Abstract
Prostate cancer is one of the most common malignancies affecting men worldwide, with bone being the most common site of metastasis in patients that progress beyond organ confinement. Bone metastases are virtually incurable and result in significant disease morbidity and mortality. Bone provides a unique microenvironment whose local interactions with tumor cells offer novel targets for therapeutic interventions. Several attractive molecules or pathways have been identified as new potential therapeutic targets for bone metastases caused by metastatic castration-resistant prostate cancer. In this review, we present the recent advances in molecular targeted therapies for prostate cancer bone metastasis focusing on therapies that target the bone cells and the bone microenvironment. The therapies covered in this review include agents that inhibit bone resorption, agents that stimulate bone formation, and agents that target the bone matrix. Suggestions to devise more effective molecular targeted therapies are proposed. Hopefully, with better understanding of the biology of the disease and the development of more robust targeted therapies, the survival and quality of life of the affected individuals could be significantly improved.
Keywords: bone metastasis, molecular targeted therapy, osteoblasts, osteoclasts, prostate cancer, RANKL
1 Introduction
Globally, 903,500 new cases of prostate cancer (PCa) and 258,400 deaths from PCa were estimated in 2011. In the United States, PCa is the most common malignancy affecting men, and is the second leading cause of cancer death among men, with an estimate of 240,890 new cases and more than 28,000 deaths in 2011 (1). In eastern countries such as China (2) and Japan (3), the incidence of PCa has dramatically increased over the past two decades, most likely due to economic development and lifestyle changes. In PCa, the development of metastasis essentially means the patient is incurable. The most common site of PCa metastasis is bone. Autopsy studies of men who died of PCa revealed radiological evidence of bone metastases in nearly 90% of the patients examined, a percentage much higher than that of other bone-metastasizing solid tumors (such as breast and lung cancer) (4). One of the hallmarks of prostate bone metastasis is the osteosclerotic (blastic) phenotype, which is the presenting manifestation for most PCa bone metastases. Even so, increased bone resorption is a prerequisite for the successful seeding of the PCa cells in blastic-predominant bone metastasis (5).
Bone metastasis significantly affects patients’ quality of life through skeletal-related events (SRE) including bone pain, pathological fractures, nerve impingement and myelophthisis. More importantly, once tumors metastasize to bone, they are virtually incurable and result in significant disease morbidity prior to a patient’s death (6). The main therapeutic option for bone metastasis in hormone-responsive PCa is androgen deprivation therapy (ADT). Despite initial response rates of 80–90%, virtually all treated patients progress to androgen-insensitive disease, a state referred to as castration-resistant PCa (CRPC) (7). Even with substantial progress in the understanding of the biology of PCa bone metastasis and the constant development of new therapeutic agents, treatments so far have had only modest effects on survival for patients with metastatic CRPC.
Several attractive molecules or pathways in the metastatic process of PCa have been identified as new potential therapeutic targets and significant progress has been made in the area of bone-targeted therapies of metastatic PCa especially in recent years. This review presents the recent advances in bone-targeted therapies for PCa bone metastasis, from preclinical in vivo investigations to clinical studies. Not covered in this review are advances in therapies that primarily target or modulate the tumor cells per se, such as androgen receptor inhibition, chemotherapy, and immunotherapy, and therapies that are currently being evaluated in other solid tumors that spread to bone but have not been tested in PCa. Interested readers may refer to other related reviews for these interesting topics (7–10).
2 Mechanisms Governing Bone Metastasis in Prostate Cancer
2.1 Why Bone?
Several theories have been proposed to explain the propensity of PCa to metastasize to bone. The most widely accepted theory is the ‘‘seed-and-soil’’ hypothesis, which was proposed more than a century ago by Stephen Paget and may be relevant in many cancer types including PCa (11). According to this theory, cancer cells (“seeds”) metastasize to locations (“soil”) that are biochemically and physiologically favorable for their implantation and growth. Strong support for this theory was demonstrated by the seminal work of Fidler et al. some 80 years later who demonstrated that although tumor cells reached the vasculature of all organs, metastases selectively developed only in certain organs (12). “Osteomimicry” is another theory proposed to explain the preferential growth of PCa cells in bone. According to this hypothesis, metastatic PCa cells take on the properties and behaviors of osteoblasts or osteoclasts upon arrival in bone. These activities lead to enhanced turnover of the bone matrix and preferential growth of PCa cells in bone (11). The bone metastatic process may involve a combination of the above, and as yet undefined, mechanisms. Understanding these mechanisms may provide a basis for identifying therapeutic targets.
2.2 RANK/RANKL/OPG as the Essential Regulators of Prostate Cancer-Bone Interactions
The interaction between osteoclasts and osteoblasts during the processes of bone resorption and formation is key to normal bone turnover. The best understood molecular link between osteoblasts and osteoclasts is the RANK/RANKL/OPG triad (6). The receptor activator of NF-κB (RANK) is a transmembrane receptor expressed on osteoclast precursor cells, while RANK ligand (RANKL) is expressed by osteoblasts and bone marrow stromal cells. Upon binding to RANK, RANKL leads to osteoclast maturation, activation, and survival. This process can be interrupted by osteoprotegerin (OPG), a soluble decoy receptor for RANKL, which is produced by mature osteoblasts and stromal cells to inhibit the maturation of osteoclasts. Upon binding RANKL, OPG inhibits RANKL’s ability to activate RANK. This, in turn, diminishes osteoclastogenesis and osteoclast activation. Increasing the ratio of OPG to RANKL has been shown to result in increased bone mass (13).
The dysregulation of the functional equilibrium in the RANK/RANKL/OPG triad is responsible for the pathological remodeling associated with malignant tumors and for the development of metastatic deposits in bone sites. Tumor cells release growth factors and/or cytokines into the bone microenvironment, which, in turn, stimulate the production of RANKL from osteoblasts. RANK stimulation by RANKL results in the differentiation of preosteoclasts into active osteoclasts, which resorb the mineralized bone matrix, thus releasing factors to promote further colonization and growth of tumor cells (14). Therefore, targeting the RANK/RANKL/OPG axis constitutes an important strategy for the management of PCa bone metastasis.
2.3 Other Molecules Important for Prostate Cancer Bone Metastasis
During the process of bone metastasis, tumor cells may acquire a specific phenotype favoring the secretion of specific cytokines and proteases that interact with the bone microenvironment (6). These factors include bone morphogenetic proteins (BMPs), transforming growth factor-β (TGF-β), protease-activated receptor (PAR), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), fibroblast growth factors (FGFs), Wnt1, parathyroid hormone-related protein (PTHrP) and prostate-specific antigen (PSA). In addition to tumor-produced factors, bone also provides a fertile “soil” for the “seeds”. Specifically, cytokines and non-collagen proteins released from the bone matrix or synthesized during bone turnover promote the colonization and growth of PCa cells in bone. This crosstalk between tumor cells and bone microenvironment creates a “vicious cycle” between the tumor cells and the bone microenvironment. For example, interactions between stromal cell-derived factor 1 (SDF-1, also known as CXCL12), which is expressed by endothelial cells, osteoblasts, and stromal cells, and its receptor CXCR4, which is expressed by PCa cells, promote the directed migration (chemotaxis) and growth of metastatic deposits of PCa cells in bone (15). Proteases such as matrix metalloproteinases (MMPs) (16), cathepsins (17), and the urokinase-type plasminogen activator (uPA) (18) also significantly contribute to the growth and expansion of the metastatic deposit of PCa in bone. Src family kinases play an important role in PCa growth, invasion, and metastatic dissemination (19). Integrin αvβ3 expression in osteoclasts is also involved in the formation of PCa bone metastasis (20). In-depth review of the molecular mechanisms of PCa bone metastasis is not the focus of this review but can be found elsewhere (6, 21).
