Abstract
The objective of this study was to determine whether radiation-induced injury to the heart after 10 Gy total body irradiation (TBI) is direct or indirect. Young male WAG/RijCmcr rats received a 10 Gy single dose using TBI, upper hemi-body (UHB) irradiation, lower hemi-body (LHB) irradiation, TBI with the kidneys shielded, or LHB irradiation with the intestines shielded. Age-matched, sham-irradiated rats served as controls. The lipid profile, kidney injury, heart and liver morphology and cardiac function were determined up to 120 days after irradiation. LHB, but not UHB irradiation, increased the risk factors for cardiac disease as well as the occurrence of cardiac and kidney injury in a way that was quantitatively and qualitatively similar to that observed after TBI. Shielding of the kidneys prevented the increases in risk factors for cardiac disease. Shielding of the intestines did not prevent the increases in risk factors for cardiac disease. There was no histological evidence of liver injury 120 days after irradiation. Injury to the heart from irradiation appears to be indirect, supporting the notion that injury to abdominal organs, principally the kidneys, is responsible for the increased risk factors for and the occurrence of cardiac disease after TBI and LHB irradiation.
Keywords: blood lipids, total body irradiation, partial body irradiation, morphology, cardiac risk factors, heart disease
Introduction
In order to develop adequate medical countermeasures, there is an urgent need to understand the extent and mechanisms of injury that may occur in vital organs such as the heart following radiation exposure from a terrorist attack or from a nuclear power plant accident. In any of these radiation/nuclear incidents, children would likely account for a significant portion of the affected population. However, the extent to which radiation exposure affects the heart of children is poorly understood.
In children, radiation exposure of the heart to 15 Gy or more increases the relative hazard of developing cardiac disease 2 to 6 fold compared with non-irradiated individuals (1). The risk factors for cardiac disease can also be detected at higher rates in survivors of cancer treatment during childhood, compared with their siblings (2). Risk factors for cardiac disease are also increased in young adults treated with hematopoietic stem cell transplantation following total body irradiation (TBI) during childhood (3). Evidence that TBI may be a risk factor for cardiac disease also comes from longitudinal studies of the Japanese atomic bomb survivors. In this population, mortality from cardiac disease was significantly increased after more than 40 years after single dose entire body exposure of 1-2 Sv (4, 5).
The impact of TBI on late injury to a child's heart has not been known until recently (6). In developing a model to study the late effects of radiation on the child's heart, a TBI dose was selected that was survivable if hematopoietic lethality was avoided. The rationale for using a dose of 10 Gy was that this dose has a high relevance after radiation/nuclear incidents, and because exposure to this dose poses risks of serious health effects, yet survival is possible with medical support (7). In these studies, it was demonstrated that a single exposure to 10 Gy TBI in the immature (5 week old) WAG/RijCmcr rat, representative of a pediatric population, increases serum total cholesterol, LDL cholesterol and triglycerides, all of which are biomarkers for an increased risk of cardiac disease. Hypercholesterolemia is associated with morphological injury to the vascular endothelium resulting in vessel stenosis, a decreased density of smaller diameter coronary vessels, and a decrease in ventricular function at 120 days after TBI. Mechanical cardiac injury is manifest as a decline in the global radial and circumferential left ventricular strain (6).
In a radiation/nuclear incident, partial or whole body radiation exposure, rather than exposure of a single organ such as the heart, would be the most likely consequence. Moreover a previous study has shown that 10 Gy, thoracic-only irradiation, did not result in hypercholesterolemia, or cardiac morphological and ventricular mechanical dysfunction over at least 240 days (6). Injury to the heart following irradiation appears to be indirect, with effects in non-thoracic organs causing (or exacerbating) an increase in risk factors for cardiac disease, injury to the coronary vasculature and ventricular dysfunction (6). However, this notion has not been conclusively demonstrated. If confirmed, then countermeasures that target injury to non-thoracic organs may be effective in decreasing injury to the heart.
The kidney is a radiosensitive abdominal organ susceptible to the development of nephropathy with hypertension, proteinuria and azotemia after irradiation (8, 9). Renal dysfunction may be part of the mechanism responsible for the increased risk for heart disease following TBI in adults (10). The intestines occupy the majority of the abdomen and are also known to be susceptible to injury after irradiation (11). However, the role of the kidneys and the intestines in the mechanisms that underlie TBI-induced late injury to the heart in children is not known. It is proposed that radiation-induced injury to the heart is indirect, mediated by radiation injury to the lower hemi-body organs. The corollary to this hypothesis is that shielding of the kidney or intestines at the time of irradiation will prevent increases in risk factors for heart disease following radiation. Shielding of the kidneys as part of 14 Gy total body irradiation protocol, in preparation for bone marrow transplantation, decreases the incidence of late renal dysfunction in adults (12), clearly demonstrating the ability of local shielding to limit radiation injury in this organ. However, the effectiveness of kidney shielding prior to irradiation in children and its impact on the development of heart disease has not been examined.
The objective of the present study was to determine whether TBI-induced increases in the risk factors for cardiac disease and the occurrence of cardiac disease are direct or indirect.
Methods and materials
Experimental animals
Young, 7-8 week old, male WAG/RijCmcr rats were maintained on sterilized rat chow and water ad libitum in a moderate-security barrier facility at the Biomedical Resource Center of the Medical College of Wisconsin (MCW), Milwaukee, Wisconsin, USA. The drinking water supplied to the rats was further purified by reverse-osmosis and then chlorinated, but not acidified. Animal care was in accordance with NIH guidelines. The Animal Care and Use Committee at MCW approved all protocols.
Irradiator and dosimetry
Irradiation was with 320 kVp orthovoltage x-rays from an XRad320 x-ray unit. The radiation dosimetry has been described in detail previously (13). The dose-rates for the total body (TBI), upper hemi-body (UHB) and lower-hemi body (LHB) irradiation studies were 1.73, 1.66 and 1.66 Gy/min, respectively. The dose-rate for the studies involving local kidney shielding was 1.71 Gy/min. The dose-rates for the studies involving an exteriorized intestine, comprising LHB irradiation with sham surgery, LHB irradiation with the exteriorized intestines irradiated, LHB irradiation with the exteriorized intestines shielded, or irradiation of the exteriorized intestines with LHB shielded were 1.60, 1.64, 1.55 or 1.38 Gy/min, respectively.
