Abstract
Background
Illicit stimulant use increases oxidative stress and oxidative stress has been found to be associated with deficits in memory, attention, and problem-solving.
Objective
To test a model of the association among oxidative DNA damage, a severe form of oxidative stress, and stimulant use, executive function, and stimulant-use outcomes.
Methods
Six sites evaluating 12-step facilitation for stimulant abusers obtained peripheral blood samples from methamphetamine-dependent (n=45) and cocaine-dependent (n=120) participants. The blood samples were submitted to a comet assay to assess oxidative DNA damage. Executive Dysfunction was assessed with the Frontal Systems Behavior Scale (FrSBe), which is a reliable and valid self-report assessment of executive dysfunction, disinhibition, and apathy. Stimulant-use measures included self-reported stimulant use and stimulant urine drug screens (UDS).
Results
While more recent cocaine use (<30 days abstinence) was associated with greater oxidative DNA damage (W=2.4, p<.05, d=.36), the results did not support the hypothesized relationship between oxidative DNA damage, executive dysfunction, and stimulant-use outcomes for cocaine-dependent patients. Support for the model was found for methamphetamine-dependent patients, with oxidative DNA damage significantly greater in methamphetamine-dependent patients with executive dysfunction (W=2.2, p<.05, d=.64) and with executive dysfunction being a significant mediator of oxidative DNA damage and stimulant use during active treatment (ab=0.089, p<.05). As predicted, neither disinhibition nor apathy were significant mediators of oxidative damage and future stimulant use.
Conclusion
These findings provide preliminary support for a model in which oxidative damage resulting from methamphetamine use results in executive dysfunction which in turn increases vulnerability to future stimulant use.
Keywords: oxidative damage, stimulant-dependence, executive function
Introduction
Methamphetamine (1) and cocaine (2) administration have both been found to increase the formation of reactive oxygen species (ROS). Under normal physiological conditions, a group of antioxidants in the body is able to detoxify ROS. However, in certain circumstances an increased release of ROS through dysfunction of the mitochondrial oxidative phosphorylation results in “oxidative stress.” Under these conditions, the ROS are not entirely detoxified and react with cellular proteins, lipids and DNA bases to form oxidized products, such as oxidative modification of DNA bases, with impaired functionality and, in severe forms, mutations. Pre-clinical research suggests that oxidative stress plays an important role in the cytotoxic effects of both cocaine and methamphetamine (1–3) and that such effects may increase vulnerability to relapse (3, 4). Moreover, the neurotoxic effects of methamphetamine have been established in a number of animal species (5) with evidence to suggest that the damage is caused, in part, by oxidative stress (1, 6). While cocaine is not neurotoxic to dopamine and serotonin neurons (7), recent evidence suggests that cocaine use may significantly speed gray matter loss associated with aging and cognitive decline (8), with oxidative stress postulated to play a role in the process (9).
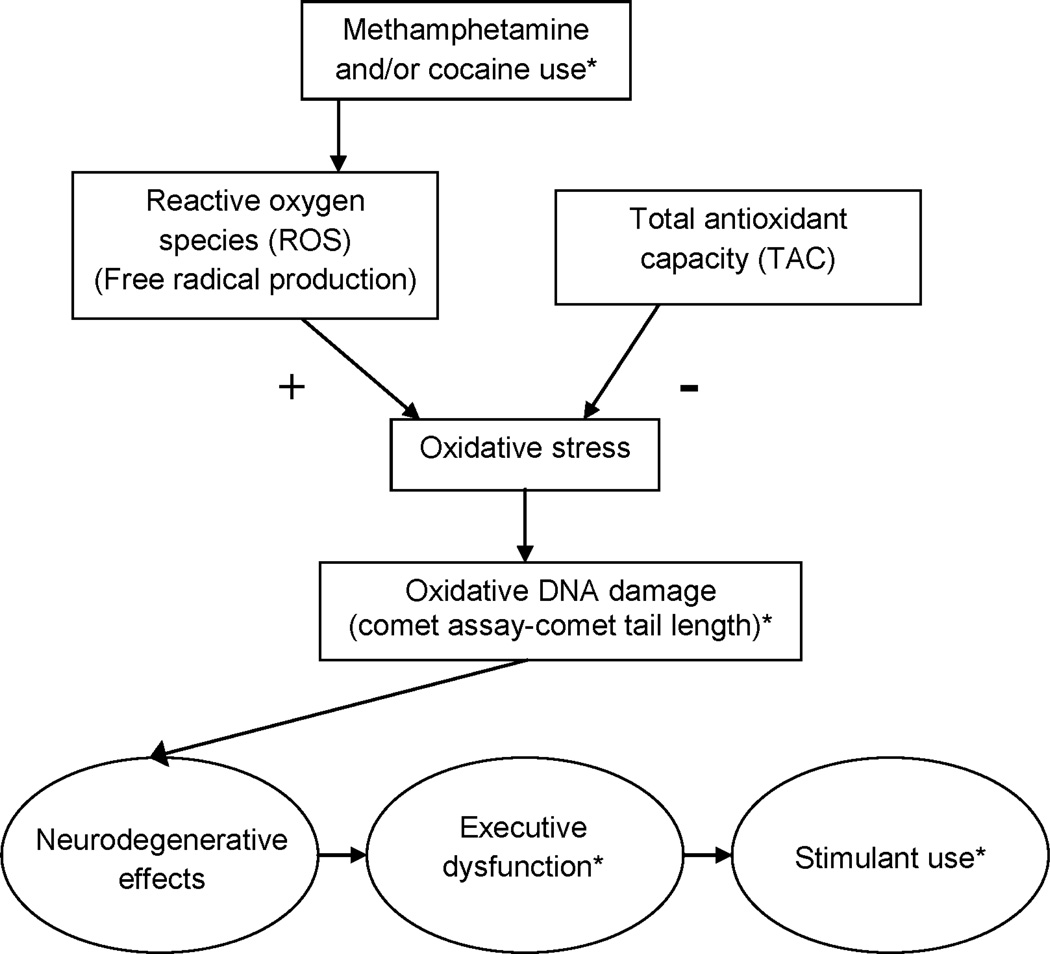
Research has found that oxidative stress/damage is associated with deficits in memory, attention, and problem-solving (10–14). Methamphetamine and cocaine use are associated with multiple brain alterations (15–19) but, based on the oxidative stress/damage literature, we postulate that oxidative stress/damage in methamphetamine/cocaine users will be associated with deficits in memory, attention, and problem solving, for which we use the term executive dysfunction, while not necessarily being associated with other changes related to cocaine/methamphetamine use such as disinhibition (19). A model of the relationships among methamphetamine and cocaine use, oxidative stress, executive dysfunction and stimulant use outcomes is provided in Figure 1. In this model, ROS production is increased by methamphetamine (1) and cocaine (2) use and, in severe cases, increases oxidative stress sufficiently to result in oxidative damage. This damage includes neuronal damage, which is evidenced in the executive dysfunction that has been observed in stimulant-dependent populations (20–22). This dysfunction, which impairs the ability to learn and apply new, more adaptive behaviors, serves to increase vulnerability to future stimulant use.
