Abstract
Rationale
Whereas cannabinoid CB1 receptors have long been known to contribute to the rewarding effects and dependence liability of many drugs of abuse, recent studies have implicated the involvement of cannabinoid CB2 receptors.
Objective
Here, we evaluated the role of CB2 receptors in the rewarding properties of nicotine, as assessed in the conditioned place preference (CPP) paradigm and mecamylamine-precipitated withdrawal in nicotine dependent mice.
Methods
Using complementary pharmacological and genetic approaches, we investigated the involvement of CB2 receptors in nicotine- and cocaine-induced CPP in mice and mecamylamine-precipitated withdrawal in nicotine-dependent mice. We also determined whether deletion of CB2 receptors affects nicotine-induced hypothermia and hypoalgesia.
Results
Nicotine-induced (0.5 mg/kg) CPP was completely blocked by selective CB2 antagonist, SR144528 (3 mg/kg) in wild-type mice, and was absent in CB2 (−/−) mice. Conversely, the CB2 receptor agonist, O-1966 (1, 3, 5, 10, 20 mg/kg) given in combination with a subthreshold dose of nicotine (0.1 mg/kg) elicited a place preference. In contrast, O-1966 (20 mg/kg) blocked cocaine (10 mg/kg)-induced CPP in wild type mice, while CB2 (−/−) mice showed unaltered cocaine CPP. CB2 (+/+) and (−/−) nicotine-dependent mice showed almost identical precipitated withdrawal responses and deletion of CB2 receptor did not alter acute somatic effects of nicotine.
Conclusions
Collectively, these results indicate that CB2 receptors are required for nicotine-induced CPP in the mouse, while it is not involved in nicotine withdrawal or acute effects of nicotine. Moreover, these results suggest that CB2 receptors play opposing roles in nicotine- and cocaine-induced CPP.
Keywords: Cannabinoid, CB2, Conditioned place preference, Nicotine, Mecamylamine, Reinforcement, Reward, Withdrawal
Introduction
Tobacco use remains a leading preventable cause of mortality and morbidity worldwide (Benowitz 2008; Changeux 2010). Nicotine, the principal psychoactive component of tobacco, significantly contributes to the reinforcing effects, as well as the dependence and addiction liability of tobacco smoking (Stolerman and Jarvis 1995; Castañé et al. 2005). Although available treatments for tobacco smoking cessation, including nicotine substitution therapy, bupropion, and varenicline, possess efficacy, relapse rates remain high. Thus, a great need remains for the development of novel and effective therapeutic approaches to treat nicotine addiction.
Accumulating evidence indicates that the endocannabinoid system (EC) plays an important role in the reinforcing properties of drugs of abuse, predominantly through neuromodulatory function in the mesolimbic system (Maldonado et al. 2006; Parolaro et al. 2007; Solinas et al. 2008; Muldoon et al. 2013). The EC system consists of two cannabinoid receptor subtypes, as well as endogenous ligands and enzymes responsible for their biosynthesis and degradation (Di Marzo 2009). Major endogenous cannabinoids: 2-arachidonoylglycerol (2-AG) (Mechoulam et al. 1995; Sugiura et al. 1995) and N-arachidonoylethanolamine (anandamide; AEA) (Devane et al. 1992) are synthesized “on demand” and act as retrograde messengers that are rapidly degraded by the respective enzymes, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) (Cravatt et al. 1996, 2001; Dinh et al. 2002). Both AEA and 2-AG bind primarily to G-protein coupled cannabinoid receptors: CB1 and CB2 (Matsuda et al. 1990; Munro et al. 1993). The CB1 cannabinoid receptor, one of most abundant G-coupled receptors in the central nervous system (CNS), contributes to various aspects of rewarding properties of drugs of abuse, including nicotine (Le Foll and Goldberg 2004; Merritt et al. 2008; Gamaleddin 2012a, b). Importantly, CB1 receptor blockade attenuates the reinforcing effects of nicotine in rodents (Cohen et al. 2005a; Merritt et al. 2008; Le Foll et al. 2008).
Although initial studies did not detect CB2 receptor expression in brain (Munro et al. 1993), growing evidence demonstrates the expression of these receptors in the CNS, with their presence detected on endothelial cells, microglia, and neurons (Pettit et al. 1998; Cabral and Marciano-Cabral 2005; Van Sickle et al. 2005; Gong et al. 2006; Onaivi et al. 2008; Atwood and Mackie 2010; Atwood et al. 2012). CB2 receptor expression has been found in several brain structures such as: hippocampus, cerebral cortex, striatum, amygdala and brain stem (Van Sickle et al. 2005; Gong et al. 2006; Onaivi et al. 2006, 2008; García-Gutiérrez et al. 2010). Emerging evidence has implicated the involvement of CB2 receptors in the rewarding properties of substances of abuse, including alcohol and cocaine (Onaivi et al. 2008; Xi et al. 2011; Aracil-Fernández et al. 2012). Chronic treatment with cocaine was shown to increase expression of CB2 receptor gene in the brain of mice (Onaivi et al. 2008). Moreover, selective CB2 receptor agonists reduced self-administration of cocaine (Xi et al. 2011) and CB2 receptor overexpression reduced cocaine motor sensitization (Aracil-Fernández et al. 2012) in mice. Curiously, the CB2 receptor antagonist SR144528 decreased cocaine-induced reinstatement of cocaine self-administration in rats (Adamczyk et al. 2012). However, neither CB2 receptor agonists nor antagonists altered nicotine self-administration or nicotine seeking behavior in rats (Gamaleddin et al. 2012a, b), suggesting a differential role of this receptor in cocaine and nicotine drug taking behavior.
The purpose of the present study was to assess the involvement of CB2 receptors in mouse models of nicotine reward and dependence. There were three primary objectives of this study. First, we assessed the effects of a selective CB2 receptor antagonist (SR144528) and agonist (O-1966) on nicotine reward using the mouse conditioned place preference (CPP) paradigm. Nicotine CPP was also determined in CB2 (−/−) and their wild type counterparts. As a comparison, we assessed O-1966 in cocaine CPP. Given the findings that CB2 receptor agonists reduced cocaine self-administration and other pharmacological effects of cocaine, we hypothesized that O-1966 would similarly attenuate nicotine CPP in the mouse. Second, we investigated the role of CB2 receptors on physical (i.e., somatic signs and hyperalgesic responses) and affective (i.e., elevated plus maze) signs of nicotine withdrawal in mice. Third, we examined whether acute pharmacological effects of nicotine (i.e., antinociception and hypothermia) would be altered in CB2 (−/−) mice.