Total body and partial body irradiation experimental design
Rats (n=9/group) received TBI, UHB or LHB irradiation with a single dose of 10 Gy carried out with a posterior-anterior field. Sham-irradiated rats (n=9) served as controls. The diaphragm was used to identify the dividing line between the upper hemi body and the lower hemi body irradiations. The model used is relevant to a potential radiological terrorism scenario. The need to use bone marrow transplantation, which was used in a previous study (14), was avoided by shielding (the bone marrow) in one leg. This had no effect on the progression of cardiac injury. Rats were irradiated and maintained in the barrier facility throughout the study. The start of the study was defined as the time rats were irradiated or sham treated.
Kidney shielding experimental design
Animals were immobilized in acrylic plastic jigs that allowed irradiation with parallel-opposed lateral fields that included both kidneys (15). To shield the kidneys, a customized 6 mm thick, lead plate was placed in the beam line at the anatomic location of the kidneys. Its correct positioning was confirmed using a PaxScan 2520V Amorphous Silicon Digital X-Ray Imager (Varian), attached to XRad320 x-ray unit to capture images of the exposed field (Figure 2A). Rats (n=5-8/group) received TBI with a single dose of 10 Gy with or without kidney shielding. Sham irradiated rats served as controls.
Figure 2.
Kidney shielding at the time of irradiation prevents the increase in risk factors for heart disease and kidney injury. (A) The location of the kidneys (yellow) and liver (red) are indicated. The liver lies directly under the diaphragm (blue), which separates the thoracic and abdominal cavities. In this lateral image, the two kidneys are overlapping with the left kidney being the more caudal than the right kidney. (B) Time-related changes in total cholesterol, triglycerides, LDL cholesterol, HDL cholesterol, creatinine and BUN following TBI with and without kidney shielding compared with sham irradiated controls. Data shown as means + SD, n=5-8/group. *=p<0.05, TBI vs. TBI with the kidneys shielded.  = TBI lateral,
= TBI lateral,  = lateral TBI with the kidneys shielded
= lateral TBI with the kidneys shielded  = sham-irradiated control.
= sham-irradiated control.
Intestinal exteriorization experimental design
Rats were anesthetized using a combination of ketamine (30 mg/kg i.p.) and pentobarbital (30 mg/kg i.p.). In some groups, a laparotomy was performed and the intestines, from the proximal duodenum to the distal descending colon, were then temporarily exteriorized and could be either irradiated or shielded from a single dose of 10 Gy using a customized 6mm thick lead plate. The exteriorized intestines were placed inside a saline soaked 10 mm×10 mm gauze sponge, preheated to body temperature to prevent desiccation and hypothermia during the irradiation period. Anesthetized rats were placed on their left side and received either LHB irradiation with sham surgery (n=6), LHB irradiation with the exteriorized intestines irradiated (n=6), LHB irradiation with the exteriorized intestines shielded (n=6), or irradiation of the exteriorized intestines with LHB shielded (n=6). In rats where a laparotomy was performed, the intestines were then reinserted into the abdomen and the incision closed using removable surgical clips. The rats were allowed to recover and the surgical clips were removed 24 - 48 h later. All anesthetized rats were placed on a heating blanket to maintain normothermia (370C) throughout surgery, irradiation and recovery. One control group of anesthetized rats received sham irradiation with sham surgery (n=4), and another group of conscious rats received sham irradiation (n=4).
Feces collection and processing for quantitative PCR
The rats were housed in groups during this study. In order to collect feces, individual rats were simply removed from their cage and handled gently. This nearly always promoted the elimination of fecal material, which was then freshly collected. Fresh fecal pellets were obtained from each rat prior to (day 0) and at days 2, 4, 7, and 14 post irradiation. Pellets were homogenized in 1 ml PBS, and 200 ul of the homogenate were used for microbial DNA isolation using the QIAamp DNA Stool Mini Kit (Qiagen, Valencia, CA). Isolated DNA samples were subjected to quantitative PCR using an iCycler (Bio-Rad, Hercules, CA) for microbial population enumeration. The PCR reaction mixture consisted of 50% iQ SYBR Green Supermix (Bio-Rad), 0.4 μM forward and reverse primers, and 3.8% template solution in RNase/DNase free water. The forward (F) and reverse (R) 16S rRNA primer pair Uni515F (GTGCCAGCMGCCGCGGTAA) and Ent826R (GCCTCAAGGGCACAACCTCCAAG) was used to quantify the Proteobacteria taxon (16). Genomic DNA from Escherichia coli were obtained from the American Type Culture Collection and used as standards.
Lipid profile
Blood was taken at the time of irradiation, and then at 20 - 120 days after TBI, UHB irradiation, LHB irradiation, TBI with the kidneys shielded, and at 20 – 170 days after LHB irradiation with the intestines shielded, and from sham irradiated rats. To sample blood, the rats were briefly anesthetized with isoflurane /oxygen and blood collected from the retro-orbital plexus. Serum was then analyzed for lipid levels [total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol and triglycerides] (Dynacare Laboratories, Milwaukee, Wisconsin, USA).
Kidney function
Blood was taken at the time of irradiation, and then at 20 - 170 days after TBI, UHB irradiation, LHB irradiation, TBI with the kidneys shielded, and at 20 – 170 days after LHB irradiation with the intestines shielded, and from sham irradiated rats. Serum was then analyzed for blood urea nitrogen (BUN) and creatinine levels (Dynacare Laboratories, Milwaukee, Wisconsin, USA).
Histology
To evaluate tissue damage at 120 days after TBI, UHB irradiation and LHB irradiation, compared with sham irradiated rats, the entire heart (n=3) and liver (n=3) were fixed in formalin (10% v/v), embedded in paraffin, and 5μm-thick sections cut from each block and stained with hematoxylin and eosin (H&E) or Masson-trichrome according to standard methods. Ten sections from each heart and liver were evaluated.
Morphometric analysis
Sections of the left ventricular of the heart (n=3/group) were analyzed in a blinded fashion. Randomly selected images of tissue were acquired using a Nikon Plan Fluor 40x/0.75 objective lens mounted on a Nikon E-55i microscope fitted with a Nikon DS-Fi1 camera and saved as 24-bit RGB images in TIFF format. Perivascular fibrotic changes in 20-30 fields containing coronary vessels, 2-50 μm in diameter for each heart were quantified using MetaMorph image analysis program version 7.7.0.0. (Molecular Devices, Downington, PA, USA). On calibrated images, the areas of both the vessel lumen and the perivascular space were measured by planimetry. Planimetry determined the true area of the coronary vessel, thereby eliminating errors that may have been caused by compression and collapse. The area of fibrosis, per luminal area, was then calculated for each coronary vessel accessed. The resultant data was then averaged per animal and then grouped by treatment for statistical analysis. The average microvasculature fibrosis was expressed as percent collagen per lumen area for each image.