Figure 1.
Proposed model of the relationships among methamphetamine and cocaine use, oxidative stress/damage, executive dysfunction, and stimulant use outcomes. *Variable measured in present study.
To test this model, we sought a peripheral measure of oxidative damage that would be associated with neurocognitive function. It is important to note that oxidative damage can be detected in peripheral tissue (23) and that peripheral measures of oxidative damage have been found to be associated with cognitive function including in mild cognitive impairment (MCI) and Alzheimer's Disease (AD) (23). It has been noted that the comet assay may be suitable for assessing the neurotoxicity and genotoxicity of drugs of abuse (24) and oxidative DNA damage, as measured by tail length of the comet assay, has been found to be related to cognitive impairment. Specifically, a study of AD, MCI, and normal control participants, found significantly greater oxidative DNA damage, as measured by tail length from peripheral blood samples, in both the AD and MCI groups compared to normal controls (25).
The present study, which was an ancillary to the National Drug Abuse Treatment Clinical Trials Network (NIDA CTN) trial on 12-step facilitation for stimulant abusers (26), was a preliminary investigation of the relationships outlined in Figure 1. It was predicted that: 1. oxidative DNA damage would be inversely associated with length of abstinence from stimulants, 2. oxidative DNA damage would be associated with deficits in executive function, and 3. executive function would mediate the relationship between oxidative DNA damage and future stimulant use.
Methods
Participants
Six of the substance abuse community treatment programs (CTPs) participating in the STAGE-12 trial, evaluating a modified 12-step facilitation for stimulant abusers, participated in this ancillary study. The 165 methamphetamine- or cocaine-dependent participants in the present study were randomized into the STAGE-12 trial, had a current diagnosis of stimulant dependence based on the DSM-IV Checklist (27), did not have a seizure disorder or a history of stroke, and provided a blood sample for the comet assay.
Procedures
See Donovan et al. (26) for a description of the STAGE-12 study procedures. Briefly, participants were randomized to either Stimulant Abuser Groups to Engage in 12-Step (STAGE-12) or to treatment as usual (TAU). TAU participants received treatment as ordinarily provided by the site. STAGE-12 participants received a combination of five group and three individual sessions that replaced the comparable number of sessions typically provided. STAGE-12 research visits were completed at screening/baseline, study weeks 2, 4, and 8, and at three and six months following randomization. Participants in the present study completed a single session in which baseline characteristics, a measure of executive function, and a blood sample were obtained. The blood was stored at −80°C and shipped on dry ice to Madison (WI), where the samples were analyzed.
Measures
Oxidative DNA Damage
The comet assay, also called single cell gel electrophoresis (SCGE), is a sensitive and rapid technique for quantifying and analyzing oxidative DNA damage. Oxidized DNA bases are translated into single strand breaks using a DNA repair enzyme, here formamidopyrimidine DNA glycosylase. The enzyme recognizes oxidized pyrimidines and purines, like 8-oxoguanine, and removes the oxidized base by a catalytic cleavage of the N-glycosydic bond. The elimination of the enzyme from the DNA results in the excision of the sugar and the formation of a one nucleoside gap in the DNA, creating a DNA single strand break (28). The DNA is stained after on-slide electrophoresis using fluorescent dyes and the resulting image resembles a "comet" with a distinct head and tail. The head is composed of intact DNA, while the tail consists of damaged or broken pieces of DNA. The extent of DNA liberated from the head of the comet is directly proportional to the amount of DNA damage (29).
