Abstract
M1 activation of macrophages promotes inflammation and immunity to intracellular pathogens, while M2 macrophage activation promotes resolution of inflammation, wound healing, and tumor growth. These divergent phenotypes are characterized, in part, by the expression of iNOS and arginase I (Arg1) in M1 vs. M2 activated macrophages, respectively. Here we demonstrate that the Ron receptor tyrosine kinase tips the balance of macrophage activation by attenuating the M1 phenotype while promoting expression of Arg1, through a Stat6-independent mechanism. Induction of the Arg1 promoter by Ron is mediated by an AP-1 site located 433 bp upstream of the transcription start site. Treatment of primary macrophages with MSP, the ligand for Ron, induces potent MAP kinase activation, upregulates Fos, and enhances binding of Fos to the AP-1 site in the Arg1 promoter. In vivo, Arg1 expression in tumor-associated macrophages (TAMs) from Ron−/− mice was significantly reduced compared with TAMs from control animals. Furthermore, we show that Ron is expressed specifically by Tie2-expressing macrophages (TEMs), a TAM subset that exhibits a markedly skewed M2 and pro-tumoral phenotype. Decreased Arg1 in TAMs from Ron−/− mice was associated with reduced syngeneic tumor growth in these animals. These findings indicate that Ron induces Arg1 expression in macrophages through a previously uncharacterized AP-1 site in the Arg1 promoter, and that Ron could be therapeutically targeted in the tumor microenvironment to inhibit tumor growth by targeting expression of Arg1
Introduction
L-arginine metabolism in macrophages is regulated by the enzymes arginase I (Arg1) and inducible nitric oxide synthase (iNOS), which produce urea and ornithine (precursor for synthesis of polyamines and pralines) or citrulline and the inflammatory mediator nitric oxide (NO), respectively(1). Divergent expression of Arg1 and iNOS has contributed to the dichotomous nomenclature of macrophages(2, 3). M1 (or classically activated) macrophages express high levels of iNOS and low levels of Arg1, and participate in the clearance of intracellular pathogens. Conversely, M2 (or alternatively activated) macrophages express the reverse pattern, and not only develop in response to parasitic infections in a Th2 cytokine-dependent manner(4), but also protect host tissue from inflammatory damage(5). While dysregulated expression of iNOS by M1 macrophages promotes inflammatory damage, as occurs in atherosclerosis(6, 7), excess expression of Arg1 by M2 macrophages thwarts effective immunity against intracellular pathogens(8) and exacerbates tumor growth(9–11). Thus, during both the initiation and resolution of immunity, expression of iNOS and Arg1 in macrophages must be tightly regulated in order to protect the host from potentially damaging inflammation.
Tumor-associated macrophages (TAMs) represent a substantial fraction of the growing tumor mass and are associated with poor prognosis in many human tumors(12–14). TAMs play disparate roles in tumor progression, depending on their phenotypic properties. M2 skewed macrophages promote tumor growth by virtue of their proangiogenic and prometastatic properties. In addition, high levels of Arg1 expression in TAMs results in depletion of its substrate, L-arginine, from the extracellular environment, decreased CD3ζ chain expression in T cells, and inhibition of antigen-specific T cell proliferation(15). Tie2-expressing macrophages (TEMs) comprise a TAM subset that express high levels of Arg1 and have an anti-inflammatory and pro-angiogenic (i.e. M2) phenotype(16). Selective depletion of this TAM subset results in inhibition of both angiogenesis and tumor growth(17). Thus, targeting the tumor-promoting activity of TAMs, including the expression of Arg1, has important therapeutic implications.