Cardiac echocardiography
Left ventricular systolic function was assessed using two dimensional strain echocardiography after TBI, UHB irradiation and LHB irradiation, and compared with sham irradiated rats (n=5/group) after 120 days. The operator was double blinded with respect to treatment allocation. An echocardiograph Vivid 7 (General Electric, Waukesha, Wisconsin, USA) was used with a M12L (11-MHz) linear-array transducer. Closed-chest imaging was performed in the short-axis view at the mid-LV level (level of papillary muscles). The image depth was 2.5 cm and acquisition was at 236 frames/second with electrocardiographic gating (17).
Echocardiography image analysis
The images were processed using EchoPAC Q analysis software (General Electric). The method has been previously described (17). Briefly, the endocardial border was manually traced at end-systole by an experienced operator blind to treatment assignment, and the software then automatically selected 6 equidistant tissue-tracking regions of interest in the myocardium. The outer border was adjusted to approximate to the epicardial border. The software provided a profile of the radial (myocardial deformation towards the center) and circumferential (myocardial deformation along the curvature) strain (expressed as %) with time. End systolic radial and circumferential strain were obtained for each of the 6 segments and global strain calculated from the average of the values. Three consecutive heart beats were measured and the average used for analysis.
Statistical analysis
All values were expressed as the mean ± standard deviation (SD). For the lipid and kidney function and echocardiography analysis, statistical significance was determined by performing a one-way analysis of variance using a Bonferroni's multiple comparison as the post hoc test (18). The Students t-test was used to find significant differences among variables in the qPCR data. Significance was attributed to those groups that had a P value <0.05.
Results
Studies were performed to determine whether TBI-induced damage to the heart is indirect, resulting from effects in non-thoracic organs that can increase risk factors for cardiac disease and the occurrence of cardiac disease.
Total vs. partial body irradiation and risk factors for cardiac disease
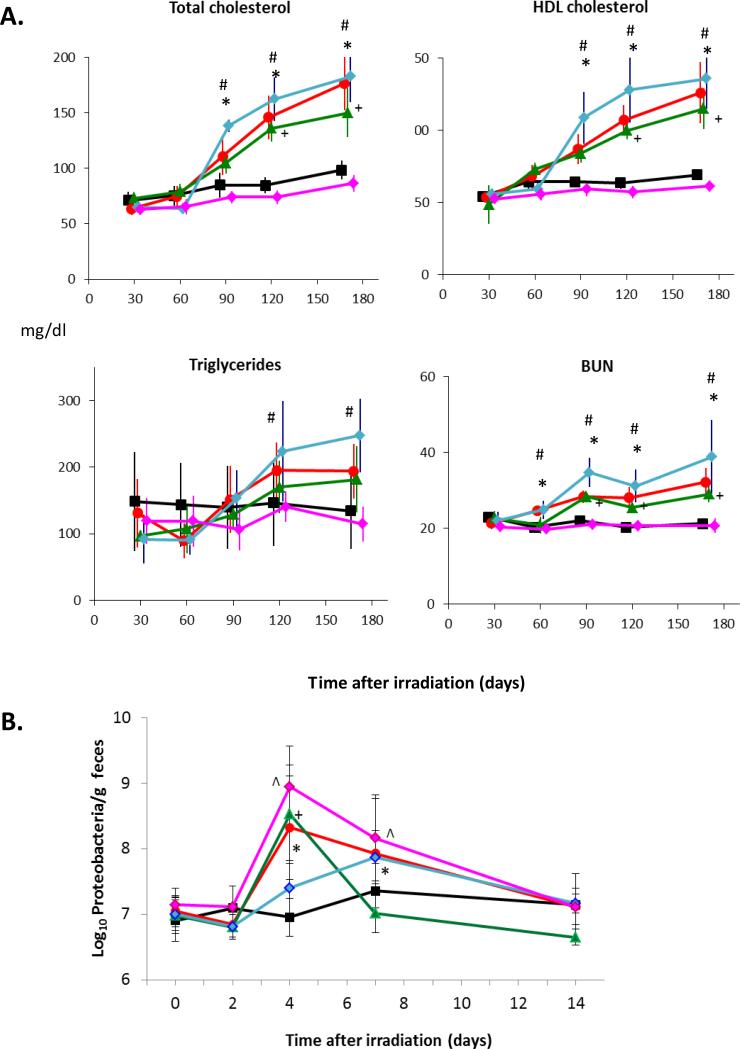
At intervals of ≥ 60 days after TBI or LHB irradiation, there was a significant increase in total cholesterol, LDL cholesterol and triglycerides compared with those rats receiving UHB radiation alone and the sham irradiated controls (Figure 1). The profile of changes for all three of these risk factors for cardiac disease was very similar for rats receiving either TBI or LHB irradiation (Figure 1). At 120 days after irradiation, the levels of total cholesterol and LDL cholesterol declined slightly, but not significantly, in rats subjected to LHB irradiation relative to TBI. However, elevated levels of triglycerides had declined after 120 days in both TBI and LHB irradiated rats. BUN levels were significantly and progressively increased from 60 days after both TBI and LHB irradiation compared with UHB irradiation and the sham irradiated controls (Figure 1). Thus LHB irradiation increased the risk factors for cardiac disease in a manner that was quantitatively and qualitatively very similar to that observed following TBI.
Figure 1.
Time-related changes in risk factors for cardiac disease and kidney injury following total and partial body irradiation: total cholesterol, LDL cholesterol, triglycerides and blood urea nitrogen (BUN) following either TBI, LHB irradiation, UHB irradiation or in sham irradiation (controls). Data shown as the means + SD (n=9/group). * = p <0.05, TBI vs. age-matched controls. # = p<0.05, LHB vs. age-matched controls.  = sham irradiated control,
= sham irradiated control,  = TBI,
= TBI,  = LHB,
= LHB,  = UHB.
= UHB.