Methods and materials
Drugs and chemicals
(−)-Nicotine hydrogen tartrate salt and mecamylamine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). The CB 2 antagonist N-[(1S)-endo-1,3,3,-trimethylbicyclo[2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide (SR144528) (1 or 3 mg/kg) and cocaine were obtained from the National Institute on Drug Abuse (Bethesda, MD). A selective CB2 receptor agonist O-1966, a dimethoxy-resorcinol-dimethylheptyl analog that shows approximately 225 fold higher CB2 selectivity (Ki=22.5 nM) over CB1 receptor, was synthesized as described by Wiley et al. (2002) (Organix, Inc., Woburn, MA). The compound was characterized on the basis of its 1H NMR (Jeol Eclipse 300 MHz; Jeol USA, Inc., Peabody, MA) profile, TLC, and elemental analyses. Purity of the compound was >98 % that was determined by elemental analysis. Biological activity of O-1966 was assessed by determining its affinity for CB1 and CB2 using [3H] CP55,940 binding to mouse and rat brain membranes and Chinese hamster ovary cells expressing the human CB2 receptor (CHO-hCB2 cells), respectively (Wiley et al. 2002). In vitro functional activity was determined in mouse brain membranes and CHO-hCB2 cells using [35S] GTPγS binding (Zhang et al. 2007). Activity of O-1699 was also verified in vivo, in behavioral and immunological assays (Wiley et al. 2002; Zhang et al. 2007; Ramirez et al. 2012).
Nicotine and mecamylamine were dissolved in physiological saline (0.9 % sodium chloride). SR144528 and O-1966 were dissolved in ethanol, followed by addition of Alkamuls-620 (Sanofi-Aventis, Bridgewater, NJ), and diluted with 0.9 % saline to form a vehicle mixture of ethanol-Alkamuls-620-saline in a ratio of 1:1:18. All injections were administered in a volume of 10 ml/kg. Nicotine and mecamylamine were administered via subcutaneous injection (s.c.), while SR144528, O-1966, and cocaine were given via the intraperitoneal (i.p.) route of administration. The doses of nicotine used were previously shown to produce reliable acute pharmacological effects (Damaj et al. 1999), reliable CPP (Walters et al. 2006; Grabus et al. 2006; Kota et al. 2007), and reliable dependence (Damaj et al. 2003; Jackson et al. 2008). The selection of 10 mg/kg cocaine was based on the results of several reports showing that it leads to CPP (Sora et al. 2001; Hnasko et al. 2007). The selection of SR144528 doses was based on our previous report showing that 3 mg/kg SR144528 significantly antagonizes the antinociceptive effects of a CB2 receptor agonist, it is frequently used dose and effective in various assays (Cravatt, et al. 2004; Kinsey et al. 2011). All doses are expressed as the free base of the drug.
Animals
Subjects consisted of male C57BL/6 J mice (The Jackson Laboratory, Bar Harbor, ME) that were approximately 10 weeks of age at the beginning of the study. In addition, male and female CB2 (−/−) mice, as well as CB2 wild type littermate control mice, were obtained from the Center Transgenic Colony at Virginia Commonwealth University. CB2 (−/−) mice were backcrossed onto a C57BL/6 J back-ground for at least 8 generations. Mutant and wild type mice were derived from CB2 (+/−) breeding pairs. PCR genotyping of the CB2 (−/−) mice was performed with DNA extracted from the mouse tail tip (about 2–3 mm) with use of KAPA Mouse Genotyping Kit (KAPABIOSYSTEM, Boston, USA). The sequences of primers used were: CB2GS1: GAC TAG AGC TTT GTA GGT AGG CGG G, CB2GS2: GGA GTT CAA CCC CAT GAA GGA GTA C, CB2NEO: GGG GAT CGA TCC GTC CTG TAA GTC T. The PCR conditions were: 95 °C for 180 s, then 35 cycles at 95 °C for 15 s, 62 °C for 15 s, and 72 °C for 15 s.
Animals were maintained on a 12/12 h light/dark cycle (0600 hours on/1800 hours off) in a temperature (20–22 °C) and humidity (55±10 %) controlled facility. Subjects were housed five mice per cage with ad libitum access to food and water. The animal facility was approved by the Association for Assessment and Accreditation of Laboratory Animal Care. Experiments were performed during the light cycle.
All animal protocols were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory and Animal Resources 2011). After testing was completed, all mice were humanely euthanized via CO2 asphyxia, followed by rapid cervical dislocation.
Nicotine and cocaine CPP studies
An unbiased mouse CPP paradigm was utilized in all studies as described in Kota et al. (2007). In brief, mice were placed in enriched environment and handled for three days prior to initiation of CPP testing. The CPP apparatus (Med-Associates, St. Albans, VT, ENV3013) consisted of white and black chambers (20×20×20 cm each), which differed in floor texture (white mesh and black rod: Med-Associates, ENV-3013WM and ENV-3013BR) to help the mice further differentiate between the two environments. Place conditioning chambers were separated by a smaller intermediate compartment with a smooth PVC floor and partitions that allowed access to the black and white chambers. On day 1 (preconditioning day), animals were confined to the intermediate compartment for a 5-min habituation period and then partitions were lifted, and mice allowed to move freely between compartments for 15 min. Time spent in each compartment was recorded and used to establish baseline chamber preferences, if any. Mice were separated into experimental and control groups such that initial chamber biases were approximately balanced. On days 2–4 (conditioning days), twice per day, mice were injected with vehicle or drug and subsequently paired with either the white or black chamber, where they were allowed to roam for 15 min. Vehicle-treated animals were paired with saline in both chambers and drug-treated animals received saline in one chamber and drug in the opposite chamber. Pairing of the drug with either the black or white chamber was randomized within the drug-treated group of mice. Animals in the drug group received drug each day. Injections were counterbalanced so that some mice received drug in the morning, others in the late afternoon. On day 5 (test day), mice did not receive an injection. They were placed into the center chamber for 5 minutes, the partitions were lifted, and they were allowed to roam freely for 15 min. Locomotor activity counts and time spent on each side were recorded. Data were expressed as time spent on the drug-paired side post-conditioning minus time spent on the drug-paired side preconditioning. A positive number indicated a preference for the drug-paired side, whereas a negative number implied an aversion to the drug-paired side. A number of zero or near zero indicated no preference for either side.
Three experiments evaluating the role of CB2 receptors in nicotine CPP were conducted and two experiments evaluated the role of CB2 receptors in cocaine CPP. The first experiment assessed the CB2 antagonist SR144528 (1 or 3 mg/kg, i.p.) versus vehicle 15 min before nicotine (0.5 mg/kg, s.c.). The second experiment examined nicotine (0.1 and 0.5 mg/kg) or cocaine (10 mg/kg, i.p.) CPP in CB2 (−/−) and CB2 (+/+) mice using the procedure described above. The third experiment evaluated the effects of vehicle or the CB2 agonist O-1966 (1, 3, 5, 10 and 20 mg/kg) 15 min before nicotine (0.1 mg/kg, s.c.) or O-1966 (20 mg/kg) 15 min before cocaine (10 mg/kg). On days 2–4 subjects received conditioning sessions in which the saline group received saline in both sides of the boxes and drug groups received nicotine or cocaine on one of the sides and saline on the opposite side.
