Fournier PE, Stein A. 2009. Wohlfahrtiimonas chitiniclastica bacteremia in homeless woman. Emerg. Infect. Dis. 15:985–987. 10.3201/eid1506.080232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tóth EM, Schumann P, Borsodi AK, Kéki Z, Kovács AL, Márialigeti K. 2008. Wohlfahrtiimonas chitiniclastica gen. nov., sp. nov., a new gammaproteobacterium isolated from Wohlfahrtia magnifica (Diptera: Sarcophagidae). Int. J. Syst. Evol. Microbiol. 58:976–981. 10.1099/ijs.0.65324-0 [DOI] [PubMed] [Google Scholar]
- 8.Farkas R, Hall MJ, Bouzagou AK, Lhor Y, Khallaayoune K. 2009. Traumatic myiasis in dogs caused by Wohlfahrtia magnifica and its importance in the epidemiology of wohlfahrtiosis of livestock. Med. Vet. Entomol. 23(Suppl 1):80–85. 10.1111/j.1365-2915.2008.00772.x [DOI] [PubMed] [Google Scholar]
- 9.Lee JK, Lee YY, Park KH, Sim J, Choi Y, Lee SJ. 2014. Wohlfahrtiimonas larvae sp. nov., isolated from the larval gut of Hermetia illucens (Diptera: Stratiomyidae). Antonie Van Leeuwenhoek 105:15–21. 10.1007/s10482-013-0048-5 [DOI] [PubMed] [Google Scholar]