Shielding of the kidneys
Shielding of the kidneys at the time of irradiation prevented increases in the total cholesterol, triglycerides, LDL cholesterol, HDL cholesterol, creatinine and BUN compared with the sham irradiated controls at intervals from 60 to 120 day after the start of the study (Figure 2B). The positioning of the lead plate to completely shield the kidneys during irradiation was confirmed by X-ray imaging. The positioning of the lead plate did not result in shielding of the liver during irradiation (Figure 2A).
Shielding of the intestines
Further studies were performed to examine the possibility that irradiation of the intestine alone could be responsible for an increase in risk factors for cardiac disease and kidney injury. At 90 – 170 days after irradiation, there was an increase in total cholesterol, HDL cholesterol and BUN in rats that received LHB irradiation with sham surgery (red circle), LHB irradiation with the exteriorized intestines irradiated (green triangle), and LHB irradiation with the exteriorized intestines shielded (blue diamond), compared with the sham irradiated and sham surgery group (black square) (Figure 3). The direction of change for triglycerides following LHB irradiation reflected the changes in total cholesterol, HDL cholesterol and BUN. However, the large within-group variation for triglycerides resulted in fewer statistically significant changes compared with the sham irradiated and sham surgery group. Irradiation of the exteriorized intestines alone with the LHB shielded (red diamond) did not result in an increase in total cholesterol, HDL cholesterol, triglycerides and BUN relative to the sham irradiated and sham surgery group (black square) (Figure 3A). Irradiation of the LHB with shielding of the exteriorized intestines (blue diamond) resulted in a greater elevation in risk factors for cardiac disease compared with irradiation of the LHB and irradiation of the exteriorized intestines (green triangle). Anesthesia delayed the LHB irradiation-induced increases in triglycerides, cholesterol and blood urea nitrogen by 30 days compared with sham treated rats not subjected to irradiation (results not shown). This protective effect of pentobarbital anesthesia is known (19, 20). To facilitate comparisons between experimental groups that received anesthesia, an equal amount of anesthesia was administered to all rats. This study showed that irradiation of the intestines alone did not increase risk factors for cardiac disease or kidney injury. However, irradiation of the LHB, with and without shielding of the exteriorized intestines, was associated with increased risk factors for cardiac disease and kidney injury.
Figure 3.
Effects of intestinal shielding at the time of irradiation. (A) Intestinal shielding does not prevent an increase in the risk factors for heart disease and kidney injury. Time-related changes in total cholesterol, HDL cholesterol, triglycerides and blood urea nitrogen after LHB irradiation and irradiation of the in situ or exteriorized intestines compared with age-matched sham irradiated controls. (B) Radiation exposure confirmed by increased intestinal bacteria. At four days post irradiation, the abundance of Proteobacteria in the feces of rats where the intestines were irradiated was increased by 20 to 100 fold above background. Data shown as means ± SD, n=4-6/group. * = p<0.05, LHB irradiation vs. sham irradiation with sham surgery. # = p< 0.05, LHB irradiation with exteriorized intestines shielded vs. Sham irradiation with sham surgery. + = p < 0.05, LHB irradiation with exteriorized intestines irradiated vs. Sham irradiation with sham surgery. ^ = LHB shielded with exteriorized intestines irradiated vs. Sham irradiation with sham surgery. HDL = high density lipoprotein. BUN = blood urea nitrogen.  = Sham irradiation with sham surgery (control), =
= Sham irradiation with sham surgery (control), =  LHB irradiation with sham surgery,
LHB irradiation with sham surgery,  = LHB irradiation with exteriorized intestines irradiated,
= LHB irradiation with exteriorized intestines irradiated,  = LHB irradiation with exteriorized intestines shielded,
= LHB irradiation with exteriorized intestines shielded,  = LHB shielded with exteriorized intestines irradiated.
= LHB shielded with exteriorized intestines irradiated.
Irradiation-induced changes in intestinal microbiota
Previous research on radiation exposure (21) has shown that exposure of the intestine to radiation induces indicative changes in the intestinal microbiome. To confirm the rats in this study were appropriately exposed to intestinal irradiation and shielding, the abundance of Proteobacteria in the feces of the rats prior to and at 2, 4, 7, and 14 days post irradiation was quantified. At 4 and 7 days post exposure, there was a characteristic increase in Proteobacteria abundance in all rats except for the LHB irradiation with the exteriorized intestines shielded (blue diamond) and the sham irradiated and sham surgery control group (black square) (Figure 3B). There was, however, a slight increase in Proteobacteria observed in the LHB irradiation with the exteriorized intestines shielded rats (blue diamond) at day 7. This smaller change in the microbiota was attributed to the incomplete shielding of the intestinal tract, i.e. the rectum and the terminal section of the sigmoid colon that could not be exteriorized and shielded. The observed changes in Proteobacteria demonstrated that the experimental setup successfully exposed or shielded the intestine from irradiation. The objective of performing the intestinal microbiota studies was to obtain an assessment of the effectiveness of the shielding of the gastrointestinal tract. The intestinal microbiota evaluation is not an absolute indication of gastrointestinal radiation injury.