Chronic nicotine administration protocol
CB2 (−/−) and (+/+) mice were anesthetized with sodium pentobarbital (45 mg/kg, i.p.) and implanted with Alzet osmotic mini pumps [model 1007D (7 days) Durect Corporation, Cupertino, CA] filled with (−)-nicotine or saline solution as described in Jackson et al. (2008). The concentration of nicotine was adjusted according to animal weight and mini pump flow rate. For withdrawal studies, mice received nicotine at 24 mg/kg/day for 7 days. We have previously demonstrated that this dosing regimen is sufficient for development of physical dependence in mice (Damaj et al. 2003; Jackson et al. 2011; Alajaji et al. 2013). Due to rapid nicotine metabolism in mice (Petersen et al. 1984; Damaj et al. 2007), we employed a relatively high nicotine dose in order to produce similar nicotine plasma levels as observed in heavy smokers (Benowitz 2010; AlSharari et al. 2013).
Nicotine withdrawal assessment
Withdrawal studies were conducted as previously described in Jackson et al. (2008). On the morning of day 8, mice were injected with the non-selective nicotinic antagonist, mecamyl-amine (2 mg/kg, s.c.) and assessed for withdrawal responses 15 min later. The mice were first evaluated for 5 min in the plus maze test for anxiety-related behavior, followed by a 20-min observation of somatic signs that included paw and body tremors, head shakes, backing, jumps, curls, and ptosis. The specific testing sequence was chosen based on our prior studies showing that this order of testing reduced within-group variability and produced the most consistent results (Jackson et al. 2008). An observer blinded to the experimental treatments evaluated the animals.
Acute nicotine assessment
CB2 (−/−) and CB2 (+/+) mice were injected with either saline or nicotine (0.5 or 2.5 mg/kg, s.c.). These two doses were chosen because they reflect approximate ED20 and ED80 values of the nicotine dose response curve in the antinociceptive response (Damaj et al. 1999). Antinociception was measured 5 min after nicotine injection and changes in body temperature were measured 30 min after injection. These pretreatment times are based on nicotine time-course actions, and reflect the Tmax responses (Damaj et al. 1999).
Tail-flick test
Antinociception was assessed using the tail-flick test developed by D’Amour and Smith (1941). Mice were lightly restrained, and a radiant heat source was directed onto the upper portion of the tail. A control response (2–4 s) was determined for each mouse before treatment, and test latency was determined 5 min after drug administration. The apparatus had an automatic cut-off of 10 s to minimize tissue damage. Antinociceptive responses were expressed as the mean±SEM of the maximum latency after drug treatment.
Body temperature
Rectal temperature was measured using a thermistor probe (inserted 24 mm) and digital thermometer (YSI Inc., Yellow Springs, OH). Readings were taken just before and at 30 min after nicotine injection. The difference in rectal temperature before and after treatment was calculated for each mouse. The ambient temperature of the laboratory varied from 21–24 °C from day to day.
Statistical analysis
Data were analyzed using two-way analysis of variance (ANOVA) with Bonferroni post hoc comparisons when appropriate. p values of <0.05 were considered to be statistically significant.
Results
Disruption of CB2 receptor signaling blocks the rewarding effects of nicotine in the CPP test
As shown in Fig. 1, nicotine (0.5 mg/kg) produced a robust CPP [nicotine main effect: F(1,53)=14.4, p<0.01]. The CB2 receptor antagonist, SR144528 partially prevented this effect at 1 mg/kg and completely blocked it at 3 mg/kg [SR144528 main effect; F(2,53)=3.6, p<0.05]. SR144528 (1 and 3 mg/kg) did not affect place preference in the absence of nicotine. Similarly, CB2 (−/−) mice did not display a nicotine CPP, while CB2 (+/+) mice showed a significant increase in the preference for the 0.5 mg/kg nicotine associated compartment [interaction between nicotine administration and genotype: F(1,33)=11.0, p<0.01]. However, 0.1 mg/kg nicotine did not produce a significant preference in either genotype [p=0.57] (Fig. 2). These results suggest that CB2 receptors play a necessary role in the development of nicotine CPP. None of the drug conditions significantly affected locomotor activity during CPA training (Table 1).
Fig. 1.
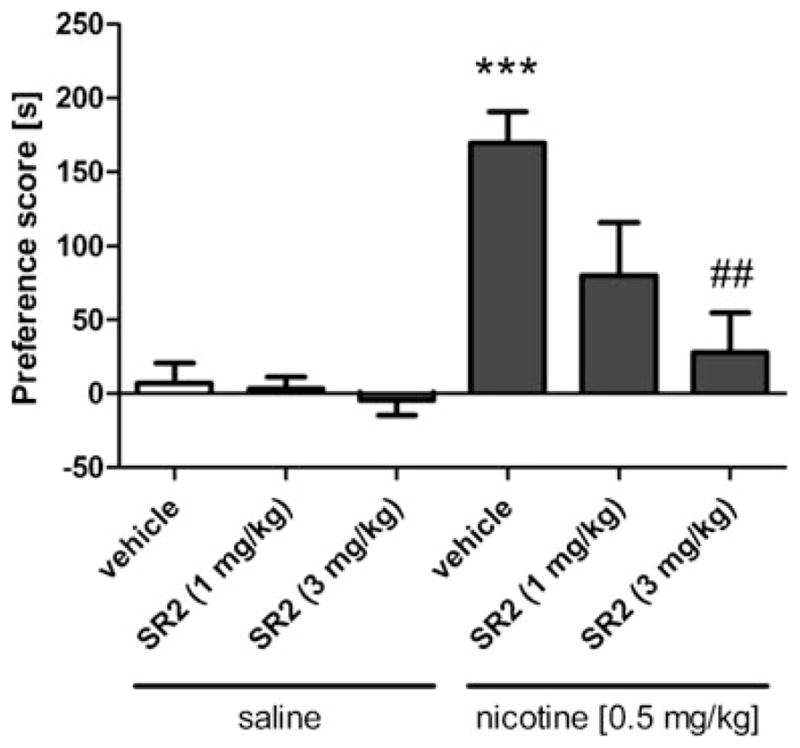
Nicotine (0.5 mg/kg) produces a conditioned place preference that is fully blocked by the CB2 receptor antagonist SR144528 at 3 mg/kg and partially blocked at 1 mg/kg. Data are depicted as mean±SEM, n=7–20 mice per group; ***p<0.01 vs vehicle + saline, ##p<0.01 vs vehicle + nicotine
Fig. 2.