Executive Function/Cognition
Past research indicates that traditional neurocognitive assessments can fail to detect deficits in individuals with frontal lobe damage whose behaviour in natural settings is clearly impaired (30). The Frontal Systems Behavior Scale (FrSBe) is a brief, valid, and reliable assessment of pre-frontal cortex functioning that can be completed as a self report (30). All participants completed the FrSBe, which assesses functioning for three neurobehavioral domains: Apathy (e.g., low energy and interest, difficulties with initiation, blunted affective expression), Disinhibition (e.g., impulsivity, emotional lability, socially inappropriate behavior), and Executive Dysfunction (e.g., problems with attention, problem solving, insight, working memory, mental flexibility) summed for a Total (30, 31). For the present study, Executive Dysfunction was the scale of interest since it assesses problems with executive function and cognition. The FrSBe is written at a 6th-grade reading level and consists of 46 self-report items, with responses in a five-point Likert-type scale. Importantly, the FrSBe includes normative data allowing for the calculation of T-scores with cut-offs for designating clinically significant neurobehavioral impairment. A body of research supports the reliability (30, 32) and validity (31, 33–37) of the FrSBe. All raw FrSBe scores were converted into T-scores using the T-score tables provided in the FrSBe manual, which are categorized according to age, gender, and educational level (30). For all FrSBe scales, T-scores ≥65 indicate clinically significant neurobehavioral abnormalities (30).
Stimulant Use
Stimulant use was measured by self-reported use assessed using the Timeline Follow-Back procedure (38) and by qualitative urine drug screen (UDS). The stimulants screened for by the UDS were cocaine, methamphetamine, and amphetamine. Since over half of the sample did not use stimulants during treatment and almost 40% were stimulant-free during follow-up, it was determined that success or failure in maintaining abstinence was more relevant than actual levels of stimulant use. Therefore, the mediation analyses evaluating the relationship between oxidative DNA damage and stimulant use outcomes were based on binary indicators of whether the respective measure (self-report or UDS) indicated success or failure in maintaining abstinence over the respective periods (treatment phase or follow-up).
Comet analysis
The whole blood sample was thawed and 10μL of the sample were added to 65μL of 0.8% low melting agarose solution on a microscope slide. For each sample, six slides were prepared. One set of three slides was used to determine the background DNA damage and the other set of three was used to determine the oxidative DNA damage, by treating the content of each slide with the enzyme formamidopyrimidine DNA glycosylase (FPG). Subsequently, the cover slips were pulled off and the gels were washed with enzyme buffer (40 mM HEPES, 0.1 M KCl, 0.5 mM EDTA, 0.2 mg/ml BSA, pH 8.0 with KOH) three times for 5min at 4°C. Then the background set was incubated with 50μL/pad enzyme buffer only and the oxidative damage set was incubated with the same volume of a 1:5000 dilution of FPG for 30min at 37°C. All slides were lysed at 4°C for at least 1h (2.5 M NaCl, 0.1 M EDTA, 10 mM Tris, pH 10). Thereafter, the samples underwent DNA unwinding at a pH ≥ 13, followed by electrophoresis at 4°C, 25V and 300mA for 20 min in electrophoresis buffer (0.3 M NaOH, 1 mM EDTA). The samples were neutralized (0.4 M Tris, pH to 7.5 with conc. HCl) and stained with 40 μl/pad ethidium bromide (50 μg/ml). The tail length analysis was done on a fluorescent microscope (Nikon, USA) supported by the COMET ASSAY IV software (Perceptive Instruments, Haverhill, UK). It should be noted that the tail length reported is the difference in tail length of the FPG treated and untreated sample resulting in the tail length representing the oxidative DNA damage only. The untreated samples had tail lengths of up to 55 μm and the FPG -treated samples of up to 75 μm.
Data analysis
A Wilcoxon was used to test the relationship between oxidative DNA damage and two dichotomous measures of baseline stimulant-use: UDS result on the day of testing (stimulant positive vs stimulant negative) and self-reported days of abstinence during the prior 90 days (<30 vs. ≥ 30, which was based on the median abstinence length of 30 days). A Wilcoxon was also used to test the relationship between oxidative DNA damage and significant neurobehavioral abnormalities as measured by the FrSBe. Mediation analyses were used to test the hypothesis that executive function would mediate the relationship between oxidative DNA damage and future stimulant use.
More specifically, relationships between oxidative DNA damage (i.e., predictor variable was comet tail length), neurocognitive function (i.e., mediating variables; includes Executive Dysfunction, Apathy, Disinhibition, and Total frontal systems function), and stimulant use (i.e., outcome variables; includes stimulant use during treatment and during follow-up, as assessed by self-report and UDS), were tested for consistency with the simple mediation model introduced by Baron and Kenny (39) and expounded upon in more contemporary approaches (40), after which we modeled our analyses. Each assessment involved a single outcome variable (Y); a single predictor variable (X), and a single neurocognitive functioning variable as a potential mediator (M). Making the appropriate adjustment for dichotomous outcomes (41), each assessment involved three regressions: 1) testing X as a predictor of Y, which gives an estimate of the overall strength of the relationship between X and Y (c); 2) testing X as a predictor of M, which gives an estimate of strength of the overall relationship between X and M (a); and, 3) jointly testing X and M as predictors of Y, which gives an estimate of both the direct effect of X on Y (c1) and the effect of M on Y when X is accounted for (b). The indirect effect of X on Y through M (also known as the mediation effect) was estimated by the product, ab. Ideally, the mediation effect (ab) plus the direct effect of X on Y independent of M (c1) should roughly equal the total effect of X on Y (c), Therefore, c – c1 was used as a confirmatory estimate of the mediation effect (42). The statistical significance of each estimate (a, b, c, c1, ab, and c – c1) was tested using a 95% confidence interval estimated using a bootstrap procedure with 5000 iterations. The mediation effect was considered statistically significant wherever the confidence interval for the ab estimate did not include zero. All analyses were completed separately for methamphetamine-dependent and cocaine-dependent participants.