Total vs. partial body irradiation and the occurrence of late cardiac disease
Cardiac histology
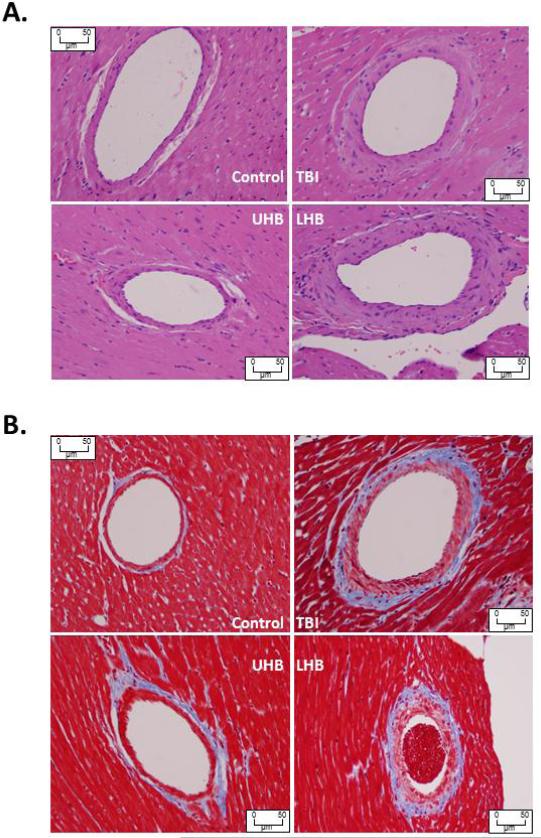
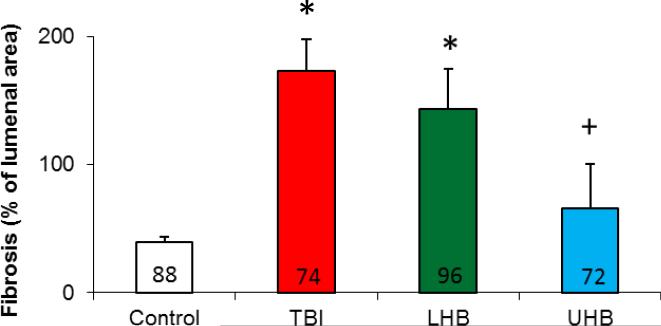
Studies were performed to examine whether TBI, LHB irradiation or UHB irradiation results in cardiac disease, as manifest by histological changes, compared with sham irradiated control rats. Histological sections were analyzed by a cardiac pathologist blinded to the identity of the specimens (RAK). TBI and LHB irradiation resulted in medial thickening and an increase in peri-arterial sclerosis of penetrating coronary arteries at 120 days after irradiation compared with age-matched sham irradiated control rats. The affected arteries had luminal narrowing due to the concentric laminar thickening of the vessel walls with the accumulation of amphophilic material in the arterial media and adventitia. The myocytes from the hearts of TBI, UHB irradiation and LHB irradiation rats remained normal in appearance (Figure 4). Trichrome staining revealed peri-arterial fibrosis and irregular collagen deposition around the penetrating coronary arteries of TBI and LHB irradiated rats. Hearts from control rats had penetrating arteries showing no medial fibrosis or accumulation of amphophilic material (Figure 4). Histological evidence of injury to the heart's penetrating coronary arteries developed following TBI and LHB irradiation, but not following UHB irradiation compared with sham irradiated controls. Quantitative morphometric analysis to quantify the extent of fibrosis present in the coronary vessels in these four experimental groups confirmed the histopathologic findings of increased fibrosis in the groups receiving TBI and LHB irradiation compared with sham irradiated controls (Figure 5).
Figure 4.
Morphological changes for heart following total and partial body irradiation. Sections of heart stained with H&E (A) and trichrome (B) showing increased peri-arterial fibrosis in a small caliber coronary vessel 120 days after TBI and LHB irradiation with 10 Gy, compared with a comparable vessel in an age-matched UHB irradiated rat and a sham irradiated control.
Figure 5.
Quantitative morphometric analysis of the extent of fibrosis in coronary vessels following total or partial body irradiation. Data are shown as means ±SD, n=3/group. The number of records shown within each bar indicates the total number of vessels analyzed for all three hearts in each group. *=p<0.05, vs. control, +=p<0.05, partial body irradiation vs. TBI.
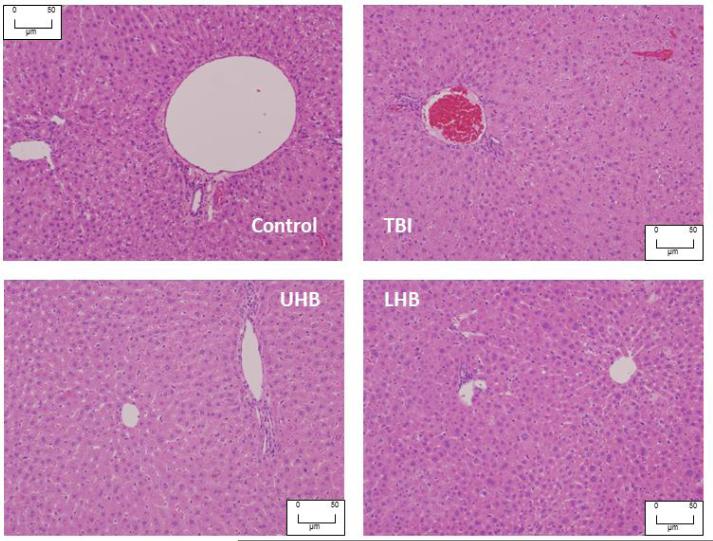
Liver histology
To determine whether total or partial body irradiation resulted in liver injury 120 days after the start of the study, liver sections were analyzed blindly by the pathologist (RAK). There was no histological evidence of necrosis or inflammation in any groups (Figure 6).
Figure 6.
Morphological images for the liver following total and partial body irradiation. Liver section stained with H&E showing normal appearance of the portal vein with a bile duct and central vein 120 days after TBI, LHB irradiation and UHB irradiation with 10 Gy, compared with an age-matched sham irradiated control sample.
Cardiac mechanical function
To determine whether TBI, UHB irradiation or LHB irradiation results in mechanical injury to the heart, global radial and circumferential strain was measured using 2D echocardiography in rats in vivo. At 120 days after TBI, hearts showed a significant decrease in global radial strain and circumferential strain compared with age-matched sham irradiated controls (Figure 7). LHB irradiation also reduced the global radial strain and circumferential strain compared with age-matched controls (Figure 7). UHB irradiation had no significant effect on these mechanical functions compared with age-matched sham irradiated control rats.
Figure 7.
Changes in global radial and circumferential strain, a measure of ventricular function, at 120 days after TBI, LHB irradiation and UHB irradiation with 10 Gy, compared with age-matched sham irradiated control. Data shown as means ± SD, n=5/group. *=p<0.05, vs. age-matched sham irradiated control.
Discussion
The results of the present study demonstrate that injury to the heart following TBI exposure to 10 Gy, a dose relevant to potential radiological terrorism, has an indirect action, with effects on abdominal organs being responsible for exporting factors that produce injury to the coronary vasculature. These findings suggest a new research paradigm whereby radiation-induced injury to the heart is indirect (22). Shielding of the kidneys during irradiation prevented the increase in risk factors for cardiac disease and also kidney injury, indicating the importance of this organ in the mechanisms that underlie radiation-induced cardiac disease. Shielding the intestines during irradiation did not prevent increases in these risk factors for cardiac disease. These findings suggest that countermeasures that target injury in abdominal organs, including the liver and kidney, could be effective in decreasing late irradiation-induced injury to the heart. The findings of the present study support the notion that radiation-induced renal dysfunction is an important component leading to cardiac disease and cardiac injury (23).