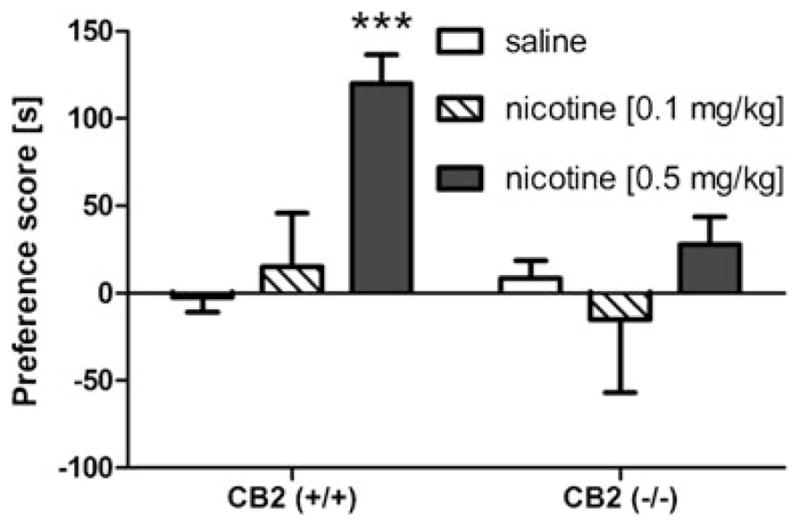
Nicotine-induced (0.5 mg/kg) conditioned place preference is absent in CB2 (−/−) mice. Low dose nicotine (0.1 mg/kg) does not produce place preference in wild-type or CB2 (−/−) mice. Data are depicted as mean±SEM, n=7–12 mice per group; ***p<0.001 vs saline
Table 1.
Activity counts (mean±SEM, n=8) in the drug-paired or control compartment during assessment of SR144528 or vehicle on nicotine CPP
| Treatment group | Activity counts |
|---|---|
| Vehicle–Saline | 513.5±73.6 |
| SR144528 (1 mg/kg)–Saline | 483.7±150.6 |
| SR144528 (3 mg/kg)–Saline | 528.3±97.6 |
| Vehicle–Nicotine (0.5) | 463.4±139.1 |
| SR144528 (1 mg/kg)–Nicotine (0.5 mg/kg) | 471.3±111.4 |
| SR144528 (3 mg/kg)–Nicotine (0.5 mg/kg) | 533.3±114.4 |
Values represent the total activity counts in the drug-paired compartment on test day for each group and are presented as the average activity count on test day (postconditioning day)±SEM
CB2 receptor agonist enhances rewarding properties of nicotine
In this experiment, the CB2 receptor selective agonist O-1966 (1–20 mg/kg, i.p.) was employed to assess whether CB2 receptor stimulation would enhance the rewarding effects of a subthreshold dose of nicotine (0.1 mg/kg) in the CPP paradigm. As can be seen in Fig. 3, O-1966 enhanced the rewarding properties of an inactive dose of nicotine in the CPP assay in a dose-related manner, with 5 or 10 mg/kg O-1966 in combination with nicotine (0.1 mg/kg) producing a significant place preference [O-1966 by nicotine interaction: F(5,94)=2.32, p<0.05]. No changes in locomotor activity were observed during CPP training (Table 2).
Fig. 3.
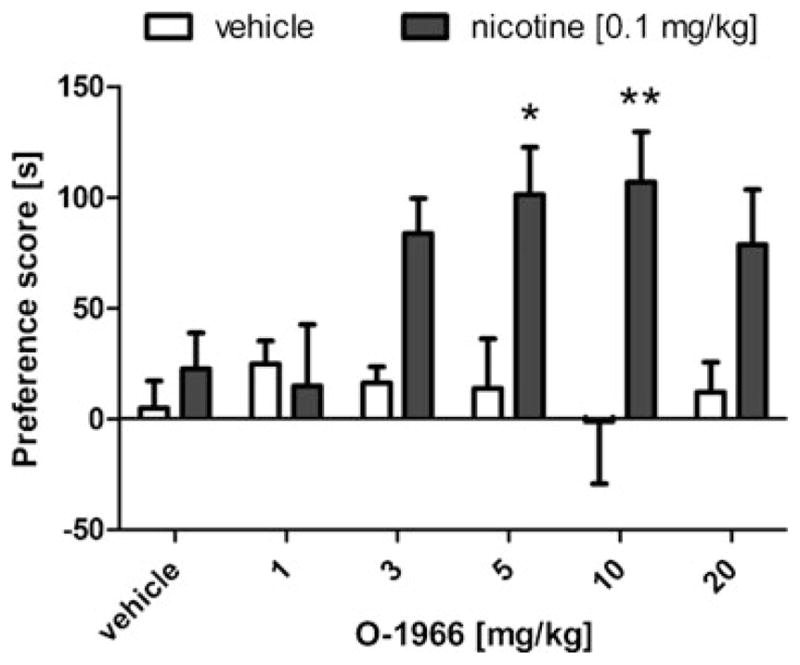
Combination of the CB2 receptor agonist, O-1966, and a subthreshold dose of nicotine (0.1 mg/kg) produces a conditioned place preference. Data are depicted as mean±SEM, n=7–20 mice per group; *p<0.05, **p<0.01 vs vehicle + O-1966
Table 2.
Activity counts (mean±SEM, n=8) in the drug-paired or control compartment during assessment of O-1966 or vehicle in nicotine CPP
| Treatment group | Activity counts |
|---|---|
| Vehicle–Saline | 489.2±41.5 |
| O-1966 (1 mg/kg)–Saline | 513.5±100.1 |
| O-1966 (10 mg/kg)–Saline | 488.3±86.4 |
| Vehicle–Nicotine (0.1 mg/kg) | 481.4±69.6 |
| O-1966 (1 mg/kg)–Nicotine (0.1 mg/kg) | 495.8±145.4 |
| O-1966 (10 mg/kg)–Nicotine (0.1 mg/kg) | 488.3±74.5 |
Values represent the total activity counts in the drug-paired or respective control compartment on the test day for each group and are presented as the average activity count on test day (postconditioning day)±SEM
CB2 receptor agonist inhibits rewarding properties of cocaine
We next assessed the effects of O-1966 on cocaine CPP. Consistent with the results of Xi et al. (2011), O-1966 (20 mg/kg) significantly blocked the development of cocaine CPP [Fig. 4a; interaction between cocaine and O-1966: F(1,27)=17.9, p< 0.001]. In the absence of O-1966, cocaine-induced CPP was present in both CB2 (+/+) and CB2 (−/−) mice [cocaine main effect: F(1,22)=20.4; p<0.001] and no significant effect of genotype [p=0.98] or interaction between genotype or cocaine treatment [p=0.73] was found (Fig. 4b). These results of the data depicted in Figs. 3 and 4, suggest opposing roles of the CB2 receptor in nicotine and cocaine CPP.
Fig. 4.