3 Agents Inhibiting Bone Resorption
3.1 Bisphosphonates as Inhibitors of Osteoclastic Activity
Bisphosphonates (BPs), synthetic non-hydrolyzable analogs of pyrophosphate with structural similarity to inorganic phosphate, are the most commonly used drugs in the management of bone complications in bone-metastasizing cancers. Nitrogen-containing BPs such as Zoledronic acid (ZA) inhibit farnesyl pyrophosphate synthase in osteoclasts, thereby preventing the formation of isoprenoid lipids required for the prenylation of small GTPases (22). In contrast, non-nitrogen-containing BPs, such as Clodronate, inhibit osteoclast activity through the intracellular formation of AppCCl2p, an ATP analog.
The FDA approved ZA in 2002 to treat hypercalcemia and prevent SREs in patients suffering from bone-metastatic breast cancer, lung cancer, multiple myeloma, and PCa (23). Although ZA was associated with significant reductions in 6-month risks of SREs in bone-metastatic PCa patients, the observed improvement in overall survival (OS) was not statistically significant (24). Clodronate has initially been reported to have no effect on OS or disease-free survival. However, a prospective study of 140 high-risk PCa patients who had clinically organ-confined disease indicated that the addition of Clodronate delayed the appearance of the first bone metastasis by seven-fold (25). Data from two aligned Medical Research Council randomized trials revealed that although there was no evidence of a benefit of OS in the non-metastatic group, Clodronate conferred an overall survival benefit when given in addition to standard hormone therapy in men with metastatic CRPC (26).
3.2 Therapies Targeting the RANK/RANKL/OPG Triad
3.2.1 RANKL-neutralizing Antibody
Denosumab (Xgeva®) is a fully human monoclonal antibody that binds to and neutralizes RANKL, thereby inhibiting osteoclast function and preventing generalized bone resorption and local bone destruction. Denosumab represents a significant advance in the treatment of bone loss associated with hormone ablation therapy in women with breast cancer and men with PCa and the prevention of SREs in patients with bone metastases from solid tumors (27).
Three main Phase III clinical studies have been published for PCa. The first study was conducted to evaluate the effect of Denosumab on bone mineral density and fractures in men receiving ADT (28). The second study compared Denosumab to ZA for the prevention of SREs in men with bone metastases from CRPC (29). The third study assessed Denosumab for the prevention of bone metastasis or death in non-metastatic CRPC patients (30). In this double-blind randomized study, Denosumab increased bone metastasis-free survival by a median of 4.2 months, compared with placebo and delayed time to first bone metastasis. However, OS was not significantly changed. The FDA voted against expanding the current indications of Denosumab since the benefits of this drug did not outweigh its risks, including osteonecrosis of jaw (ONJ) (http://articles.latimes.com/2012/feb/08/business/la-fi-amgen-20120208).
3.2.2 RANK-Fc
The efficacy of the extracellular domain of the recombinant soluble RANK fused with the immunoglobulin Fc domain (RANK-Fc) was tested as an adjuvant therapy for PCa bone metastasis in preclinical models. In a mouse intratibial injection model, the combination of RANK-Fc and Docetaxel reduced tumor burden in bone more efficiently than either treatment alone (31). In an osteolytic SCID mouse model of bone metastasis induced by intratibial injection of PC-3 cells, Virk et al. studied the effect of simultaneous blockade of the RANK/RANKL axis using subcutaneous administration of RANK-Fc and the BMP pathway using retrovirus-mediated overexpression of noggin, a cognate binding protein and an antagonist of BMP4. The combination therapy with RANK-Fc and noggin effectively delayed the development of osteolytic lesions and decreased the bone loss and tumor burden compared with noggin overexpression alone (32). Further, in a mixed lytic/blastic PCa lesion in bone induced by intratibial injection of C42b cells, the same group found that treatment with both RANK-Fc and noggin demonstrated delayed development of bone lesions, attenuation of osteolysis, inhibition of small soft tissue tumors, and preservation of bone architecture with less tumor-induced new bone formation (33). These studies suggest that combined inhibition of RANKL and the BMP pathway may be an effective biologic therapy to inhibit the progression of established osteolytic as well as mixed lytic/blastic PCa lesions in bone. The clinical significance of this combination therapy awaits further investigations.
3.2.3 OPG-Fc
In a murine model of PCa bone metastasis using luciferase expressing PC-3 cells, OPG fused to Fc (OPG-Fc) alone reduced bone resorption, inhibited progression of established osteolytic lesions, and reduced tumor area (34). OPG-Fc in combination with Docetaxel (Taxotere), the most commonly used chemotherapeutic drug for the treatment of metastatic hormone-refractory PCa, suppressed skeletal tumor burden and increased median survival time by 16.7% compared with Docetaxel alone. RANKL inhibition by OPG-Fc may enhance Docetaxel effects by increasing tumor cell apoptosis as evidenced by increased active caspase-3 (35). These studies show that inhibition of RANKL provides an additive benefit to Docetaxel treatment and support clinical evaluation of this treatment option in patients.
3.3 Agents Targeting the Src Family Kinases
The Src family kinases (SFKs) are a family of non-receptor tyrosine kinases involved in the regulation of a wide range of cellular activities including proliferation, adhesion, motility, and survival. High levels of Src are found in mature osteoclasts and Src activity is essential for bone remodeling through positively regulating osteoclasts and negatively regulating osteoblasts. Several lines of evidence indicate that the SFKs contribute significantly to the increased osteoclastic activity associated with PCa bone metastases and play an important role in PCa growth, invasion, and metastatic dissemination (19). Therefore, Src/SFK inhibition represents a potentially useful therapeutic strategy for patients with advanced-stage PCa.
Of the Src inhibitors examined in clinical studies, the broadest range of data is available for Dasatinib (BMS-354825, SPRYCEL®), the dual Src and Bcr-Abl tyrosine kinase inhibitor. In Phase II monotherapy trials for CRPC patients, Dasatinib (100 mg once daily (36) or twice daily (37)) showed similar and encouraging results in a subset of patients. One Phase I-II trial assessed the combination of Dasatinib and Docetaxel, in which a 50% decline in serum PSA levels was observed in 57% of the patients and a partial response observed in 60% of the patients with measurable disease. Urinary N-telopeptide (NTx) levels decreased in 87% of the patients, and toxicity was considered manageable (38). An ongoing Phase III trial is assessing Dasatinib (100 mg once daily) in combination with Docetaxel, and the results are expected very soon (http://clinicaltrials.gov_NCT00744497).
Saracatinib (AZD-0530), another Src and Bcr-Abl tyrosine kinase inhibitor, has been tested in a Phase II trial for the treatment of patients with PCa that did not respond to hormone therapy (http://clinicaltrials.gov_NCT00513071). A further randomized Phase II trial evaluated the efficacy of Saracatinib in comparison with ZA on patients with metastatic breast cancer and PCa (http://clinicaltrials.gov_NCT00558272). Both of these studies have been completed, pending publication of the trial results.
KX2-391, the third Src inhibitor, is the only Src-specific inhibitor. Unlike other Src inhibitors, KX2-391 binds to the peptide substrate-binding side of Src rather than its ATP-binding site. A Phase II study is evaluating the safety and efficacy of KX2-391 (40 mg twice daily) in bone-metastatic CRPC patients who have not received chemotherapy (http://clinicaltrials.gov_NCT01074138). In a single-arm Phase II study in men with metastatic CRPC, KX2-391 used at the same dosage did not show antitumor activity, but had modest effects on bone turnover markers such as urinary NTx and osteocalcin. Higher once-daily dosing is planned for future trials using KX2-391 (39). Bosutinib (SKI-606), the fourth inhibitor of Src and Bcr-Abl, was shown to block PCa invasion, growth, and metastasis in preclinical studies (40). This leads to the proposal for its future evaluation as a potential therapeutic in PCa bone metastasis in clinic trials.