Results
Sample Characteristics
Table 1 provides the demographic and clinical characteristics for the 45 methamphetamine-dependent and 120 cocaine-dependent participants. Analyses evaluating the model in Figure 1 were conducted separately for each group and, thus, the significant differences in sample characteristics between the methamphetamine-dependent and cocaine-dependent groups did not require statistical adjustment.
Table 1.
Demographics and clinical characteristics of stimulant-dependent participants
| Characteristic | Methamphetamine Dependent (n=45) |
Cocaine Dependent (n=120) |
Test Statistica | p-Value |
|---|---|---|---|---|
| Age, years | 35.0 (8.6) | 40.4 (9.1) | W = −3.4 | < 0.01 |
| Education, years | 11.7 (1.4) | 12.1 (1.6) | W = −1.2 | 0.23 |
| Gender, % Male | 17.8% | 38.3% | X2 (1) = 6.3 | 0.01 |
| Race, % | F = 0.0 | < 0.01 | ||
| White | 86.7% | 26.1% | ||
| African-American | 2.2% | 67.2% | ||
| Other/Mixed | 11.1% | 6.7% | ||
| Ethnicity, % Hispanic | 6.7% | 2.5% | F = 0.2 | 0.35 |
| Cigarette smoker, % | 82.2% | 77.5% | X2 (1) = 0.4 | 0.51 |
| Stimulant use, years | 10.2 (7.0) | 13.1 (7.6) | W = −2.1 | 0.03 |
| Stimulant Positive UDSb , % | 35.6% | 14.2% | X2 (1) = 9.4 | < 0.01 |
| Stimulant use days in last 30 | 5.1 (7.6) | 3.0 (4.7) | T (57.0) = 1.7 | 0.09 |
| Non-Stimulant SUDc Diagnosis, % | 51.1% | 80.0% | X2 (1) = 13.6 | < 0.01 |
| Alcohol Diagnosis, % | 40.0% | 69.2% | X2 (1) = 11.7 | < 0.01 |
| Marijuana Diagnosis, % | 22.2% | 41.7% | X2 (1) = 5.3 | 0.02 |
| Opiate Diagnosis, % | 8.9% | 16.7% | X2 (1) = 1.6 | 0.21 |
| Benzodiazapine Diagnosis, % | 4.4% | 7.5% | F = 0.2 | 0.73 |
Note: Where not specifically indicated, numbers represent means (standard deviations).
W: Wilcoxon, X2(df): Pearson Chi-square, F: Fisher’s exact, T(df): Student’s T;
Urine drug screen;
Substance use disorder.
Oxidative DNA damage and recent stimulant use
Table 2 provides the results of analyses testing the hypothesis that oxidative DNA damage would be inversely associated with length of abstinence from stimulants. The sample size for the methamphetamine-dependent participants, n=45, was relatively small and, thus, Cohen's d (43) effect sizes are provided in addition to the results from the analyses. Consistent with the prediction that oxidative DNA damage would be inversely associated with length of abstinence from stimulants, the Wilcoxon analysis revealed that cocaine-dependent participants with < 30 days of abstinence during the 90-day pre-test day period had significantly greater DNA damage compared to those with ≥ 30 days of abstinence (Table 2). In contrast, the relationship between oxidative DNA damage and stimulant UDS result was not significant for cocaine-dependent participants. For methamphetamine-dependent participants, there was not a significant relationship between oxidative DNA damage and self-reported days of stimulant abstinence. Oxidative DNA damage was greater in methamphetamine-dependent participants with a positive, relative to negative, baseline stimulant UDS result but not to a statistically-significant degree; however, the effect size for the comparison was d=.45, which is a medium-sized effect (Table 2).
Table 2.
Mean oxidative DNA damage levels (comet tail length) as a function of recent stimulant-use and neurobehavioral abnormalities
| Methamphetamine-dependent participants (N=45) |
Cocaine-dependent participants (N=120) |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | % | Oxidative DNA Damage, (µm) |
Test Statistica / effect size |
N | % | Oxidative DNA Damage, (µm) |
Test Statistica/ effect size |
|
| Recent stimulant use | ||||||||
| Days of abstinence at baseline (SRb) | W = 0.5 | W = 2.4* | ||||||
| < 30 days | 22 | 48.9 | 16.7 (6.9) | d = 0.10 | 61 | 50.8 | 19.0 (10.1) | d = 0.36 |
| ≥ 30 days | 23 | 51.1 | 15.9 (9.3) | 59 | 49.2 | 15.2 (10.5) | ||
| Stimulant UDS c result on day of testing | W = 1.3 | W = 0.6 | ||||||
| Positive | 16 | 35.6 | 18.6 (8.5) | d = 0.45 | 17 | 14.2 | 15.2 (8.0) | d = 0.21 |
| Negative | 29 | 64.4 | 15.0 (7.8) | 103 | 85.8 | 17.5 (10.8) | ||
| Neurobehavioral abnormalities as assessed by the FrSBe d | ||||||||
| Apathy | W = 0.8 | W = 0.5 | ||||||
| Significant | 34 | 79.1 | 16.8 (7.9) | d = 0.28 | 104 | 88.9 | 16.8 (10.0) | d = 0.26 |
| Non-significant | 9 | 20.9 | 14.5 (9.4) | 13 | 11.1 | 19.6 (14.2) | ||
| Disinhibition | W = 0.3 | T(82) = −0.7 | ||||||
| Significant | 37 | 84.1 | 16.2 (7.8) | d = 0.06 | 86 | 72.9 | 17.4 (11.4) | d = 0.12 |
| Non-significant | 7 | 15.9 | 15.7 (10.5) | 32 | 27.1 | 16.1 (7.7) | ||
| Executive Dysfunction | W = 2.2* | W = 0.7 | ||||||
| Significant | 25 | 56.8 | 18.3 (8.0) | d = 0.64 | 87 | 73.7 | 17.3 (10.6) | d = 0.10 |
| Non-significant | 19 | 43.2 | 13.3 (7.7) | 31 | 26.3 | 16.3 (10.4) | ||
| Total | W = 1.3 | W = 0.4 | ||||||
| Significant | 34 | 79.1 | 17.0 (7.6) | d = 0.42 | 99 | 85.3 | 17.1 (11.0) | d = 0.01 |
| Non-significant | 9 | 20.9 | 13.6 (10.2) | 17 | 14.7 | 17.0 (8.0) | ||
Note: Where not specifically indicated, numbers represent means (standard deviations).