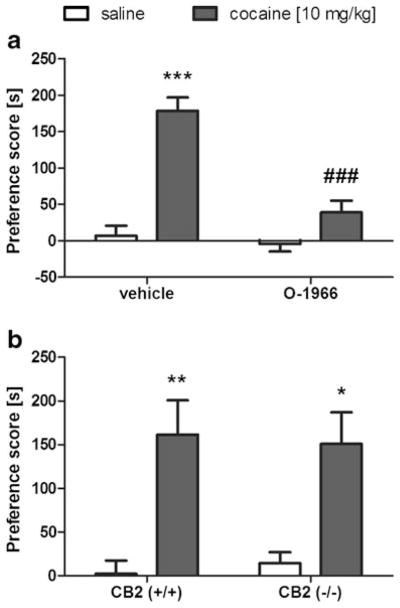
Involvement of CB2 receptor in cocaine-induced CPP. a O-1966 (20 mg/kg) suppresses cocaine-induced conditioned place preference. b Cocaine-induced conditioned place preference (10 mg/kg, i.p.) is present in wild-type and CB2 (−/−) mice. Data are depicted as mean±SEM, n=5–8; *p<0.05, **p<0.01, ***p<0.001 vs saline, and ###p<0.01 vs cocaine pretreated with vehicle
Disruption of CB2 signaling does not affect nicotine withdrawal
In this experiment, we examined whether CB2 receptors play a role in somatic and affective signs of withdrawal in nicotine-dependent mice. CB2 (−/−) and (+/+) mice were implanted with nicotine mini pumps and then challenged with mecamylamine (2 mg/kg, s.c.) to precipitate withdrawal. Subjects were tested for somatic withdrawal responses, anxiogenic-like effects in the elevated plus maze, and hyperalgesia in the hot plate test. As shown in Fig. 5a, CB2 receptors are not required for the expression of anxiogenic-like withdrawal responses in the elevated plus maze, as indicated by pronounced decreases in open arm time, irrespective of genotype [nicotine main effect: F(1,20)=255; p<0.001; genotype main effect: p=0.30; and nicotine by genotype interaction: p=0.11 (Fig. 5a). Time spent in the closed arm, time spent in the center, and numbers of head dips and crosses between arms (indicating no effect on locomotor activity) were not affected by either nicotine withdrawal or genotype (data not shown). In addition, mice implanted with nicotine mini pumps and challenged with mecamylamine exhibited an equivalent magnitude of total withdrawal signs [Fig. 5b; F(1,20)=137.0, p< 0.001] compared to wild-type animals (no effect of genotype or interaction was found, p=0.65). Similarly, separate analyses of the individual somatic withdrawal signs (i.e., paw tremors, backing, head shakes, body tremors) did not differ between both genotypes (data not shown). Finally, CB2 (−/−) mice displayed significant hyperalgesic effects [F(1,20)=64.6; p<0.001], regardless of genotype [genotype main effect: p=1; genotype by nicotine interaction; p=1] (Fig. 5c). These results suggest that CB2 receptors are not necessary for the expression of precipitated nicotine withdrawal signs.
Fig. 5.
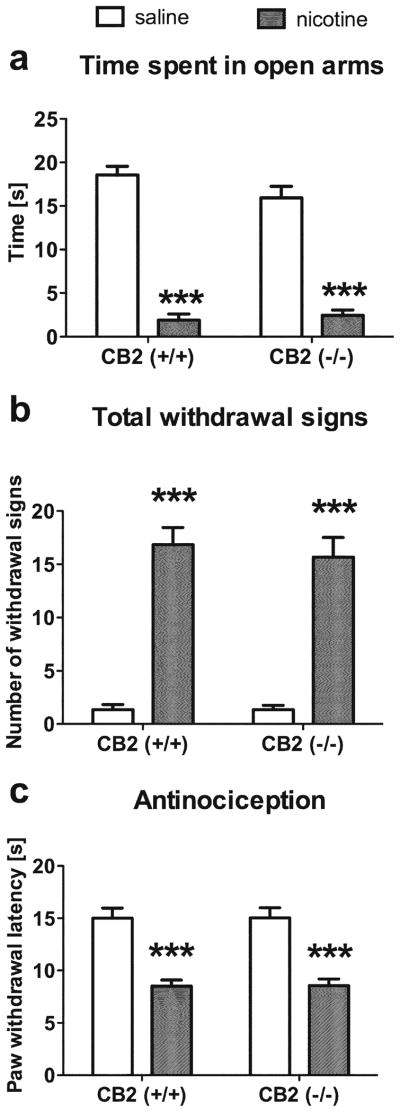
CB2 receptors are not required for the expression of mecamylamine-precipitated withdrawal responses in nicotine-dependent mice. CB2 (−/−) and (+/+) mice implanted with nicotine mini pumps and challenged with mecamylamine (2 mg/kg, s.c.) displayed similar: a anxiogenic-like responses, as reflected by a pronounced decrease of time spent in the open arms of elevated plus maze test, b total number of somatic withdrawal signs, and c hyperalgesic responses in the hot plate test. Data are depicted as mean± SEM, n=6 per group; ***p<0.001 vs saline for each respective genotype
CB2 receptors are not required for nicotine-induced antinociception and hypothermia
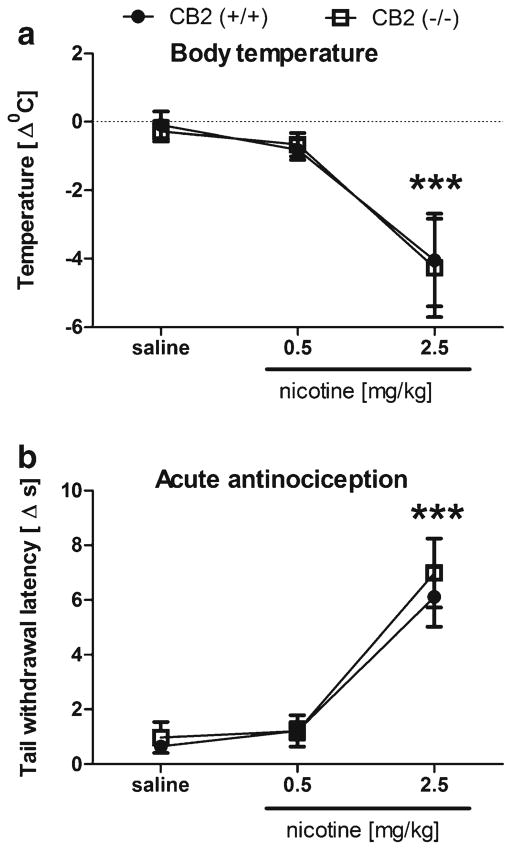
To determine whether CB2 receptors are necessary for acute pharmacological effects of nicotine, we examined whether nicotine would elicit antinociceptive and hypothermic effects in CB2 (−/−) mice. As shown in Fig. 6, nicotine elicited significant decreases in body temperature [F(2,38)=97.9; p<0.001] and elevations in tail-flick latency [F(2,39)=37.2; p<0.001], regardless of genotype.
Fig. 6.