3.4 Agents Targeting Integrins
Integrins are heterodimeric adhesion receptors that mediate cell–matrix interaction. Integrin αvβ3 is known to be expressed on tumor cells as well as on osteoclasts and has been shown to induce RANKL expression in PCa cells (41). Integrin αvβ3 expression is involved in bone metastasis and subsequent bone resorption in PCa (20). Abegrin™ (Etaracizumab, previously known as Vitaxin® or MEDI-522) is a novel humanized monoclonal antibody directed against the αvβ3 integrin. A Phase II, randomized, multicenter study has been launched to evaluate the effect of Abegrin in combination with Docetaxel, Prednisone, or ZA for the treatment of patients with metastatic CRPC. This clinical trial has been completed, pending publication of the trial result (http://clinicaltrials.gov_NCT00072930).
MK-0429 (Merck, NJ, US) is an αvβ3 integrin inhibitor under evaluation for its effect on bone turnover and disease activity in men with CRPC and bone metastases. There was some evidence of an early reduction of bone turnover, indicating a potential for clinical use in the treatment of bone-metastatic disease. MK-0429 was generally well tolerated, with the most common side effects being nausea and an unexpected increase in serum PSA level (20). EMD 525797 (DI17E6) is a novel humanized monoclonal antibody targeting the αv integrin. A multicenter Phase I study in progressive CRPC with bone metastases revealed no dose-limiting toxicities (42). Further studies are warranted to evaluate the clinical activity of EMD 525797.
GLPG0187 is a non-peptide αv-integrin antagonist. In vitro and in vivo data showed that GLPG0187 is a potent inhibitor of osteoclastic bone resorption and angiogenesis. In animal models of PCa bone metastasis, GLPG0187 markedly reduced the metastatic tumor growth of PC-3M-Pro4/luc cells in both preventive and curative protocols (43). These results suggested that GLPG0187 can inhibit PCa bone metastases by antitumor, antiresorptive, and antiangiogenic mechanisms.
4 Agents Stimulating Bone Formation
4.1 Endothelin A Receptor
Endothelins exert their activity through binding to two distinct G protein-coupled receptors (GPCRs): Endothelin A receptor (ETAR) and Endothelin B receptor (ETBR). Endothelin 1 (ET-1) is a proliferation signal for many cells including PCa cells and osteoblasts and is known to participate in the process by which PCa forms metastases in bone (44). It has been shown that higher tissue ETAR levels in PCa patients correlate with advanced tumor stage, grade, and metastases. Although preclinical data support the concept that inhibition of the endothelin signaling axis may be useful in controlling tumor growth in bone, clinical results to date have been mixed and are sometimes disappointing.
Atrasentan (ABT-627 or Xinlay™), which inhibits the binding of ET-1 to ETAR without affecting the receptor density, is the first oral selective ETAR antagonist in clinical use. A Phase I-II study of Atrasentan and Docetaxel in men with metastatic CRPC determined the maximum tolerated dose of every-3-week Docetaxel with 10 mg Atrasentan was 70–75 mg/m2. OS and progression-free survival were not significantly improved compared to those seen with Docetaxel alone, whereas the rate of PSA decline was slightly lower than expected (45). In a Phase III study in CRPC, the only significant finding with Atrasentan administration (10 mg/day) was the suppression of BALP and PSA levels (46). Another phase III trial in non-metastatic CRPC that randomized 467 men to Atrasentan and 474 to placebo also failed to improve time-to-disease progression or OS (47). These unfavorable clinical outcomes and a correlation with increased adverse cardiovascular events such as vasodilation and fluid retention led the FDA to reject the approval of Atrasentan for the treatment of CRPC.
Zibotentan (ZD-4054) is a specific oral ETAR inhibitor that, unlike Atrasentan, demonstrates no detectable inhibition of the ETBR (48). In Phase II trials, in patients with asymptomatic or mildly symptomatic metastatic CRPC, Zibotentan (10–15 mg/day) did not delay time to progression, but, conversely to Atrasentan, it was associated with a promising improvement in OS (49, 50). However, a recent Phase III study of 594 patients did not demonstrate any clinical benefit in patients with bone-metastatic CRPC (51). Another Phase III study has been performed to further evaluate the efficacy of Zibotentan in combination with Docetaxel in metastatic CRPC. Primary results indicated that Zibotentan plus Docetaxel did not result in a significant improvement in OS compared with Docetaxel plus placebo in patients with metastatic CRPC (52). These disappointing results have cast a shadow over the future of the endothelin receptor antagonists in PCa therapy.
5 Agents Targeting the Bone Matrix
5.1 Bone-homing Radionuclides
Bone-homing radionuclides are systemically administered radiopharmaceuticals currently under investigation for the palliative treatment of severe pain in osteosclerotic metastatic bone disease (53). Certain radionuclides such as the β-emitter Strontium-89 (89Sr) and the α-emitter Radium-223 (Alpharadin or Xofigo®) integrate into the bone cavity, thus irradiating tumor cells that colonize in bone; others radionuclides, e.g., the β-emitter Samarium-153 (153Sm), can be conjugated to ligands, such as ethylenediaminetetramethylene phosphonate (EDTMP), to direct selective delivery to bone lesions (54). 153Sm-EDTMP, which was approved by FDA in 1997, is the preferred β-emitting radiopharmaceutical of many clinicians. The half-life is 1.9 days and pain relief is rapid, generally between 2 and 7 days. Although 153Sm-EDTMP can control pain, it has failed to affect the survival of PCa patients when used alone (55). However, several Phase I–II trials have demonstrated that, when combined with other systemic therapies, such as Docetaxel, the efficiency of 153Sm therapy can be increased, leading to a marked improvement in bone pain and a trend towards increased OS (56–58).
The α-emitter Alpharadin, which has a half-life of 11.43 days, naturally targets the hydroxyapatite in the bone matrix because it simulates the stoichiometry of calcium. Alpharadin is different from the β-particles in that it carries higher radioactive energy but travels shorter distances (<100 μm), allowing for greater killing to tumor cells but less toxicity to the surrounding healthy tissue (59). Another advantage of Alpharadin is that it does not require the cancer cell to cycle in order to achieve its antitumor effect. This distinct feature is of particular benefit in the treatment of PCa, which has a low overall proliferative rate. Alpharadin has been systematically evaluated for highly localized treatment of osteoblastic bone metastases and shown to be a promising candidate for managing bone metastases in patients with CRPC.
In a Phase II trial of 64 CRPC patients scheduled to receive local-field external-beam radiation therapy to relieve pain from bone metastasis, Alpharadin has been shown to prolong the time to SRE and increase OS compared with placebo. Importantly, very few patients had grade 3–4 hematological toxicities, with the most common adverse effects being nausea, bone pain, fatigue, diarrhea, vomiting, and constipation (60). Less hematological toxicities compared with the β-emitters 153Sm and 89Sr were confirmed in a more recent clinical study (61). The latest Phase III clinical trial results have been published, which indicated that, compared with placebo, Alpharadin significantly improved OS (median 14.0 vs. 11.2 months) and was associated with low myelosuppression rates and fewer adverse effects in metastatic PCa (62). In May, 2013, the US FDA approved Alpharadin for the treatment of patients with CRPC, symptomatic bone metastases and no known visceral metastatic disease (http://www.prnewswire.com/news-releases/bayer-receives-us-fda-approval-for-xofigo-radium-ra-223-dichloride-injection-as-a-new-treatment-for-castration-resistant-prostate-cancer-with-bone-metastases-207545191.html).