W: Wilcoxon, T(df): Student’s T;
Self-reported;
Urine drug screen;
Frontal Systems Behavior Scale.
p < 0.05
Oxidative DNA damage and executive function/cognition
Table 2 displays the results of analyses evaluating whether oxidative DNA damage differed significantly between those with and without clinically significant neurobehavioral abnormalities. As predicted, oxidative DNA damage was significantly greater in methamphetamine-dependent participants with, than without, significant Executive Dysfunction (W=2.2, p<0.05; d=0.64). The other comparisons for the methamphetamine-dependent participants revealed no significant differences. Contrary to prediction, oxidative DNA damage was not significantly greater in cocaine-dependent participants with, than without, significant Executive Dysfunction (W=0.7, p>0.05; d=0.10). The other comparisons for the methamphetamine-dependent participants revealed no significant differences.
Executive Dysfunction as a mediator of oxidative DNA damage and stimulant use outcomes
Primary tests of mediation (i.e., tests of the ab effect) revealed only one significant effect - Executive Dysfunction was a significant mediator of oxidative DNA damage and self-reported stimulant use during the active treatment phase in the methamphetamine-dependent participants. As shown in Table 3, the accuracy of ab parameter estimate was confirmed by the secondary test of mediation, the effect estimate for c – c1, though the statistical significance of this estimate was not confirmed.
Table 3.
Summary of analyses testing FrSBe scores as a mediator (M) of the association between comet tail length (X) and substance use outcomes (Y), for methamphetamine- and cocaine-dependent participants
| Methamphetamine-dependent participants | Cocaine-dependent participants | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment phase | Follow-up phase | Treatment phase | Follow-up phase | ||||||
| FrSBe Score (M) |
Mediation Coefficient |
Stimulant Positive UDS (Y) |
SR Stimulant Use Days (Y) |
Stimulant Positive UDS (Y) |
SR Stimulant Use Days (Y) |
Stimulant Positive UDS (Y) |
SR Stimulant Use Days (Y) |
Stimulant Positive UDS (Y) |
SR Stimulant Use Days (Y) |
| Apathy | c | −0.012 | −0.020 | 0.024 | 0.378 | 0.082 | 0.078 | −0.071 | 0.055 |
| a | 0.196 | 0.196 | 0.193 | 0.211 | −0.058 | −0.098 | 0.165 | 0.168 | |
| b | −0.264 | −0.101 | −0.153 | −0.149 | 0.076 | −0.092 | 0.166 | 0.175 | |
| c' | 0.031 | −0.006 | 0.047 | 0.434 | 0.086 | 0.072 | −0.085 | 0.041 | |
| ab | −0.052 | −0.020 | −0.030 | −0.031 | −0.004 | 0.009 | 0.027 | 0.029 | |
| c – c' | −0.044 | −0.014 | −0.023 | −0.056 | −0.004 | 0.007 | 0.014 | 0.014 | |
| Disinhibition | c | −0.012 | −0.046 | −0.012 | 0.334 | 0.088 | 0.084 | −0.081 | 0.063 |
| a | 0.072 | 0.055 | −0.004 | 0.011 | 0.113 | 0.085 | 0.104 | 0.108 | |
| b | 0.079 | 0.393* | 0.072 | 0.088 | 0.121 | 0.188 | 0.109 | 0.110 | |
| c' | −0.016 | −0.063 | −0.012 | 0.339 | 0.076 | 0.070 | −0.090 | 0.054 | |
| ab | 0.006 | 0.022 | 0.000 | 0.001 | 0.014 | 0.016 | 0.011 | 0.012 | |
| c – c' | 0.004 | 0.017 | 0.000 | −0.005 | 0.012 | 0.014 | 0.009 | 0.009 | |
| Executive | c | −0.012 | −0.046 | −0.012 | 0.334 | 0.084 | 0.086 | −0.089 | 0.069 |
| Dysfunction | a | 0.311 | 0.333 | 0.305 | 0.331 | 0.115 | 0.088 | 0.178 | 0.182 |
| b | 0.207 | 0.268* | −0.139 | −0.052 | 0.123 | 0.085 | 0.074 | −0.060 | |
| c' | −0.067 | −0.135 | 0.025 | 0.361 | 0.074 | 0.080 | −0.099 | 0.078 | |
| ab | 0.064 | 0.089* | −0.043 | −0.017 | 0.014 | 0.007 | 0.013 | −0.011 | |
| c – c' | 0.055 | 0.089 | −0.037 | −0.027 | 0.010 | 0.006 | 0.011 | −0.009 | |
| Total | c | −0.012 | −0.020 | 0.024 | 0.378* | 0.079 | 0.080 | −0.077 | 0.059 |
| a | 0.284 | 0.284 | 0.252 | 0.269 | 0.095 | 0.049 | 0.056 | 0.060 | |
| b | −0.076 | 0.330* | 0.005 | −0.072 | 0.053 | 0.046 | 0.210* | −0.038 | |
| c' | 0.004 | −0.093 | 0.023 | 0.405* | 0.075 | 0.078 | −0.084 | 0.061 | |
| ab | −0.021 | 0.094 | 0.001 | −0.019 | 0.005 | 0.002 | 0.012 | −0.002 | |
| c – c' | −0.016 | 0.073 | 0.001 | −0.027 | 0.004 | 0.002 | 0.006 | −0.002 | |
Note: Values shown in table are regression coefficient estimates. FrSBe = Frontal Systems Behavior Scale; UDS = Urine Drug Screen; SR = Self-report; M=Mediator, X=Predictor, Y=Outcome; c = Total Effect of X on Y; a = Effect of X on M; b = Effect of M on Y; c' = Direct Effect of X on Y; ab = Indirect Effect of X on Y through Mediator M (main effect of interest, shown in bold);
95% bootstrap confidence intervals indicate alpha = 0.05 statistical significance.