The CB2 receptor is not necessary for nicotine-induced a hypothermia and b antinociception. CB2 (−/−) and (+/+) mice displayed nearly identical responses to nicotine. Data are depicted as mean ± SEM, n=6–9; ***p<0.001 vs saline
Discussion
The results of the present study provide the first evidence supporting the involvement of CB2 receptors in the rewarding properties of nicotine as measured in the CPP assay. Using pharmacological and genetic approaches, we demonstrate that the CB2 receptor is required for nicotine-induced CPP. Conversely, administration of the CB2 receptor agonist O-1966 increased the rewarding properties of a subthreshold dose of nicotine. Similarly, previous studies showed that disruption of CB1 receptor function through pharmacological blockade or genetic deletion inhibits nicotine-induced CPP (Le Foll and Goldberg 2004; Cohen et al. 2005a; Merritt et al. 2008; Muldoon et al. 2013). Thus, both the CB1 receptor and CB2 receptor appear to contribute to the rewarding properties of nicotine.
The role that CB2 receptors play in modulating the pharmacological effects of nicotine appears to be limited to CPP, as these receptors were not required for the acute antinociceptive and hypothermic effects of nicotine. Additionally, CB2 receptors were not necessary for various aspects of nicotine withdrawal, including anxiogenic-like responses, hyperalgesia, and somatic signs of withdrawal. Likewise, CB1 receptors are required for rewarding properties of nicotine in CPP paradigm, but do not appear necessary for the expression of nicotine withdrawal responses (Merritt et al. 2008).
In contrast to our observation that CB2 receptors are required for rewarding properties of nicotine in the CPP test, Gamaleddin et al. (2012a, b) reported no effects of a CB2 receptor agonist or antagonist on nicotine taking and nicotine seeking in a rat self-administration model. The apparent contradictory results between Gamaleddin et al. and the present study may be a consequence of species differences. Similarly, species differences have been found with respect to FAAH inhibitors on the effects of nicotine in various models of drug reward. For example, while Merritt et al. (2008) found that FAAH compromised mice displayed enhanced nicotine-induced CPP in the mouse, Scherma et al. (2008) reported that FAAH inhibition interfered with the rewarding properties of nicotine in the rat. Interestingly, CB1 receptor activation drives enhanced nicotine-induced CPP in mice treated with FAAH inhibitors, while PPARα receptors mediate the anti-reward-like effects in the rat (Luchicchi et al. 2010; Muldoon et al. 2013). Accordingly, differential distribution and function of cannabinoid receptors throughout the CNS (McPartland et al. 2007) may contribute to the opposing roles of the CB2 receptor in the rewarding properties of nicotine. Alternatively, the opposing role of the CB2 receptor between the two studies may be accounted by distinct neural substrates mediating the behaviors assessed in the models. Both paradigms differ substantially, since CPP represents a Pavlovian conditioning paradigm in which the drug is paired with a specific chamber and rewarding properties are inferred if subjects spend more time in the drug paired chamber than the vehicle paired chamber. Thus, the CB2 receptor may play a role in learning the association between nicotine and the context and/or the rewarding properties of nicotine. On the other hand, the self-administration paradigm evaluates drug taking, which is strongly associated with motivation to consume the stimulus and “wanting” of the drug (Berridge et al. 2009). Self-administration studies require extensive operant training of at least 15–20 days of nicotine administration. However, in the present CPP study, mice received only three injections of nicotine prior to testing. Several studies indicate dissociation between CPP and self-administration paradigm (Bardo et al. 1999; Deroche et al. 1999; Bardo and Bevins 2000), including different neurochemical substrates of those behaviors. For example, multiple studies (for review: Bardo and Bevins 2000) have demonstrated that D2 receptor antagonists attenuate cocaine self-administration, but do not affect cocaine-induced CPP. Further studies are necessary to understand the differences of CB2 receptor agonists between nicotine self-administration in the rat and nicotine-induced CPP.
Based on the report of Xi et al. (2011), showing that a CB2 receptor agonist reduce cocaine self-administration, we initially hypothesized that O-1966 would reduce nicotine CPP. Surprisingly, this CB2 receptor agonist enhanced nicotine CPP. Because the facilitatory effect of O-1966 on nicotine CPP is contradictory to the observation that CB2 receptor agonists attenuate cocaine self-administration (Xi et al. 2011,) we evaluated the effects of O-1966 in cocaine CPP to ascertain whether the effects of O-1966 in modulating place preferences are drug dependent. The results of the present study showing that the CB2 agonist O-1966 blocks cocaine-induced CPP are consistent with a recent report by Xi et al. (2011), who demonstrated that the CB2 receptor agonist JWH133 inhibited intravenous cocaine self-administration. Moreover, both studies show that cocaine-induced CPP or cocaine self-administration is not altered in CB2 (−/−) mice or wild-type mice treated with a CB2 receptor antagonist. In addition, the finding of Aracil-Fernández et al. (2012) that CB2 receptor overexpressing mice display phenotypic decrements in the acquisition of cocaine self-administration is consistent with the notion that enhanced CB2 signaling reduces reward-like effects of cocaine. Thus, CB2 receptors differentially regulate the rewarding properties of cocaine and nicotine.
These findings taken together indicate that distinct mechanisms of action account for the differential role that CB2 receptors play in the rewarding properties of cocaine and nicotine. Cocaine directly inhibits transport of dopamine (DA), serotonin, and norepinephrine, which results in a prolonged signaling of these monoamines (Giros et al. 1996; Sora et al. 2001). On the other hand, nicotine stimulates nicotinic cholinergic receptors located on dopaminergic cells in the VTA. Nicotinic receptors are also present on GABA-ergic interneurons or glutamatergic cells localized both within (i.e., VTA and prefrontal cortex) as well as outside the mesolimbic system (e.g., habenula), that indirectly regulate DA release in NAc (Changeux 2010; Baldwin et al. 2011; McCallum et al. 2012). Therefore, the effects of nicotine in the different models of reward may be a consequence of neurochemical pathways involving structures outside the mesolimbic system or monoaminergic pathways (Cohen et al. 2005b). In addition, the disparate effects of CB2 receptors in nicotine and cocaine models of reward may result from direct versus indirect regulation of DA release from receptor pools located at different levels of mesolimbic circuitry. Future studies are required to elucidate the underlying mechanisms mediating the differential effects of CB2 receptors on nicotine and cocaine reward in conditioned place preference, drug taking, and drug seeking models.
Similarly, the CB1 receptor plays distinct roles in preclinical models of nicotine and cocaine reward. While CB1 receptor antagonists inhibit the reinforcing effects of nicotine, as manifested by decreases in nicotine self-administration and seeking behavior (Cohen et al. 2002, 2005a, b), as well as nicotine-induced CPP (Le Foll and Goldberg 2004), rimonabant does not affect cocaine self-administration in rats and monkeys (Fattore et al. 1999; Tanda et al. 2000; Filip et al. 2006), or cocaine CPP (Chaperon et al. 1998; but see Yu et al. 2011). In addition, CB1 (−/−) mice show unaltered cocaine-induced CPP (Martin et al. 2000). On the other hand, AM404, an inhibitor of the putative anandamide transporter, counteracted cocaine facilitated intracranial self-stimulation through a CB1 receptor-dependent mechanism (Vlachou et al. 2008). In contrast, CB1 receptor antagonists inhibit both nicotine–induced CPP and nicotine self-administration (Cohen et al. 2002, 2005a; Le Foll and Goldberg 2004). Thus, the CB1 receptor appears to play an important role for nicotine reward, but is largely dispensable for the rewarding properties of cocaine.