5.2 Cathepsin K
Cathepsins have been shown to be important mediators of metastasis across a range of tumors, including PCa. The primary cathepsins found in the bone microenvironment are cathepsins D, K, and L. Cathepsin K is responsible for the production of NTx, a marker of bone turnover, which is generated from degradation of bone matrix collagen I. Increased type I collagen degradation of NTx associated with cathepsin K activity was demonstrated in the sera of patients with PCa bone metastases (17). The critical role of cathepsin K in degradation and/or mobilization of mineralized collagen I and other metastasis-related proteins, such as osteonectin, VEGF, osteopontin, SDF-1, during bone resorption makes the inhibition of its activity an attractive antiresorptive strategy in the treatment of metastatic bone disease.
Most of the investigations of cathepsin K inhibitors have been performed in breast cancer and osteoporosis patients, with novel inhibitors only recently being applied to PCa bone metastases. The only Phase III trial of testing the cathepsin K inhibitor, Odanacatib (MK-0822), which was initiated to assess its effect in prolonging time to first bone metastasis in men with CRPC, was closed before its completion and no further evaluation is ongoing in the oncology setting, likely owing to commercial considerations (http://clinicaltrials.gov_NCT00691899.).
5.3 The MMP/TIMP System
The extracellular matrix (ECM) is a barrier for cancer cell spreading. Solubilization of the ECM is a prerequisite for cancer cell migration, invasion, and formation of metastatic deposits. MMPs are a family of zinc-dependent proteolytic enzymes that collectively degrade most of the components of the ECM (63). MMPs are expressed by cancer cells, stromal cells, infiltrating inflammatory cells, as well as osteoclasts. In general, the expression and activation of MMPs correlate with advanced tumor stage, increased tumor angiogenesis, invasion, metastasis, and poor prognosis. MMPs, especially MMP-1, MMP-2, MMP-7, MMP-9, and MT1-MMP, are expressed in human PCa tissues and are involved in the establishment and growth of metastatic PCa in bone (64). Therefore, MMPs constitute an attractive target for intervention in PCa bone metastasis.
A large number of MMP inhibitors (MMPIs) have been developed in recent two or three decades. Several generations of synthetic MMPIs were tested in clinical trials since the 1990’s. These include the first-generation peptidomimetics (such as Batimastat and Marimastat), the second-generation nonpeptidomimetics (such as Tanomastat and Prinomastat), and the third-generation tetracycline derivatives (such as Minocycline and Metastat). These MMPIs have shown to be effective in blocking PCa bone metastasis in preclinical studies. However, clinical trials were basically unsuccessful mainly because of the lack of overall response and the presence of dose-limiting toxicity. Consequently, all clinical trials on the use of synthetic MMPIs have been terminated (65). Inhibition of MMPs using PCK3145 (66), a synthetic peptide, or Silibinin (67), a naturally occurring small molecule inhibitor, has been reported and the results showed that these agents inhibited PCa cell-induced osteolysis in preclinical studies.
In a chimeric SCID-human model of PCa bone metastasis, we investigated the effect of MMP inhibition by tissue inhibitors of metalloproteinases (TIMPs), the most important endogenous inhibitors of MMPs, on the interaction between PCa cells and the implanted bone. We found that recombinant adenoviruses expressing TIMP-1 or TIMP-2 (AdTIMP-1 or AdTIMP-2) in cultured bone fragments inhibited MMP-2 activity, bone turnover and degradation induced by PC-3 cancer cells. In vivo, AdTIMP-2 expression in bone significantly reduced PC-3-induced osteolysis, osteoclast recruitment, and bone turnover (68). This establishes that the TIMP pathway is an intriguing target for developing gene therapy in hopes of preventing PCa growth and spread in bone. However, strategies by which an adenoviral vector can survive the immunosurveillance of an affected individual and by which the body’s entire bone marrow stroma can be transduced need to be carefully planned.
5.4 The uPA–uPAR System
uPA is a serine protease that binds to its cell surface receptor uPAR and promotes pericellular proteolysis and intracellular signaling, which both coordinate multiple processes including cell migration, proliferation, and survival during cancer progression (18). The clinical evaluation of uPA and uPAR in the serum of patients with PCa has revealed strong associations with multiple states of disease progression (69). The finding that the amino-terminal fragment (ATF) of uPA, which antagonizes the actions of uPA, drives osteoblastic responses in three dimensional co-cultures of PCa cells and osteoblasts led to its consideration as a target in osteoblastic metastatic bone disease.
In a mouse model of intratibial injection of PC-3 cells, Fritz et al. used murine mesenchymal stem cells (MSCs) engineered to express hATF to evaluate the effect of MSC-mediated delivery of uPA antagonist on PCa progression in bone (70). They demonstrated a significant decrease in tumor-associated angiogenesis and protection from tumor-induced osteolysis in MSC-hATF-treated mice. These studies suggested that the regulation of the uPA–uPAR system could provide potential treatments for bone-metastatic PCa.
5.5 TGF-β Signaling
TGF-β is a major bone-derived factor, which contributes to the “vicious cycle” of cancer bone metastasis. TGF-β released from the bone matrix stimulates tumor cells to produce osteolytic factors that further stimulate adjacent bone resorption. In addition, TGF-β also regulates key components of the metastatic cascade including epithelial-mesenchymal transition (EMT), tumor cell invasion, angiogenesis, and immunosuppression (71).
In an intracardiac injection model of PCa bone metastasis, the effect of TGF-β signaling inhibition on osteoblastic tumor growth was investigated. The results indicated that stable knockdown of TGF-β1 with an shRNA resulted in decreased tumor incidence and bone formation. In addition, systemic administration of either a small inhibitor of TGF-β receptor type I kinase (TβRI-KI) or a pan TGF-β binding protein (BGERII) decreased bone tumor growth and osteoblastic bone formation (72). More recently, Hu et al. examined whether Ad.sTβR-Fc, an oncolytic virus expressing soluble TGF-β receptor type II fused with human Fc, and TAd.sTβR-Fc, a variant of Ad.sTβR-Fc, can treat bone metastasis of PCa. Both Ad.sTβR-Fc and TAd.sTβR-Fc caused significant inhibition of tumor growth and inhibited tumor-induced hypercalcemia, with the former being more effective in inhibiting tumor growth (73).
LY2109761 is a selective TGF-β receptor type I kinase inhibitor. LY2109761 treatment significantly inhibited the growth of MDA PCa 2b (osteoblastic) and PC-3 (osteolytic) cells in the bone of SCID mice; moreover, it resulted in significantly less bone loss in the PC-3 tumor-bearing bones as well as an increase in normal bone mass. This increased mass in non-tumorous bone may be a desirable side effect of LY2109761 treatment for osteopenia or osteoporosis secondary to ADT (74). These results support the development of therapies targeting TGF-β for advanced PCa. It is anticipated that TGF-β signaling inhibitors will shortly enter clinical evaluation for metastatic PCa.
6 The Role of Androgens in Bone Metastasis
Androgens impact both PCa growth and bone remodeling. Thus, they have potential to modulate the progression of bone metastasis directly, through a direct proliferative effect on PCa cells and indirectly, through altering the bone metastatic microenvironment through bone remodeling. Androgen status can impact the signaling state and survival of PCa cells. For example, androgen deprivation therapy has been shown to alter the subcellular localization of ErbB3, a receptor tyrosine kinase, that has pro-tumorigenic effects and is upregulated in bone metastasis (75). It is not clear if androgen ablative therapies have any specific impact specifically on bone metastasis as opposed to metastasis in soft tissue sites. In one report, adding androgen ablation therapy to docetaxel treatment had no additive or synergistic effect on tumor response in a mouse model compared to docetaxel alone (76). In contrast, intratibial tumors in mice of the androgen-responsive PCa cell line, C42, responded to castration, providing evidence that PCa skeletal metastases can maintain an androgen sensitive state (77). There are no good clinical data to suggest that androgen deprivation therapy prevents bone metastasis; albeit, hormone naïve (i.e., no androgen deprivation therapy received by the patient) bone metastases can respond to androgen deprivation therapy.