Discussion
This preliminary investigation provides support for the model outlined in Figure 1 for methamphetamine-dependent, but not cocaine-dependent, participants. For cocaine-dependent participants, those reporting fewer days of stimulant-abstinence (< 30 days) had significantly greater oxidative DNA damage relative to those with greater abstinence (≥ 30 days) but the results did not support the hypothesized relationship between oxidative DNA damage, executive dysfunction, and stimulant use outcomes. For methamphetamine-dependent participants, the results revealed that oxidative DNA damage was significantly greater in participants with, relative to without, executive dysfunction and that executive dysfunction was a mediator for oxidative DNA damage at baseline and stimulant use during active treatment; no significant association was found between oxidative DNA damage and the other scales of the FrSBe.
The present results are consistent with pre-clinical research suggesting that oxidative stress plays a role in the toxic effects of stimulants (1, 2) in that oxidative DNA damage was significantly greater in cocaine-dependent patients with shorter, relative to longer, days of stimulant-abstinence. While not statistically significant, oxidative DNA damage was also greater in methamphetamine-dependent participants with a positive, relative to negative, baseline stimulant UDS result, with an effect size of d=.45. While the lack of relationship between oxidative DNA damage and executive dysfunction in cocaine-dependent participants is counter to our hypothesis and to the postulated role of oxidative stress in speeding gray matter loss associated with aging and cognitive decline in cocaine-dependent patients (8, 9), it is consistent with research finding that cocaine is not neurotoxic to dopamine and serotonin neurons (7). Likewise, the present results finding a relationship between methamphetamine use and executive dysfunction are consistent with a body of research which has documented that methamphetamine is neurotoxic across a range of species (5).
The finding of significantly greater oxidative DNA damage in methamphetamine-dependent participants with, than without, significant Executive Dysfunction is also consistent with past research finding a relationship between oxidative damage and deficits in memory, attention, and problem-solving (10–14). The present study did not find that Executive Dysfunction was a significant mediator between oxidative DNA damage stimulant use during follow-up but the significant mediation of Executive Dysfunction between baseline oxidative DNA damage and stimulant use during active treatment may suggest that methamphetamine-dependent participants with executive dysfunction benefited less from treatment relative to participants without such dysfunction.
The present findings should be considered in light of several limitations. First, the sample size for the methamphetamine-dependent participants was small and so the present results need to be replicated in a larger sample. Second, comet assays are associated inter-lab variability and there are currently no normative standards for these tests. The present study did not include a normal control group with which to compare the results from the methamphetamine-dependent participants; this limitation should be corrected in any future research seeking to replicate and expand upon the present results. Another important limitation is that this study is correlational in nature and, thus, cause and effect determinations cannot be made. In addition, the study was conducted with a stimulant-dependent sample that abused other substances and, thus, the observed associations cannot be attributed solely to stimulant use. Moreover, statistical adjustment for multiple analyses was not used which might have resulted in Type I errors. However, effect sizes for significant results were provided, which is consistent with the recommendation that effect sizes be provided rather than using the Bonferroni procedure to adjust for multiple-comparisons (44). A final limitation was the reliance on self-reported executive functioning rather than obtaining both self- and informant-reports. A study with Spanish poly-substance abusers revealed that FrSBe scores from patient self-report did not differ significantly from informant-report when reporting about periods of abstinence but that self-report, relative to informant-report, of neurobehavioral abnormalities was significantly lower when a period of substance use was rated, suggesting that substance abusers may be less self-aware of their problematic functioning during use periods (45). Other research has also found evidence of impaired insight in stimulant-dependent patients (46). Future research should thus obtain both self- and informant-reports to assess inter-rater agreement.
In summary, this is the first study to evaluate oxidative damage, an extreme form of oxidative stress, in stimulant-dependent patients. Consistent with pre-clinical research suggesting that oxidative stress plays a role in the cytotoxic effects of stimulants, the present study found an inverse relationship between length of abstinence from cocaine and methamphetamine and oxidative damage level. The present results also suggest that methamphetamine is neurotoxic as assessed by executive dysfunction while cocaine is not, which is consistent with research finding that methamphetamine, but not cocaine, is toxic to dopamine and serotonin neurons (5, 7). The present findings also indicate that the executive dysfunction observed in the methamphetamine-dependent participants is of clinical import in that it was a significant mediator between baseline oxidative DNA damage and self-reported stimulant use during active treatment. Future research to replicate and extend the findings of this preliminary investigation may be warranted.