While we determined that CB2 receptors are required for nicotine-induced CPP, we found that somatic and affective signs of withdrawal were not affected in nicotine-dependent CB2 (−/−) or (+/+) mice, indicating that CB2 receptors do not play a necessary role in nicotine withdrawal. Likewise, the antinociceptive and hypothermic effects of an acute inject of nicotine was not altered by deletion of the CB2 receptors. Thus, CB2 receptors play a differential role in the pharmacological effects of nicotine.
In conclusion, the present study reveals surprising data showing that CB2 receptors play opposing roles in nicotine-and cocaine-induced CPP. Specifically, blocking the CB2 receptor disrupts nicotine-induced CPP, while CB2 receptor agonism enhances nicotine-induced CPP. Conversely, CB2 receptor agonists reduce cocaine-induced CPP. In contrast to the finding that CB2 receptors are required for nicotine-induced CPP in the mouse, CB2 receptors are not necessary for both the expression of withdrawal in nicotine-dependent mice and acute pharmacological effects of nicotine.
Acknowledgments
This work was supported by the National Institute of Drug Abuse grant DA-05274 to MID, P01DA009789, and P50DA005274. The authors thank Cindy Evans and Tie Han for their technical assistance with this study. All experiments comply with the current laws of USA.
Abbreviations
- CNS
central nervous system
- CPP
conditioned place preference
- DA
dopamine
- i.p
intraperitoneal injection
- NAc
nucleus accumbens
- s.c
subcutaneous injection
- VTA
ventral tegmental area
Footnotes
There are no conflicts of interest to disclose for this research.
References
- Adamczyk P, Miszkiel J, McCreary AC, Filip M, Papp M, Przegaliński E. The effects of cannabinoid CB1, CB2 and vanilloid TRPV1 receptor antagonists on cocaine addictive behavior in rats. Brain Res. 2012;1444:45–54. doi: 10.1016/j.brainres.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Alajaji M, Bowers MS, Knackstedt L, Damaj MI. Effects of the beta-lactam antibiotic ceftriaxone on nicotine withdrawal and nicotine-induced reinstatement of preference in mice. Psychopharmacology (Berlin) 2013 doi: 10.1007/s00213-013-3047-3. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlSharari SD, Akbarali HI, Abdullah RA, Shahab O, Auttachoat W, Ferreira GA, White KL, Lichtman AH, Cabral GA, Damaj MI. Novel insights on the effect of nicotine in a murine colitis model. J Pharmacol Exp Ther. 2013;344(1):207–217. doi: 10.1124/jpet.112.198796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aracil-Fernández A, Trigo JM, García-Gutiérrez MS, Ortega-Álvaro A, Ternianov A, Navarro D, Robledo P, Berbel P, Maldonado R, Manzanares J. Decreased cocaine motor sensitization and self-administration in mice overexpressing cannabinoid CB2 receptors. Neuropsychopharmacology. 2012;37(7):1749–1763. doi: 10.1038/npp.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160(3):467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Straiker A, Mackie K. CB2 cannabinoid receptors inhibit synaptic transmission when expressed in cultured autaptic neurons. Neuropharmacology. 2012;63(4):514–523. doi: 10.1016/j.neuropharm.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin PR, Alanis R, Salas R. The Role of the Habenula in Nicotine Addiction. J Addict Res Ther. 2011;S1(2):002. doi: 10.4172/2155-6105.S1-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153(1):31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Valone JM, Bevins RA. Locomotion and conditioned place preference produced by acute intravenous amphetamine: role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacology. 1999;143:39–46. doi: 10.1007/s002130050917. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83(4):531–541. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362(24):2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9(1):65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral GA, Marciano-Cabral F. Cannabinoid receptors in microglia of the central nervous system: immune functional relevance. J Leukoc Biol. 2005;78(6):1192–1197. doi: 10.1189/jlb.0405216. [DOI] [PubMed] [Google Scholar]
- Castañé A, Berrendero F, Maldonado R. The role of the cannabinoid system in nicotine addiction. Pharmacol Biochem Behav. 2005;81:381–386. doi: 10.1016/j.pbb.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Changeux JP. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci. 2010;11(6):389–401. doi: 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Soubrié P, Puech AJ, Thiébot MH. Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology (Berl) 1998;135(4):324–332. doi: 10.1007/s002130050518. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Voltz C, Steinberg R, Soubrié P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13(5–6):451–463. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- Cohen C, Kodas E, Griebel G. CB1 receptor antagonists for the treatment of nicotine addiction. Pharmacol Biochem Behav. 2005a;81(2):387–395. doi: 10.1016/j.pbb.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrié P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716) Neuropsychopharmacology. 2005b;30(1):145–155. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Saghatelian A, Hawkins EG, Clement AB, Bracey MH, Lichtman AH. Functional disassociation of the central and peripheral fatty acid amide signaling systems. Proc Natl Acad Sci U S A. 2004;101(29):10821–10826. doi: 10.1073/pnas.0401292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- Damaj MI, Glassco W, Aceto MD, Martin BR. Antinociceptive and pharmacological effects of metanicotine, a selective nicotinic agonist. J Pharmacol Exp Ther. 1999;291:390–388. [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307(2):526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Siu EC, Sellers EM, Tyndale RF, Martin BR. Inhibition of nicotine metabolism by methoxysalen: Pharmacokinetic and pharmacological studies in mice. J Pharmacol Exp Ther. 2007;320(1):250–257. doi: 10.1124/jpet.106.111237. [DOI] [PubMed] [Google Scholar]
- Deroche V, Le Moal M, Piazza PV. Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci. 1999;11:2731–2736. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res. 2009;60(2):77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99(16):10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Martellotta MC, Cossu G, Mascia MS, Fratta W. CB1 cannabinoid receptor agonist WIN 55,212-2 decreases intravenous cocaine self-administration in rats. Behav Brain Res. 1999;104(1–2):141–146. doi: 10.1016/s0166-4328(99)00059-5. [DOI] [PubMed] [Google Scholar]
- Filip M, Gołda A, Zaniewska M, McCreary AC, Nowak E, Kolasiewicz W, Przegaliński E. Involvement of cannabinoid CB1 receptors in drug addiction: effects of rimonabant on behavioral responses induced by cocaine. Pharmacol Rep. 2006;58(6):806–819. [PubMed] [Google Scholar]
- Gamaleddin I, Wertheim C, Zhu AZ, Coen KM, Vemuri K, Makryannis A, Goldberg SR, Le Foll B. Cannabinoid receptor stimulation increases motivation for nicotine and nicotine seeking. Addict Biol. 2012a;17(1):47–61. doi: 10.1111/j.1369-1600.2011.00314.x. [DOI] [PubMed] [Google Scholar]