7 Concluding Remarks and Perspectives
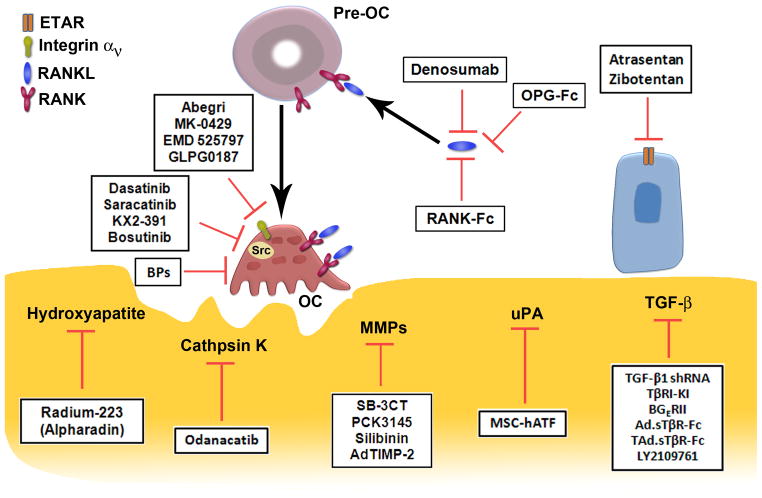
Novel therapies that target the bone cells and the bone matrix during PCa metastasis are summarized in Figure 1 and Table 1. Even with these developments, the management of PCa bone metastases still remains a dilemma for researchers as well as clinicians. In order to achieve the goal of curing bone metastasis in PCa patients, we propose that several factors be taken into consideration. First, treatment of bone metastasis in CRPC is complicated by the fact that both osteoblastic and osteolytic responses are involved in different stages of bone metastasis. Therefore, overemphasizing on either arm will result in unsatisfactory outcome. Logically, one could expect that better disease control could be achieved by combination therapies that target both of the osteoblastic and osteolytic pathways during PCa progression. Second, PCa metastasis to bone is a complex progress involving tumor-stomal cell, tumor-matrix, and tumor-vasculature interactions. Simultaneous targeting of the molecules that facilitate interactions between PCa cells and the bone microenvironment could significantly influence future therapeutic approaches. Third, current animal models of cancer metastasis (78) do not faithfully reflect what’s really going on inside the patients’ bone. Therefore, more robust models are needed that mimic the complex nature of PCa bone metastasis. These models should be more representative of patient disease progression. Hopefully, with better understanding of the biology of PCa bone metastasis and the development of more robust molecular therapies, the survival and quality of life of the affected individuals could be significantly improved.
Figure 1. Summary of the agents used to target the bone for prostate cancer metastasis.
A schematic overview of the recent development of bone-targeted therapeutic strategies for prostate cancer metastasis that have been investigated in clinical or preclinical studies. A detailed description is provided in the text.
Table 1.
Development of Agents for Bone-targeted Therapies of Metastatic Prostate Cancer
| Agents | Mechanism of Action | Biochemical Property | Molecular Targets | Stage of Development | Refs. |
|---|---|---|---|---|---|
| Zoledronic acid | Inhibiting resorption | Synthetic analog of pyrophosphate (N-containing) | Farnesyl pyrophosphate synthase | Approved | 23, 24 |
| Clodronate | Inhibiting resorption | Synthetic analog of pyrophosphate (non-N-containing) | ? | PR04/PR05 | 25, 26 |
| Denosumab | Inhibiting resorption | Humanized monoclonal antibody | RANKL | Phase III | 28–30 |
| RANK-Fc | Inhibiting resorption | Recombinant protein | RANKL | Preclinical | 31–33 |
| OPG-Fc | Inhibiting resorption | Recombinant protein | RANKL | Preclinical | 34, 35 |
| Dasatinib (SPRYCEL®) | Inhibiting resorption | Tyrosine kinase inhibitor | Src, Abl | Phase III | 36–38 |
| Saracatinib (AZD-0530) | Inhibiting resorption | Tyrosine kinase inhibitor | Src, Abl | Phase II | * |
| KX2-391 | Inhibiting resorption | Tyrosine kinase inhibitor | Src | Phase II | 39, # |
| Bosutinib (SKI-606) | Inhibiting resorption | Tyrosine kinase inhibitor | Src, Abl | Preclinical | 40 |
| Abegri (Etaracizumab, Vitaxin® or MEDI-522) | Inhibiting resorption | Humanized monoclonal antibody | Integrin alpha(nu)beta(3) | Phase II | & |
| MK-0429 | Inhibiting resorption | Small molecule inhibitor | Integrin alpha(nu)beta(3) | Phase I | 20 |
| EMD 525797 (DI17E6) | Inhibiting resorption | Humanized monoclonal antibody | Integrin alpha(nu) | Phase I | 42 |
| GLPG0187 | Inhibiting resorption | Non-peptide integrin antagonist | Integrin alpha(nu) | Preclinical | 43 |
| Atrasentan (ABT-627 or Xinlay) | Stimulating bone formation | Small molecule inhibitor | ETAR>ETBR | Phase III, further approval rejected | 46, 47 |
| Zibotentan (ZD-4054) | Stimulating bone formation | Small molecule inhibitor | ETAR | Phase III | 49–52 |
| Samarium-153-EDTMP | Targeting bone matrix | Radiopharmaceutical (β-emitter) | ? | Approved; Phase II for combination with Docetaxel | 56–58 |
| Radium-223 (Alpharadin, or Xofigo®) | Targeting bone matrix | Radiopharmaceutical (a-emitter) | Hydroxyapatite | Approved | $ |
| Odanacatib (MK-0822) | Targeting bone matrix | Small molecule inhibitor | Cathepsin K | Phase III | ▲ |
| PCK3145 | Targeting bone matrix | Synthetic peptide | MMPs | Preclinical | 66 |
| Silibinin | Targeting bone matrix | Small molecule inhibitor | MMPs | Preclinical | 67 |
| AdTIMP-2 | Targeting bone matrix | Recombinant adenovirus expressing TIMP-2 | MMPs | Preclinical | 68 |
| MSC-hATF | Targeting bone matrix | MSCs engineered to express uPA antagonist hATF | uPA–uPAR | Preclinical | 70 |
| TGF-β1 shRNA | Targeting bone matrix | shRNA | TGF-β | Preclinical | 74 |
| TβRI-KI | Targeting bone matrix | Receptor kinase inhibitor | TGF-β receptor type I | Preclinical | 74 |
| BGERII | Targeting bone matrix | Pan TGF-β binding protein | TGF-β | Preclinical | 74 |
| Ad.sTβR-Fc | Targeting bone matrix | Oncolytic virus expressing soluble TGF-β receptor type II fused with Fc | TGF-β | Preclinical | 75 |
| TAd.sTβR-Fc | Targeting bone matrix | A variant of Ad.sTβR-Fc | TGF-β | Preclinical | 75 |
| LY2109761 | Targeting bone matrix | Small molecule inhibitor | TGF-β receptor type I | Preclinical | 76 |
Abbreviations
EDTMP, ethylenediaminetetramethylene phosphonate; ETAR, endothelin A receptor; ETBR, endothelin B receptor; hATF, human amino-terminal fragment; MMPs, matrix metalloproteinases; MSC, mesenchymal stem cell; OPG, osteoprotegerin; RANK, receptor activator of NF-κB; RANKL, receptor activator of NF-κB ligand; TGF-β, transforming growth factor β; TIMP-2, tissue inhibitor of metalloproteinase 2; TβRI, TGF-β receptor type I; uPA, urokinase-type plasminogen activator; uPAR, urokinase-type plasminogen activator receptor
http://www.clinicaltrials.gov/ct2/results?term=NCT00513071&Search=Search; http://www.clinicaltrials.gov/ct2/results?term=NCT00558272&Search=Search
Acknowledgments
We would like to thank Dr. Fei Liu and Ms. Hui Yao at Hunan Normal University Medical College for assistance in preparation of the illustrations and the manuscript. This work was supported by funding from the Construct Program of the Key Discipline of Basic Medicine in Hunan Province, Hunan Normal University Startup Fund for Returned Overseas Scholars (130608), the National Institutes of Health P01 CA093900, the National Natural Science Foundation of China (81071755), National Science & Technology Pillar Program of China (2012BAD34B02), Special Fund for Agro-scientific Research in the Public Interest of China (201303071-2-1), and S&T Plan Key Project of Hunan Province (2013WK4006).