Acknowledgments
This study was supported by the following grants from the National Institute on Drug Abuse (NIDA) Clinical Trials Network: U10-DA013036 to Oregon Health and Science University (Dr. McCarty); U10-DA013732 to the University of Cincinnati (Drs. Somoza/Winhusen); U10-DA013720 to the University of Miami School of Medicine (Dr. Szapocznik); U10-DA020024 to the University of Texas Southwestern Medical Center (Drs. Adinoff/Trivedi); and U10-DA013714 to the University of Washington (Dr. Donovan). The data and safety monitoring board (DSMB) of the Center Clinical Trials Network (CCTN) of the National Institute on Drug Abuse (NIDA) provided guidance and final approval for the study design. The director and deputy director of the CCTN, the DSMB of the CCTN, and a quality assurance subcontractor to the CCTN monitored study conduct, data collection, and data management. A subcontractor to the CCTN was responsible for data management. The publications committee of the Clinical Trials Network (CTN) gave final approval of the analysis and interpretation of the data and approved the manuscript.
Footnotes
Declarations of Interest: All authors declare that they have no conflicts of interest.
References
- 1.Yamamoto BK, Bankson MG. Amphetamine neurotoxicity: cause and consequence of oxidative stress. Crit Rev Neurobiol. 2005;17:87–117. doi: 10.1615/critrevneurobiol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- 2.Poon HF, Abdullah L, Mullan MA, Mullan MJ, Crawford FC. Cocaine-induced oxidative stress precedes cell death in human neuronal progenitor cells. Neurochem Int. 2007;50:69–73. doi: 10.1016/j.neuint.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Cunha-Oliveira T, Rego AC, Oliveira CR. Cellular and molecular mechanisms involved in the neurotoxicity of opioid and psychostimulant drugs. Brain Res Rev. 2008;58:192–208. doi: 10.1016/j.brainresrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- 5.Yuan J, Hatzidimitriou G, Suthar P, Mueller M, McCann U, Ricaurte G. Relationship between temperature, dopaminergic neurotoxicity, and plasma drug concentrations in methamphetamine-treated squirrel monkeys. J Pharmacol Exp Ther. 2006;316(3):1210–1218. doi: 10.1124/jpet.105.096503. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto BK, Moszczynska A, Gudelsky GA. Uhl GR. Addiction Reviews 2. Vol 1187. 2010. Amphetamine toxicities: Classical and emerging mechanisms; pp. 101–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumann MH, Raley TJ, Partilla JS, Rothman RB. Biosynthesis of dopamine and serotonin in the rat brain after repeated cocaine injections: a microdissection mapping study. Synapse. 1993;14(1):40–50. doi: 10.1002/syn.890140107. [DOI] [PubMed] [Google Scholar]
- 8.Ersche KD, Jones PS, Williams GB, Robbins TW, Bullmore ET. Cocaine dependence: a fast-track for brain ageing? Mol Psychiatry. 2012 doi: 10.1038/mp.2012.31. In Press: doi: http://dx.doi.org/10.1038/mp.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappas S. Cocaine eats up brain twice as fast as normal aging. [accessed 30 October 2012];Interview with Ersche. 2012 Available at: http://www.foxnews.com/health/2012/04/25/cocaine-eats-up-brain-twice-as-fast-as-normal-aging/. (Archived at http://www.webcitation.org/6CIM15ctW on 19 November 2012).
- 10.Ancelin ML, Christen Y, Ritchie K. Is antioxidant therapy a viable alternative for mild cognitive impairment? Examination of the evidence. Dement Geriatr Cogn Disord. 2007;24:1–19. doi: 10.1159/000102567. [DOI] [PubMed] [Google Scholar]
- 11.Berr C. Cognitive impairment and oxidative stress in the elderly: results of epidemiological studies. Biofactors. 2000;3:205–209. doi: 10.1002/biof.5520130132. [DOI] [PubMed] [Google Scholar]
- 12.Berr C, Balansard B, Arnaud J, Roussel AM, Alperovitch A. Cognitive decline is associated with systemic oxidative stress: the EVA study. Etude du Vieillissement Arteriel. J Am Geriatr Soc. 2000;48:1285–1291. doi: 10.1111/j.1532-5415.2000.tb02603.x. [DOI] [PubMed] [Google Scholar]
- 13.Fukui K, Omoi NO, Hayasaka T, Shinnkai T, Suzuki S, Abe K, Urano S. Cognitive impairment of rats caused by oxidative stress and aging, and its prevention by vitamin E. Ann NY Acad Sci. 2002;959:275–284. doi: 10.1111/j.1749-6632.2002.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 14.Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev. 2004;3:431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein RZ, Moeller SJ, Volkow ND. Cognitive disruptions in drug addiction: a focus on the prefrontal cortex. In: Adinoff B, Stein EA, editors. Neuroimaging in Addiction. Chichester, West Sussex, UK: John Wiley & Sons, Ltd.; 2011. pp. 179–210. [Google Scholar]