- Gamaleddin I, Zvonok A, Makriyannis A, Goldberg SR, Le Foll B. Effects of a selective cannabinoid CB2 agonist and antagonist on intravenous nicotine self-administration and reinstatement of nicotine seeking. PLoS One. 2012b;7(1):e29900. doi: 10.1371/journal.pone.0029900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Gutiérrez MS, Pérez-Ortiz JM, Gutiérrez-Adán A, Manzanares J. Depression-resistant endophenotype in mice overexpressing cannabinoid CB2 receptors. Br J Pharmacol. 2010;160(7):1773–1784. doi: 10.1111/j.1476-5381.2010.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071(1):10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology (Berl) 2006;184(3–4):456–463. doi: 10.1007/s00213-006-0305-7. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Sotak BN, Palmiter RD. Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J Neurosci. 2007;46:12484–12488. doi: 10.1523/JNEUROSCI.3133-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008;325:302–312. doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Chen X, Miles MF, Harenza J, Damaj MI. The neuropeptide galanin and variants in the GalR1 gene are associated with nicotine dependence. Neuropsychopharmacology. 2011;36(11):2339–2348. doi: 10.1038/npp.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Mahadevan A, Zhao B, Sun H, Naidu PS, Razdan RK, Selley DE, Imad Damaj M, Lichtman AH. The CB2 cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Neuropharmacology. 2011;60(2–3):244–251. doi: 10.1016/j.neuropharm.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Rimonabant, a CB1 antagonist, blocks nicotine-conditioned place preferences. Neuroreport. 2004;15(13):2139–2143. doi: 10.1097/00001756-200409150-00028. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Forget B, Aubin HJ, Goldberg SR. Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: insights from pre-clinical and clinical studies. Addict Biol. 2008;13(2):239–252. doi: 10.1111/j.1369-1600.2008.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchicchi A, Lecca S, Carta S, Pillolla G, Muntoni AL, Yasar S, Goldberg SR, Pistis M. Effects of fatty acid amide hydrolase inhibition on neuronal responses to nicotine, cocaine and morphine in the nucleus accumbens shell and ventral tegmental area: involvement of PPAR-alpha nuclear receptors. Addict Biol. 2010;15(3):277–288. doi: 10.1111/j.1369-1600.2010.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29(4):225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci. 2000;12(11):4038–4046. doi: 10.1046/j.1460-9568.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Cowe MA, Lewis SW, Glick SD. α3β4 nicotinic acetylcholine receptors in the medial habenula modulate the mesolimbic dopaminergic response to acute nicotine in vivo. Neuropharmacology. 2012;63(3):434–440. doi: 10.1016/j.neuropharm.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland JM, Glass M, Pertwee RG. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br J Pharmacol. 2007;152(5):583–593. doi: 10.1038/sj.bjp.0707399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Merritt LL, Martin BR, Walters C, Lichtman AH, Damaj MI. The endogenous cannabinoid system modulates nicotine reward and dependence. J Pharmacol Exp Ther. 2008;326(2):483–492. doi: 10.1124/jpet.108.138321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon PP, Lichtman AH, Parsons LH, Damaj MI. The role of fatty acid amide hydrolase inhibition in nicotine reward and dependence. Life Sci Life Sci. 2013;92:458–462. doi: 10.1016/j.lfs.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- National Research Council of the National Academies, Institute for Laboratory Animal Research. Guide for the care and use of laboratory animals. 8. The National Academies Press; Washington, D.C: 2011. [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS One. 2008;3(2):e1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolaro D, Vigano D, Realini N, Rubino T. Role of endocannabinoids in regulating drug dependence. Neuropsychiatr Dis Treat. 2007;3(6):711–721. doi: 10.2147/ndt.s976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos. 1984;12(6):725–731. [PubMed] [Google Scholar]
- Pettit DA, Harrison MP, Olson JM, Spencer RF, Cabral GA. Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J Neurosci Res. 1998;51(3):391–402. doi: 10.1002/(SICI)1097-4547(19980201)51:3<391::AID-JNR12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Ramirez SH, Haskó J, Skuba A, Fan S, Dykstra H, McCormick R, Reichenbach N, Krizbai I, Mahadevan A, Zhang M, Tuma R, Son YJ, Persidsky Y. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood–brain barrier dysfunction under inflammatory conditions. J Neurosci. 2012;32(12):4004–4016. doi: 10.1523/JNEUROSCI.4628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherma M, Panlilio LV, Fadda P, Fattore L, Gamaleddin I, Le Foll B, Justinová Z, Mikics E, Haller J, Medalie J, Stroik J, Barnes C, Yasar S, Tanda G, Piomelli D, Fratta W, Goldberg SR. Inhibition of anandamide hydrolysis by cyclohexyl carbamic acid 3′-carbamoyl-3-yl ester (URB597) reverses abuse-related behavioral and neurochemical effects of nicotine in rats. J Pharmacol Exp Ther. 2008;327(2):482–490. doi: 10.1124/jpet.108.142224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. Br J Pharmacol. 2008;154(2):369–383. doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci U S A. 2001;98(9):5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215(1):89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3(11):1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310(5746):329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Stamatopoulou F, Nomikos GG, Panagis G. Enhancement of endocannabinoid neurotransmission through CB1 cannabinoid receptors counteracts the reinforcing and psychostimulant effects of cocaine. Int J Neuropsychopharmacol. 2008;11(7):905–923. doi: 10.1017/S1461145708008717. [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 2006;184(3–4):339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Beletskaya ID, Ng EW, Dai Z, Crocker PJ, Mahadevan A, Razdan RK, Martin BR. Resorcinol derivatives: a novel template for the development of cannabinoid CB(1)/CB(2) and CB(2)-selective agonists. J Pharmacol Exp Ther. 2002;301(2):679–689. doi: 10.1124/jpet.301.2.679. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL. Brain cannabinoid CB2 receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011;14(9):1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LL, Zhou SJ, Wang XY, Liu JF, Xue YX, Jiang W, Lu L. Effects of cannabinoid CB1 receptor antagonist rimonabant on acquisition and reinstatement of psychostimulant reward memory in mice. Behav Brain Res. 2011;217(1):111–116. doi: 10.1016/j.bbr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Zhang M, Martin BR, Adler MW, Razdan RK, Jallo JI, Tuma RF. Cannabinoid CB(2) receptor activation decreases cerebral infarction in a mouse focal ischemia/reperfusion model. J Cereb Blood Flow Metab. 2007;27(7):1387–1396. doi: 10.1038/sj.jcbfm.9600447. [DOI] [PMC free article] [PubMed] [Google Scholar]