List of Abbreviations
- ADT
Androgen deprivation therapy
- BALP
Bone alkaline phosphatase
- BMPs
Bone morphogenetic proteins
- BPs
Bisphosphonates
- CRPC
Castration-resistant prostate cancer
- ECM
Extracellular matrix
- ET-1
Endothelin-1
- ETAR
Endothelin A receptor
- ETBR
Endothelin B receptor
- FDA
The US Food and Drug Administration
- hATF
Human amino-terminal fragment
- MMPs
Matrix metalloproteinases
- MMPIs
Matrix metalloproteinase inhibitors
- MSCs
Mesenchymal stem cells
- NTx
N-telopeptide
- OPG
Osteoprotegerin
- OS
Overall survival
- PCa
Prostate cancer
- PSA
Prostate-specific antigen
- RANK
Receptor activator of NF-κB
- RANKL
Receptor activator of NF-κB ligand
- SFKs
Src family kinases
- SREs
Skeletal-related events
- TGF-β
Transforming growth factor β
- TIMP
Tissue inhibitor of metalloproteinases
- TβRI
Transforming growth factor β receptor type I
- uPA
Urokinase-type plasminogen activator
- uPAR
Urokinase-type plasminogen activator receptor
- ZA
Zoledronic acid
Footnotes
Conflict of Interest
None of the authors have conflicts of interest with the information presented in this manuscript.
Authors’ contributions
All named authors conceived for the study, and were involved in collecting the data and drafting and revising the manuscript. All authors read and approved the final manuscript.
References
- 1.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Dai B, Ye DW, Kong YY, Shen YJ, Wang BH. Individualized prostate biopsy strategy for Chinese patients with different prostate-specific antigen levels. Asian J Androl. 2008;10:325–31. doi: 10.1111/j.1745-7262.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- 3.Clinicopathological statistics on registered prostate cancer patients in Japan: 2000 report from the Japanese Urological Association. Int J Urol. 2005;12:46–61. doi: 10.1111/j.1442-2042.2004.00984.x. [DOI] [PubMed] [Google Scholar]
- 4.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–83. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 5.Yonou H, Ochiai A, Goya M, Kanomata N, Hokama S, Morozumi M, et al. Intraosseous growth of human prostate cancer in implanted adult human bone: relationship of prostate cancer cells to osteoclasts in osteoblastic metastatic lesions. Prostate. 2004;58:406–13. doi: 10.1002/pros.10349. [DOI] [PubMed] [Google Scholar]
- 6.Ye L, Kynaston HG, Jiang WG. Bone metastasis in prostate cancer: molecular and cellular mechanisms (Review) Int J Mol Med. 2007;20:103–11. [PubMed] [Google Scholar]
- 7.Madan RA, Arlen PM. Recent advances revolutionize treatment of metastatic prostate cancer. Future Oncol. 2013;9:1133–44. doi: 10.2217/fon.13.65. [DOI] [PubMed] [Google Scholar]
- 8.Yin L, Hu Q, Hartmann RW. Recent progress in pharmaceutical therapies for castration-resistant prostate cancer. Int J Mol Sci. 2013;14:13958–78. doi: 10.3390/ijms140713958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidegger I, Massoner P, Eder IE, Pircher A, Pichler R, Aigner F, et al. Novel therapeutic approaches for the treatment of castration-resistant prostate cancer. J Steroid Biochem Mol Biol. 2013;138:248–56. doi: 10.1016/j.jsbmb.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal N, Sonpavde G, Sternberg CN. Novel molecular targets for the therapy of castration-resistant prostate cancer. Eur Urol. 2012;61:950–60. doi: 10.1016/j.eururo.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Cher ML. Mechanisms governing bone metastasis in prostate cancer. Curr Opin Urol. 2001;11:483–8. doi: 10.1097/00042307-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197:893–5. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- 13.Trouvin AP, Goeb V. Receptor activator of nuclear factor-kappaB ligand and osteoprotegerin: maintaining the balance to prevent bone loss. Clin Interv Aging. 2010;5:345–54. doi: 10.2147/CIA.S10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baud’huin M, Duplomb L, Ruiz Velasco C, Fortun Y, Heymann D, Padrines M. Key roles of the OPG-RANK-RANKL system in bone oncology. Expert Rev Anticancer Ther. 2007;7:221–32. doi: 10.1586/14737140.7.2.221. [DOI] [PubMed] [Google Scholar]
- 15.Chinni SR, Yamamoto H, Dong Z, Sabbota A, Bonfil RD, Cher ML. CXCL12/CXCR4 transactivates HER2 in lipid rafts of prostate cancer cells and promotes growth of metastatic deposits in bone. Mol Cancer Res. 2008;6:446–57. doi: 10.1158/1541-7786.MCR-07-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonfil RD, Chinni S, Fridman R, Kim HR, Cher ML. Proteases, growth factors, chemokines, and the microenvironment in prostate cancer bone metastasis. Urol Oncol. 2007;25:407–11. doi: 10.1016/j.urolonc.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Brubaker KD, Vessella RL, True LD, Thomas R, Corey E. Cathepsin K mRNA and protein expression in prostate cancer progression. J Bone Miner Res. 2003;18:222–30. doi: 10.1359/jbmr.2003.18.2.222. [DOI] [PubMed] [Google Scholar]
- 18.Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 2010;11:23–36. doi: 10.1038/nrm2821. [DOI] [PubMed] [Google Scholar]
- 19.Edwards J. Src kinase inhibitors: an emerging therapeutic treatment option for prostate cancer. Expert Opin Investig Drugs. 2010;19:605–14. doi: 10.1517/13543781003789388. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal MA, Davidson P, Rolland F, Campone M, Xue L, Han TH, et al. Evaluation of the safety, pharmacokinetics and treatment effects of an alpha(nu)beta(3) integrin inhibitor on bone turnover and disease activity in men with hormone-refractory prostate cancer and bone metastases. Asia Pac J Clin Oncol. 2010;6:42–8. doi: 10.1111/j.1743-7563.2009.01266.x. [DOI] [PubMed] [Google Scholar]
- 21.Clarke NW, Hart CA, Brown MD. Molecular mechanisms of metastasis in prostate cancer. Asian J Androl. 2009;11:57–67. doi: 10.1038/aja.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, et al. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci. 2007;1117:209–57. doi: 10.1196/annals.1402.089. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim A, Scher N, Williams G, Sridhara R, Li N, Chen G, et al. Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clin Cancer Res. 2003;9:2394–9. [PubMed] [Google Scholar]
- 24.Prakash G, Gautam G. Optimal bone health management strategies in patients with prostate cancer. Indian J Urol. 2013;29:89–99. doi: 10.4103/0970-1591.114024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigues P, Hering FO, Meller A. Adjuvant Effect of IV Clodronate on the Delay of Bone Metastasis in High-Risk Prostate Cancer Patients: A Prospective Study. Cancer Res Treat. 2011;43:231–5. doi: 10.4143/crt.2011.43.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dearnaley DP, Mason MD, Parmar MK, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10:872–6. doi: 10.1016/S1470-2045(09)70201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goessl C, Katz L, Dougall WC, Kostenuik PJ, Zoog HB, Braun A, et al. The development of denosumab for the treatment of diseases of bone loss and cancer-induced bone destruction. Ann N Y Acad Sci. 2012;1263:29–40. doi: 10.1111/j.1749-6632.2012.06674.x. [DOI] [PubMed] [Google Scholar]