- 17.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet JL, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend. 2007;90:2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winhusen TM, Somoza EC, Lewis DF, Kropp FB, Horigian VE, Adinoff B. Frontal systems deficits in stimulant-dependent patients: Evidence of pre-illness dysfunction and relationship to treatment response. Drug Alcohol Depend. 2013;127(1–3):94–100. doi: 10.1016/j.drugalcdep.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- 22.Marshall JF, O'Dell SJ. Methamphetamine influences on brain and behavior: unsafe at any speed? Trends Neurosci. 2012;35:536–545. doi: 10.1016/j.tins.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangialasche F, Polidori MC, Monastero R, Ercolani S, Camarda C, Cecchetti R, Mecocci P. Biomarkers of oxidative and nitrosative damage in Alzheimer's disease and mild cognitive impairment. Ageing Res Rev. 2009;8:285–305. doi: 10.1016/j.arr.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Frenzilli G, Scarcelli V, Fornai F, Paparelli A, Nigro M. The comet assay as a method of assessment of neurotoxicity - usefulness for drugs of abuse. In: Ali S, Fornai FF, editors. Cellular and Molecular Mechanisms of Drugs of Abuse and Neurotoxicity: Cocaine, GHB, Substituted Amphetamines. Boston: Blackwell Publishing; 2006. pp. 478–481. [DOI] [PubMed] [Google Scholar]
- 25.Migliore L, Fontana I, Trippi F, Colognato R, Coppede F, Tognoni G, Nucciarone B, Siciliano G. Oxidative DNA damage in peripheral leukocytes of mild cognitive impairment and AD patients. Neurobiol Aging. 2005;26:567–573. doi: 10.1016/j.neurobiolaging.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Donovan DM, Daley DC, Brigham GS, Hodgkins CC, Perl HI, Garrett SB, Doyle SR, Floyd AS, Knox PC, Botero C, Kelly TM, Killeen TK, Hayes C, Kau'ibaumhofer N, Seamans C, Zammarelli L. Stimulant abuser groups to engage in 12-Step: A multisite trial in the National Institute on Drug Abuse Clinical Trials Network. J Subst Abuse Treat. 2013;44(1):103–114. doi: 10.1016/j.jsat.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hudziak J, Helzer JE, Wetzel MW, Kessel KB, McGee B, Janca A, Przybeck T. The use of the DSM-III-R checklist for initial diagnostic assessments. Compr Psychiatry. 1993;34:375–383. doi: 10.1016/0010-440x(93)90061-8. [DOI] [PubMed] [Google Scholar]
- 28.Le Bihan YV, Izquierdo MA, Coste F, Aller P, Culard F, Gehrke TH, Essalhi K, Carell T, Castaing B. 5-Hydroxy-5-methylhydantoin DNA lesion, a molecular trap for DNA glycosylases. Nucleic Acids Res. 2011;39:6277–6290. doi: 10.1093/nar/gkr215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 30.Grace J, Malloy P. Frontal Systems Behavior Scale (FrSBe): Professional Manual. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 31.Malloy P, Grace J. A review of rating scales for measuring behavior change due to frontal systems damage. Cogn Behav Neurol. 2005;18:18–27. doi: 10.1097/01.wnn.0000152232.47901.88. [DOI] [PubMed] [Google Scholar]
- 32.Velligan DI, Ritch JL, Sui D, DiCocco M, Huntzinger CD. Frontal Systems Behavior Scale in schizophrenia: relationships with psychiatric symptomatology, cognition and adaptive function. Psychiatry Res. 2002;113:227–236. doi: 10.1016/s0165-1781(02)00264-0. [DOI] [PubMed] [Google Scholar]
- 33.Cahn-Weiner DA, Grace J, Ott BR, Fernandez HH, Friedman JH. Cognitive and behavioral features discriminate between Alzheimer's and Parkinson's disease. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:79–87. [PubMed] [Google Scholar]
- 34.Chiaravalloti ND, DeLuca J. Assessing the behavioral consequences of multiple sclerosis: an application of the Frontal Systems Behavior Scale (FrSBe) Cogn Behav Neurol. 2003;16:54–67. doi: 10.1097/00146965-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Lane-Brown AT, Tate RL. Measuring apathy after traumatic brain injury: psychometric properties of the Apathy Evaluation Scale and the Frontal Systems Behavior Scale. Brain Inj. 2009;23:999–1007. doi: 10.3109/02699050903379347. [DOI] [PubMed] [Google Scholar]
- 36.Malloy P, Tremont G, Grace J, Frakey L. The Frontal Systems Behavior Scale discriminates frontotemporal dementia from Alzheimer's disease. Alzheimers Dement. 2007;3:200–203. doi: 10.1016/j.jalz.2007.04.374. [DOI] [PubMed] [Google Scholar]
- 37.Paulsen JS, Stout JC, DelaPena J, Romero R, Tawfik-Reedy Z, Swensen MR, Grace J, Malloy PF, Hospital B. Frontal behavioral syndromes in cortical and subcortical dementia. Assessment. 1996;3:327–337. [Google Scholar]
- 38.Fals-Stewart W, O'Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- 39.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 40.MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Eval Rev. 1993;17(2):144–158. [Google Scholar]
- 41.Herr NR. Mediation with dichotomous outcomes. 2011 Retrieved from http://www.nrhpsych.com/mediation/logmed.html.
- 42.Kenny DA. Mediation. 2011 Sept. Retrieved from http://davidakenny.net/cm/mediate.htm.
- 43.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 44.Nakagawa S. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav Ecol. 2004;15(6):1044–1045. [Google Scholar]
- 45.Verdejo-Garcia A, Perez-Garcia M. Substance abusers’ self-awareness of the neurobehavioral consequences of addiction. Psychiatry Res. 2008;158:172–180. doi: 10.1016/j.psychres.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Moeller SJ, Maloney T, Parvaz MA, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Impaired insight in cocaine addiction: laboratory evidence and effects on cocaine-seeking behaviour. Brain. 2010;133:1484–1493. doi: 10.1093/brain/awq066. [DOI] [PMC free article] [PubMed] [Google Scholar]