The dose of 10 Gy used in the present study is lower than those used in previous studies of radiation-induced cardiac injury. Cardiac radiation exposure at doses greater than 15 Gy in animals results in injury that includes myocardial fibrosis and a decreased density of the smaller diameter coronary vessels (24-27). Cardiac exposure to 15 Gy or more in children also increases the risk of adverse cardiac outcomes, including congestive heart failure, myocardial infarction, pericardial disease and vascular abnormalities as long as 30 years after therapy (1). Clinical studies in older patients suggest that radiation exposure at doses below 20 Gy can result in heart disease (28). The present study using UHB irradiation confirms a previous finding that direct exposure of the heart to 10 Gy (6) does not result in adverse cardiac outcomes. However, LHB irradiation with 10 Gy did result in adverse cardiac events, suggesting that radiation-induced injury to the heart can be both direct and indirect with the dose received being critical in determining the underlying mode of injury. Thus injury to the heart from radiation exposure to 10 Gy, in contrast with a higher dose (>15 Gy), appears to be mainly indirect with effects in nonthoracic organs causing (or exacerbating) increased risk factors for cardiac disease and ventricular dysfunction.
TBI and LHB irradiation resulted in decreased myocardial strain. Myocardial strain is a direct measure of regional myocardial function as it represents myocardial deformation during systole (with radial strain representing percentage change in myocardial segment deformation towards the left ventricle center on short axis view). It has been shown to be more sensitive method for detecting early myocardial dysfunction than traditional measures of cardiac function such as fractional shortening or left ventricular ejection fraction in various forms of cardiomyopathy such as doxorubicin cardiomyopathy (29), transplant cardiomyopathy (30), remote injury in ischemic cardiomyopathy (31) as well as in detection of residual myocardial viability following ischemic injury (17, 32). Impairment in myocardial strain occurs early in cardiac injury from various causes; therefore it is a nonspecific change as far as underlying etiology is concerned. The arterial changes and changes in cardiac risk factors are also associational, not causal, and the exact cause of the arterial changes is not known. However, a decreased density of smaller caliber coronary vessels, which results in chronic hypoxia where the heart is deprived of molecular oxygen for extended periods of time and may result in ischemic changes (33), is a possible cause of the reduced strain following TBI or LHB irradiation. Increased collagen deposition following irradiation can result in the increased stiffness of the coronary vessels that could also contribute to decreased myocardial strain.
The kidney is known to be a highly radiosensitive organ, susceptible to the development of nephropathy, proteinuria and hypertension following irradiation (34). Recently, renal dysfunction has been proposed as part of the mechanism causing increased cardiac disease after TBI (10) and in the atomic bomb survivors (23). The present study demonstrates for the first time in animals, the effectiveness of kidney shielding during irradiation to prevent increases in the risk factors for cardiac disease, and further supports the notion that radiation-induced injury to the heart is indirect.
Other than radiation-induced injury to the bone marrow, the intestine is the organ radioresponsive to moderate doses of radiation. The present study demonstrates that irradiation of the intestines per se had no effect on total cholesterol levels (Figure 3) indicating that effects to the intestines were not responsible for radiation-induced increases in the risk factors for cardiac disease. The finding of irradiation-induced increases in abundance of Proteobacteria confirm and extend the recent discovery that specific intestinal microbiota present in the feces can be used as biomarkers of prior radiation exposure. Maximal changes were observed 4 days after irradiation. There was a 100-fold increase in Proteobacteria. A potential advantage of using intestinal microbiota as biomarkers of prior radiation exposure is that this biodosimetry technique is non-invasive, the level of the reporting signals change by several orders of magnitude, and intestinal microbiota unaffected by radiation can serve as internal controls (21).
The liver was not histologically affected at 120 days after 10 Gy, confirming previous reports in rat (35). In a previous study, that used the same experimental design as in the present study, serum alanine transaminase activity was decreased by 21% at 100–120 days following 10 Gy total body irradiation, while aspartate transaminase activity was decreased by 15% at 40–60 days after total body irradiation (6). Based on the evaluation of alanine transaminase and aspartate transaminase activity levels as biomarkers of hepatocyte health, liver function remained fairly stable following irradiation. The liver is responsible for cholesterol synthesis and increased cholesterol levels are a known risk factor for cardiac disease. Cholesterol can be obtained from the diet or it can be synthesized de novo. As the level of cholesterol in the rats’ diet is constant, the elevation in blood cholesterol following irradiation likely results from increased uptake from the gut, increased synthesis or decreased clearance of cholesterol from the circulation. All animal cells manufacture cholesterol with relative production rates varying by cell type and organ function. The liver and intestines are the primary sources of cholesterol production; other sites of synthesis include the adrenal glands and the skin.
In normal humans and rats, an intricate balance is maintained between the biosynthesis, utilization, and the transport of cholesterol, keeping its harmful deposition to a minimum. Exposure to ionizing radiation appears to uncouple this balance, resulting in a late sustained elevation of total cholesterol, LDL-cholesterol as well as triglycerides. However, the increase in risk factors for cardiac disease and damage to the heart resulting from exposure to ionizing radiation may not be solely attributed to increased synthesis of cholesterol by organs such as the liver. Shielding of the kidney effectively prevented the increases in total cholesterol, LDL-cholesterol and triglycerides following radiation. Radiation nephropathy may one component responsible for elevated circulating cholesterol levels because of the associated proteinuria that adversely affects hepatic lipoprotein synthesis. Further studies are needed to understand whether synthesis, metabolism or transport of cholesterol are responsible for the observed increases in cholesterol levels in the circulation following irradiation, or whether cholesterol clearance from the circulation is decreased. The blood borne component(s) affected by ionizing irradiation that damage the heart need to be identified so that strategies can be developed to mitigate its damaging effect.
Partial or total body radiation affects many tissues and can result in multiple organ dysfunction including hematopoietic failure, renal failure, pulmonary fibrosis, and an accelerated onset of coronary artery disease and mechanical cardiac dysfunction. Therefore, any effective approach to mitigate or treat radiation-induced injury to the heart has to target several effects of radiation injury and should not target just one pathway. Furthermore, cardiac injury from radiation may occur by multiple mechanisms. Therefore, targeting multiple mechanisms may be the most effective strategy to combat radiation-induced cardiac injury.