Receptor tyrosine kinases (RTKs) are involved in the initiation and progression of a variety of human malignancies, and are targets for molecular intervention. Expression of the Ron RTK is markedly elevated in a large percentage of epithelial cancers derived from breast (56%), colon (51%), lung (48%), thyroid (42%), skin (37%), bladder (36%) and pancreas (33%)(18), and constitutively active splice variants of Ron have been isolated from human colorectal carcinoma cells(19). Increased expression and constitutive phosphorylation of Ron has been observed in primary breast carcinomas, while normal breast epithelial cells, as well as cells in benign lesions (adenomas and papillomas), express comparatively low levels of Ron(20). Moreover, increased expression of Ron or its ligand, macrophage stimulating protein (MSP), in human breast carcinomas is associated with aggressive disease and poor prognosis(21). Thus, Ron is a promising target for therapeutic intervention against malignant phenotypes(22).
Ron is also expressed on tissue-resident macrophages and suppresses hallmarks of M1 activation, including expression of iNOS, IL-12p40 and TNFα in primary macrophages stimulated with IFNγ and LPS(23, 24). This inhibition is due to the ability of Ron to inhibit Stat1 phosphorylation in response to IFNγ(23, 25) and to inhibit NFκB activation in response to LPS(24, 26). Ron−/− mice express elevated levels of IL-12p40 following endotoxin challenge, resulting in enhanced IFNγ production by NK cells and increased susceptibility to septic shock(25). Furthermore, the absence of Ron leads to enhanced inflammation in animal models of multiple sclerosis(27), acute lung injury(28), and delayed-type hypersensitivity(29). Taken together, these observations indicate that Ron plays a central role in limiting Th1-mediated inflammatory responses.
In addition to its role in suppressing inflammation, we have demonstrated that Ron promotes hallmarks of an M2, or anti-inflammatory phenotype in primary macrophages, as exemplified by the upregulation of Arg1, scavenger receptor, and IL-1 receptor antagonist expression(30). While numerous studies have addressed the role of RTKs, including Ron, in the promotion of tumor cell growth and metastasis in a cell-autonomous manner, the potential role of these receptors in the tumor microenvironment remains poorly defined. Here, we demonstrate that Ron induces Arg1 expression through a previously uncharacterized AP-1 binding site in the Arg1 promoter. We further demonstrate that Ron promotes Arg1 expression in vivo in TAMs, and that Ron expression in the tumor microenvironment promotes tumor growth. In addition to the well-established role of RTKs in promoting tumor growth in a cell-autonomous manner, our findings indicate that therapeutic targeting of an RTK in the tumor microenvironment could retard tumor growth and improve clinical outcome.
Materials and Methods
Mice, Cells and Reagents
6–10 week-old C57BL/6 mice or Ron−/− mice, described previously (29), were used for this study. HEK293T, 3LL, and B16-F10 cells were purchased from ATCC (Manassas, VA). EG.7 cells were kindly provided by Dr. Avery August (The Pennsylvania State University). Cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with L-glutamine, non-essential amino acids, sodium pyruvate (CellGro, Mediatech, Manassas, VA), 10% FBS (Gibco, Gaithersburg, MD) and 10 µg/ml ciprofloxacin (Serologicals Proteins, Inc. Kankakee, IL). Antibodies for Western blot analysis were phospho-p44/42, phospho-Gab1 (Y307), phospho-Gab2 (Y452 and S159), p44/42, Gab1, Gab2 (Cell Signaling, Beverly, MA), actin (Sigma, St. Louis, MO), c-Fos (sc-253, Santa Cruz Biotechnology, CA) and arginase I (BD-Transduction Laboratories, San Jose, CA). Antibodies for flow cytometry and FACS were CD16/32 Fc-block, CD11b APC-750, Gr1-FITC, CD4-Cy5, CD8-FITC, CD31-FITC, Streptavidin-PE (BD Pharmingen Inc., San Diego, CA), Gr1-FITC, CD11b'APC-eFluor™780, MRC1-Biotin (BioLegend, San Diego, CA), CD11cPC7 (eBioscience, San Diego, CA) F4/80 RPE (Serotec, Raleigh NC), anti-Ron (R&D Systems, Minneapolis, MN), and anti-goat Alexa Fluor 647 (Molecular Probes, Eugene, OR). Antibodies used for ChIP analysis were Stat6 (sc-1698x) and Fos (sc-52x) from Santa Cruz Biotechnology. Recombinant human MSP-C632A and murine IL-4 were purchased from R&D Systems (Minneapolis, MN). Map kinase inhibitors used were U0126 (Cell Signaling, Beverly, MA), SB2035801 and SP600125 (Cayman Chemicals, Ann Arbor, MI). The Pennsylvania State University Institutional Animal Care and Use Committee (IACUC) approved all animal experiments.