- 28.Smith MR, Egerdie B, Hernandez Toriz N, Feldman R, Tammela TL, Saad F, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–55. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–22. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ignatoski KM, Escara-Wilke JF, Dai JL, Lui A, Dougall W, Daignault S, et al. RANKL inhibition is an effective adjuvant for docetaxel in a prostate cancer bone metastases model. Prostate. 2008;68:820–9. doi: 10.1002/pros.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Virk MS, Petrigliano FA, Liu NQ, Chatziioannou AF, Stout D, Kang CO, et al. Influence of simultaneous targeting of the bone morphogenetic protein pathway and RANK/RANKL axis in osteolytic prostate cancer lesion in bone. Bone. 2009;44:160–7. doi: 10.1016/j.bone.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Virk MS, Alaee F, Petrigliano FA, Sugiyama O, Chatziioannou AF, Stout D, et al. Combined inhibition of the BMP pathway and the RANK-RANKL axis in a mixed lytic/blastic prostate cancer lesion. Bone. 2011;48:578–87. doi: 10.1016/j.bone.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong AP, Miller RE, Jones JC, Zhang J, Keller ET, Dougall WC. RANKL acts directly on RANK-expressing prostate tumor cells and mediates migration and expression of tumor metastasis genes. Prostate. 2008;68:92–104. doi: 10.1002/pros.20678. [DOI] [PubMed] [Google Scholar]
- 35.Miller RE, Roudier M, Jones J, Armstrong A, Canon J, Dougall WC. RANK ligand inhibition plus docetaxel improves survival and reduces tumor burden in a murine model of prostate cancer bone metastasis. Mol Cancer Ther. 2008;7:2160–9. doi: 10.1158/1535-7163.MCT-08-0046. [DOI] [PubMed] [Google Scholar]
- 36.Yu EY, Massard C, Gross ME, Carducci MA, Culine S, Hudes G, et al. Once-daily dasatinib: expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology. 2011;77:1166–71. doi: 10.1016/j.urology.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu EY, Wilding G, Posadas E, Gross M, Culine S, Massard C, et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7421–8. doi: 10.1158/1078-0432.CCR-09-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Araujo JC, Mathew P, Armstrong AJ, Braud EL, Posadas E, Lonberg M, et al. Dasatinib combined with docetaxel for castration-resistant prostate cancer: results from a phase 1–2 study. Cancer. 2012;118:63–71. doi: 10.1002/cncr.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antonarakis ES, Heath EI, Posadas EM, Yu EY, Harrison MR, Bruce JY, et al. A phase 2 study of KX2-391, an oral inhibitor of Src kinase and tubulin polymerization, in men with bone-metastatic castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2013;71:883–92. doi: 10.1007/s00280-013-2079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabbani SA, Valentino ML, Arakelian A, Ali S, Boschelli F. SKI-606 (Bosutinib) blocks prostate cancer invasion, growth, and metastasis in vitro and in vivo through regulation of genes involved in cancer growth and skeletal metastasis. Mol Cancer Ther. 2010;9:1147–57. doi: 10.1158/1535-7163.MCT-09-0962. [DOI] [PubMed] [Google Scholar]
- 41.Gupta A, Cao W, Chellaiah MA. Integrin alphavbeta3 and CD44 pathways in metastatic prostate cancer cells support osteoclastogenesis via a Runx2/Smad 5/receptor activator of NF-kappaB ligand signaling axis. Mol Cancer. 2012;11:66. doi: 10.1186/1476-4598-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wirth M, Heidenreich A, Gschwend JE, Gil T, Zastrow S, Laniado M, et al. A Multicenter Phase 1 Study of EMD 525797 (DI17E6), a Novel Humanized Monoclonal Antibody Targeting alphav Integrins, in Progressive Castration-resistant Prostate Cancer with Bone Metastases After Chemotherapy. Eur Urol. 2013 doi: 10.1016/j.eururo.2013.05.051. [DOI] [PubMed] [Google Scholar]
- 43.van der Horst G, van den Hoogen C, Buijs JT, Cheung H, Bloys H, Pelger RC, et al. Targeting of alpha(v)-integrins in stem/progenitor cells and supportive microenvironment impairs bone metastasis in human prostate cancer. Neoplasia. 2011;13:516–25. doi: 10.1593/neo.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinto A, Merino M, Zamora P, Redondo A, Castelo B, Espinosa E. Targeting the endothelin axis in prostate carcinoma. Tumour Biol. 2012;33:421–6. doi: 10.1007/s13277-011-0299-6. [DOI] [PubMed] [Google Scholar]
- 45.Armstrong AJ, Creel P, Turnbull J, Moore C, Jaffe TA, Haley S, et al. A phase I-II study of docetaxel and atrasentan in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2008;14:6270–6. doi: 10.1158/1078-0432.CCR-08-1085. [DOI] [PubMed] [Google Scholar]
- 46.Carducci MA, Saad F, Abrahamsson PA, Dearnaley DP, Schulman CC, North SA, et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110:1959–66. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 47.Nelson JB, Love W, Chin JL, Saad F, Schulman CC, Sleep DJ, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008;113:2478–87. doi: 10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warren R, Liu G. ZD4054: a specific endothelin A receptor antagonist with promising activity in metastatic castration-resistant prostate cancer. Expert Opin Investig Drugs. 2008;17:1237–45. doi: 10.1517/13543784.17.8.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.James ND, Caty A, Borre M, Zonnenberg BA, Beuzeboc P, Morris T, et al. Safety and efficacy of the specific endothelin-A receptor antagonist ZD4054 in patients with hormone-resistant prostate cancer and bone metastases who were pain free or mildly symptomatic: a double-blind, placebo-controlled, randomised, phase 2 trial. Eur Urol. 2009;55:1112–23. doi: 10.1016/j.eururo.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 50.James ND, Caty A, Payne H, Borre M, Zonnenberg BA, Beuzeboc P, et al. Final safety and efficacy analysis of the specific endothelin A receptor antagonist zibotentan (ZD4054) in patients with metastatic castration-resistant prostate cancer and bone metastases who were pain-free or mildly symptomatic for pain: a double-blind, placebo-controlled, randomized Phase II trial. BJU Int. 2010;106:966–73. doi: 10.1111/j.1464-410X.2010.09638.x. [DOI] [PubMed] [Google Scholar]
- 51.Nelson JB, Fizazi K, Miller K, Higano C, Moul JW, Akaza H, et al. Phase 3, randomized, placebo-controlled study of zibotentan (ZD4054) in patients with castration-resistant prostate cancer metastatic to bone. Cancer. 2012;118:5709–18. doi: 10.1002/cncr.27674. [DOI] [PubMed] [Google Scholar]
- 52.Fizazi KS, Higano CS, Nelson JB, Gleave M, Miller K, Morris T, et al. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2013;31:1740–7. doi: 10.1200/JCO.2012.46.4149. [DOI] [PubMed] [Google Scholar]