This study has shown that organs within the LHB are responsible for increases in risk factors for cardiac disease as well as for cardiac injury following 10 Gy irradiation. Thus pharmaceuticals that decrease injury in LHB organs such as the kidney and liver through multiple mechanisms may be developed as medical countermeasures against radiation-induced injury to the heart. There is an urgent and immediate need for practical therapies to mitigate radiation injury to the heart following a radiologic terrorist associated event or nuclear plant accident (7). In the scenario of a radiation/nuclear incident, pretreatment of individuals prior to radiation exposure is not possible. Therefore, a medical countermeasure will need to be effective when administered after radiation exposure.
Although this study showed that TBI and LHB, but not UHB radiation were associated with worsened lipid profile and deterioration of cardiac function and structure, the exact mechanisms by which cardiac dysfunction occurs remain to be further studied. It is possible that increases in traditional cardiac risk factors such as hyperlipidemia, in addition to other yet to be determined nontraditional blood factors, may be contributing to radiation-induced cardiomyopathy. Although the present findings indicate radiation injury to the heart is indirect, it is possible that the direct effects from 10 Gy may occur at later times. However, there was no indication from the upper hemi body irradiation studies that this is present in the first 120 days.
The findings of the present study may be relevant to the increased risk of developing degenerative cardiac disease from exposure to space radiation. During exploratory missions to Lunar and Martian environments, astronauts will be continuously exposed to penetrating ions and energetic protons in galactic cosmic-rays (GCRs), and to intense periods of lower energy proton irradiation in the period of solar particle events (SPEs). The composition, energy and biological effects of GCRs and SPEs are different than for X-rays. Space radiation poses risks to mission success and risks to health following return to earth. NASA has established lifetime radiation exposure limits for heart disease to minimize or prevent risks of degenerative changes in the heart and vasculature. Dose limits for the heart are based on direct irradiation of the heart and adjacent arteries (36). The present study shows irradiation of the lower hemi body, especially the kidneys, with 10 Gy of X-rays increases the risk factors for, and the occurrence of, cardiac disease in rats. Irradiation of the upper hemi body with 10 Gy of X-rays had no effect on cardiac disease. These findings suggest a new non-cardiac source of risk for cardiac disease following exposure to ionizing radiation that may contribute to the overall mortality risks. Understanding the long-term effects of radiation on the heart is essential before undertaking space exploration for extended periods of time.
Conclusion
Injury to the immature rat heart following exposure to a TBI dose of 10 Gy is indirect with abdominal organs being responsible for exporting factors that affect the coronary vasculature and results in the development of cardiac disease.
Acknowledgements
The secretarial support of Mary Lynne Koenig, technical support of Mary Lou Mader for animal husbandry, Glen Slocum for the morphometry studies and Vladimir Semenenko, PhD for the dosimetry studies, and stimulating discussions with Eric P. Cohen, MD and Garrett J. Gross, PhD are gratefully acknowledged.
This work was supported in part by cooperative agreement AI067734 and grants HL54075 and AI091173 from the National Institutes of Health.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and preparation of this paper.
REFERENCES
- 1.Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009:339b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meacham LR, Chow EJ, Ness KK, Kamdar KY, Chen Y, Yasui Y, et al. Cardiovascular risk factors in adult survivors of pediatric cancer--a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2010;19(1):170–81. doi: 10.1158/1055-9965.EPI-09-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frisk P, Arvidson J, Larsson M, Naessen T. Risk factors for cardiovascular disease are increased in young adults treated with stem cell transplantation during childhood. Pediatr Transplant. 2012;16(4):385–91. doi: 10.1111/j.1399-3046.2012.01693.x. [DOI] [PubMed] [Google Scholar]
- 4.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950-1997. Radiat Res. 2003;160(4):381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- 5.Yamada M, Wong FL, Fujiwara S, Akahoshi M, Suzuki G. Noncancer disease incidence in atomic bomb survivors, 1958-1998. Radiat Res. 2004;161(6):622–32. doi: 10.1667/rr3183. [DOI] [PubMed] [Google Scholar]
- 6.Baker JE, Fish BL, Su J, Haworth ST, Strande JL, Komorowski RA, et al. 10 Gy total body irradiation increases risk of coronary sclerosis, degeneration of heart structure and function in a rat model. Int J Radiat Biol. 2009;85(12):1089–100. doi: 10.3109/09553000903264473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman CN, Blakely WF, Fike JR, Macvittie TJ, Metting NF, Mitchell JB, et al. Molecular and cellular biology of moderate-dose (1-10 Gy) radiation and potential mechanisms of radiation protection: report of a workshop at Bethesda, Maryland, December 17-18, 2001. Radiat Res. 2003;159(6):812–34. doi: 10.1667/rr3021. [DOI] [PubMed] [Google Scholar]
- 8.Cohen EP. Radiation nephropathy after bone marrow transplantation. Kidney Int. 2000;58(2):903–18. doi: 10.1046/j.1523-1755.2000.00241.x. [DOI] [PubMed] [Google Scholar]
- 9.O'donoghue J. Relevance of external beam dose-response relationships to kidney toxicity associated with radionuclide therapy. Cancer Biother Radiopharm. 2004;19(3):378–87. doi: 10.1089/1084978041425025. [DOI] [PubMed] [Google Scholar]
- 10.Adams MJ, Grant EJ, Kodama K, Shimizu Y, Kasagi F, Suyama A, et al. Radiation dose associated with renal failure mortality: a potential pathway to partially explain increased cardiovascular disease mortality observed after whole-body irradiation. Radiat Res. 2012;177(2):220–8. doi: 10.1667/rr2746.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Zheng H, Ou X, Albertson CM, Fink LM, Herbert JM, et al. Hirudin ameliorates intestinal radiation toxicity in the rat: support for thrombin inhibition as strategy to minimize side-effects after radiation therapy and as countermeasure against radiation exposure. J Thromb Haemost. 2004;2(11):2027–35. doi: 10.1111/j.1538-7836.2004.00960.x. [DOI] [PubMed] [Google Scholar]
- 12.Lawton CA, Barber-Derus SW, Murray KJ, Cohen EP, Ash RC, Moulder JE. Influence of renal shielding on the incidence of late renal dysfunction associated with T-lymphocyte deplete bone marrow transplantation in adult patients. Int J Radiat Oncol Biol Phys. 1992;23(3):681–6. doi: 10.1016/0360-3016(92)90035-g. [DOI] [PubMed] [Google Scholar]
- 13.Cohen EP, Fish BL, Sharma M, Li XA, Moulder JE. Role of the angiotensin II type-2 receptor in radiation nephropathy. Transl Res. 2007;150(2):106–15. doi: 10.1016/j.trsl.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moulder JE, Fish BL. Late toxicity of total body irradiation with bone marrow transplantation in a rat model. Int J Radiat Oncol Biol Phys. 1989;16(6):1501–9. doi: 10.1016/0360-3016(89)90955-3. [DOI] [PubMed] [Google Scholar]
- 15.Moulder JE, Holcenberg JS, Kamen BA, Cheng M, Fish BL. Renal irradiation and the pharmacology and toxicity of methotrexate and cisplatinum. Int J Radiat Oncol Biol Phys. 1986;12(8):1415–8. doi: 10.1016/0360-3016(86)90184-7. [DOI] [PubMed] [Google Scholar]
- 16.Croswell A, Amir E, Teggatz P, Barman M, Salzman NH. Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infect Immun. 2009;77(7):2741–53. doi: 10.1128/IAI.00006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Migrino RQ, Zhu X, Pajewski N, Brahmbhatt T, Hoffmann R, Zhao M. Assessment of segmental myocardial viability using regional 2-dimensional strain echocardiography. J Am Soc Echocardiogr. 2007;20(4):342–51. doi: 10.1016/j.echo.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Cabral HJ. Multiple comparisons procedures. Circulation. 2008;117(5):698–701. doi: 10.1161/CIRCULATIONAHA.107.700971. [DOI] [PubMed] [Google Scholar]
- 19.Andrews HL, Brace KC. Modification of early radiation death in guinea pigs. Am. J. Physiol. 1956;187(2):378–80. doi: 10.1152/ajplegacy.1956.187.2.378. [DOI] [PubMed] [Google Scholar]
- 20.Mack HP, Figge FH. Sodium Pentobarbital Anesthesia and Mortality from X-Radiation in C57 Black Mice. Science. 1952;115(2994):547–8. doi: 10.1126/science.115.2994.547. [DOI] [PubMed] [Google Scholar]
- 21.Lam V, Moulder JE, Salzman NH, Dubinsky EA, Andersen GL, Baker JE. Intestinal microbiota as novel biomarkers of prior radiation exposure. Radiat Res. 2012;177(5):573–83. doi: 10.1667/rr2691.1. [DOI] [PubMed] [Google Scholar]
- 22.Chen MH, Colan SD, Diller L. Cardiovascular disease: cause of morbidity and mortality in adult survivors of childhood cancers. Circ Res. 2011;108(5):619–28. doi: 10.1161/CIRCRESAHA.110.224519. [DOI] [PubMed] [Google Scholar]
- 23.Sera N, Hida A, Imaizumi M, Nakashima E, Akahoshi M. The association between chronic kidney disease and cardiovascular disease risk factors in atomic bomb survivors. Radiat Res. 2013;179(1):46–52. doi: 10.1667/RR2863.1. [DOI] [PubMed] [Google Scholar]
- 24.Boerma M, Roberto KA, Hauer-Jensen M. Prevention and treatment of functional and structural radiation injury in the rat heart by pentoxifylline and alpha-tocopherol. Int J Radiat Oncol Biol Phys. 2008;72(1):170–7. doi: 10.1016/j.ijrobp.2008.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boerma M, Wang J, Wondergem J, Joseph J, Qiu X, Kennedy RH, et al. Influence of mast cells on structural and functional manifestations of radiation-induced heart disease. Cancer Res. 2005;65(8):3100–7. doi: 10.1158/0008-5472.CAN-04-4333. [DOI] [PubMed] [Google Scholar]
- 26.Fajardo LF, Stewart JR. Cardiovascular radiation syndrome. N Engl J Med. 1970;283(7):374. doi: 10.1056/NEJM197008132830716. [DOI] [PubMed] [Google Scholar]
- 27.Yeung TK, Hopewell JW. Effects of single doses of radiation on cardiac function in the rat. Radiother Oncol. 1985;3(4):339–45. doi: 10.1016/s0167-8140(85)80047-5. [DOI] [PubMed] [Google Scholar]
- 28.Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, Kodama K, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76(3):656–65. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migrino RQ, Aggarwal D, Konorev E, Brahmbhatt T, Bright M, Kalyanaraman B. Early detection of doxorubicin cardiomyopathy using two-dimensional strain echocardiography. Ultrasound Med Biol. 2008;34(2):208–14. doi: 10.1016/j.ultrasmedbio.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pieper GM, Shah A, Harmann L, Cooley BC, Ionova IA, Migrino RQ. Speckle-tracking 2-dimensional strain echocardiography: a new noninvasive imaging tool to evaluate acute rejection in cardiac transplantation. J Heart Lung Transplant. 2010;29(9):1039–46. doi: 10.1016/j.healun.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Migrino RQ, Zhu X, Morker M, Brahmbhatt T, Bright M, Zhao M. Myocardial dysfunction in the periinfarct and remote regions following anterior infarction in rats quantified by 2D radial strain echocardiography: an observational cohort study. Cardiovasc Ultrasound. 2008:617. doi: 10.1186/1476-7120-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Migrino RQ, Ahn KW, Brahmbhatt T, Harmann L, Jurva J, Pajewski NM. Usefulness of two-dimensional strain echocardiography to predict segmental viability following acute myocardial infarction and optimization using bayesian logistic spatial modeling. Am J Cardiol. 2009;104(8):1023–9. doi: 10.1016/j.amjcard.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neben-Wittich MA, Wittich CM, Mueller PS, Larson DR, Gertz MA, Edwards WD. Obstructive intramural coronary amyloidosis and myocardial ischemia are common in primary amyloidosis. Am J Med. 2005;118(11):1287. doi: 10.1016/j.amjmed.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Moulder JE, Fish BL, Cohen EP. Impact of angiotensin II type 2 receptor blockade on experimental radiation nephropathy. Radiat Res. 2004;161(3):312–7. doi: 10.1667/rr3129. [DOI] [PubMed] [Google Scholar]
- 35.Geraci JP, Mariano MS. Radiation hepatology of the rat: parenchymal and nonparenchymal cell injury. Radiat Res. 1993;136(2):205–13. [PubMed] [Google Scholar]
- 36.Huff J, Cucinotta FA. Risk of degenerative tissue or other health effects from radiation exposure. In: Mcphee J, Charles J, editors. Human Health and Performance Risks of Space Exploration Missions. National Aeronautics and Space Administration, NASA; SP-2009-3405, Houston, TX: 2009. pp. 213–35. [Google Scholar]