- 53.Vengalil S, O’Sullivan JM, Parker CC. Use of radionuclides in metastatic prostate cancer: pain relief and beyond. Curr Opin Support Palliat Care. 2012;6:310–5. doi: 10.1097/SPC.0b013e328355e082. [DOI] [PubMed] [Google Scholar]
- 54.Longo J, Lutz S, Johnstone C. Samarium-153-ethylene diamine tetramethylene phosphonate, a beta-emitting bone-targeted radiopharmaceutical, useful for patients with osteoblastic bone metastases. Cancer Manag Res. 2013;5:235–42. doi: 10.2147/CMAR.S35789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sartor O, Reid RH, Hoskin PJ, Quick DP, Ell PJ, Coleman RE, et al. Samarium–153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology. 2004;63:940–5. doi: 10.1016/j.urology.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 56.Fizazi K, Beuzeboc P, Lumbroso J, Haddad V, Massard C, Gross-Goupil M, et al. Phase II trial of consolidation docetaxel and samarium-153 in patients with bone metastases from castration-resistant prostate cancer. J Clin Oncol. 2009;27:2429–35. doi: 10.1200/JCO.2008.18.9811. [DOI] [PubMed] [Google Scholar]
- 57.Morris MJ, Pandit-Taskar N, Carrasquillo J, Divgi CR, Slovin S, Kelly WK, et al. Phase I study of samarium-153 lexidronam with docetaxel in castration-resistant metastatic prostate cancer. J Clin Oncol. 2009;27:2436–42. doi: 10.1200/JCO.2008.20.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tu SM, Mathew P, Wong FC, Jones D, Johnson MM, Logothetis CJ. Phase I study of concurrent weekly docetaxel and repeated samarium-153 lexidronam in patients with castration-resistant metastatic prostate cancer. J Clin Oncol. 2009;27:3319–24. doi: 10.1200/JCO.2008.20.5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jadvar H, Quinn DI. Targeted alpha-particle therapy of bone metastases in prostate cancer. Clin Nucl Med. 2013;38:966–71. doi: 10.1097/RLU.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nilsson S, Franzen L, Parker C, Tyrrell C, Blom R, Tennvall J, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–94. doi: 10.1016/S1470-2045(07)70147-X. [DOI] [PubMed] [Google Scholar]
- 61.Nilsson S, Strang P, Aksnes AK, Franzen L, Olivier P, Pecking A, et al. A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer. 2012;48:678–86. doi: 10.1016/j.ejca.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 62.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 63.Okazaki I, Nabeshima K. Introduction: MMPs, ADAMs/ADAMTSs research products to achieve big dream. Anticancer Agents Med Chem. 2012;12:688–706. doi: 10.2174/187152012802650200. [DOI] [PubMed] [Google Scholar]
- 64.Sottnik JL, Zhang J, Macoska JA, Keller ET. The PCa Tumor Microenvironment. Cancer Microenviron. 2011;4:283–97. doi: 10.1007/s12307-011-0073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stellas D, Patsavoudi E. Inhibiting matrix metalloproteinases, an old story with new potentials for cancer treatment. Anticancer Agents Med Chem. 2012;12:707–17. doi: 10.2174/187152012802650246. [DOI] [PubMed] [Google Scholar]
- 66.Annabi B, Bouzeghrane M, Currie JC, Dulude H, Daigneault L, Garde S, et al. Inhibition of MMP-9 secretion by the anti-metastatic PSP94-derived peptide PCK3145 requires cell surface laminin receptor signaling. Anticancer Drugs. 2006;17:429–38. doi: 10.1097/01.cad.0000203388.68034.06. [DOI] [PubMed] [Google Scholar]
- 67.Singh RP, Raina K, Deep G, Chan D, Agarwal R. Silibinin suppresses growth of human prostate carcinoma PC-3 orthotopic xenograft via activation of extracellular signal-regulated kinase 1/2 and inhibition of signal transducers and activators of transcription signaling. Clin Cancer Res. 2009;15:613–21. doi: 10.1158/1078-0432.CCR-08-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deng X, He G, Levine A, Cao Y, Mullins C. Adenovirus-mediated expression of TIMP-1 and TIMP-2 in bone inhibits osteolytic degradation by human prostate cancer. Int J Cancer. 2008;122:209–18. doi: 10.1002/ijc.23053. [DOI] [PubMed] [Google Scholar]
- 69.Shariat SF, Roehrborn CG, McConnell JD, Park S, Alam N, Wheeler TM, et al. Association of the circulating levels of the urokinase system of plasminogen activation with the presence of prostate cancer and invasion, progression, and metastasis. J Clin Oncol. 2007;25:349–55. doi: 10.1200/JCO.2006.05.6853. [DOI] [PubMed] [Google Scholar]
- 70.Fritz V, Noel D, Bouquet C, Opolon P, Voide R, Apparailly F, et al. Antitumoral activity and osteogenic potential of mesenchymal stem cells expressing the urokinase-type plasminogen antagonist amino-terminal fragment in a murine model of osteolytic tumor. Stem Cells. 2008;26:2981–90. doi: 10.1634/stemcells.2008-0139. [DOI] [PubMed] [Google Scholar]
- 71.Juarez P, Guise TA. TGF-beta in cancer and bone: implications for treatment of bone metastases. Bone. 2011;48:23–9. doi: 10.1016/j.bone.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 72.Mishra S, Tang Y, Wang L, deGraffenried L, Yeh IT, Werner S, et al. Blockade of transforming growth factor-beta (TGFbeta) signaling inhibits osteoblastic tumorigenesis by a novel human prostate cancer cell line. Prostate. 2011;71:1441–54. doi: 10.1002/pros.21361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu Z, Gupta J, Zhang Z, Gerseny H, Berg A, Chen YJ, et al. Systemic delivery of oncolytic adenoviruses targeting transforming growth factor-beta inhibits established bone metastasis in a prostate cancer mouse model. Hum Gene Ther. 2012;23:871–82. doi: 10.1089/hum.2012.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wan X, Li ZG, Yingling JM, Yang J, Starbuck MW, Ravoori MK, et al. Effect of transforming growth factor beta (TGF-beta) receptor I kinase inhibitor on prostate cancer bone growth. Bone. 2012;50:695–703. doi: 10.1016/j.bone.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng CJ, Ye XC, Vakar-Lopez F, Kim J, Tu SM, Chen DT, et al. Bone microenvironment and androgen status modulate subcellular localization of ErbB3 in prostate cancer cells. Mol Cancer Res. 2007;5:675–84. doi: 10.1158/1541-7786.MCR-06-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fizazi K, Sikes CR, Kim J, Yang J, Martinez LA, Olive MC, et al. High efficacy of docetaxel with and without androgen deprivation and estramustine in preclinical models of advanced prostate cancer. Anticancer Res. 2004;24:2897–903. [PubMed] [Google Scholar]
- 77.Pfitzenmaier J, Quinn JE, Odman AM, Zhang J, Keller ET, Vessella RL, et al. Characterization of C4-2 prostate cancer bone metastases and their response to castration. J Bone Miner Res. 2003;18:1882–8. doi: 10.1359/jbmr.2003.18.10.1882. [DOI] [PubMed] [Google Scholar]
- 78.Rosol TJ, Tannehill-Gregg SH, Corn S, Schneider A, McCauley LK. Animal models of bone metastasis. Cancer Treat Res. 2004;118:47–81. doi: 10.1007/978-1-4419-9129-4_3. [DOI] [PMC free article] [PubMed] [Google Scholar]