Flow Cytometry
Single cell suspensions of splenocytes were achieved using a Dounce homogenizer. RBCs were lysed for 15 minutes on ice using ACK buffer (0.15M NH4CL, 1 mM KHCO3, 0.1 mM Na2EDTA pH7.4), and resuspended in FACS buffer (ice cold PBS + 2% FBS). 3×106 cells were stained in 100 µL FACS buffer with indicated antibodies. Dissected and minced tumors were digested with 0.05% collagenase type IV (Sigma) for 30 minutes at 37°C, and filtered through 70 µm nylon mesh (BD Falcon) to achieve single cell suspensions. Cells were stained with the indicated antibodies and analyzed on a Beckman FC500 flow cytometer.
Macrophage Isolation
Resident peritoneal macrophages were extracted from 6–10 week old C57BL/6 or FVB mice as previously described(30) and incubated at 37°C for 2 hours prior to stimulation. Thioglycollate elicited macrophages were prepared by injecting 2-month old C57BL/6 mice with 2 mL of 3% aged thioglycollate media (Difco, Sparks, MD). On day 4, cells were isolated by lavage and prepared as above for resident macrophages. MDSCs and TAMs were isolated from spleen or tumor cell suspensions from day 15 3LL tumor-bearing mice by FACS with antibodies to CD11b, Gr1 and F4/80 using a Cytopeia influx cell sorter. TEMs and inflammatory TAMs(16) were isolated from MMTV-PyMT spontaneous mammary tumors (14 weeks of age). Tumors were excised and made into single-cell suspensions by collagenase IV (0.2 mg/ml, Worthington), dispase (2 mg/ml, Gibco) and DNaseI (0.1 mg/ml, Roche) treatment. Before sorting, all cell suspensions were incubated with rat anti-mouse FcγIII/II receptor (CD16/CD32) blocking antibodies together with monoclonal antibodies and 7-AAD to stain nonviable cells. TEMs were isolated as 7-AAD− CD11b+ GR1− CD31low/− MRC1+ CD11c− cells and inflammatory TAMs as 7-AAD− CD11b+ GR1− CD31low/− MRC1low/− CD11c+ cells(16). This gating formula excludes CD31+ endothelial cells, Gr1+granulocytes and 7-AAD+ nonviable cells from the analysis. Cells were sorted using a MoFlo apparatus (Dako). After sorting, purity was >95%.
Plasmid Construction
Identification of Arg1 promoter binding sites was performed using MatInspector software by Genomatix (Ann Arbor, MI). Arg1 reporter constructs, except the AP-1 mutant, were described previously(31). Mutagenesis of the AP-1 site was performed using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). Forward primer, 5’-CCCAGAACTTGAAGCCTTGTTGCAGGATGCTCAACAGGAGG-3’, reverse primer, 5’-CCTCCTGTTGAGCATCCTGCAACAAGGCTTCAAGTTCTGGG-3’. Ron and Src constructs were described previously(32, 33).
Luciferase Assay
6×105 293T cells were plated per well in 24-well plates. After 16 hours, cells were transfected with 15 ng/well Arg1 promoter, PCI-Ron or PCI-neo control, and 0.5 ng/well Renilla luciferase plasmids using Mirus Transit-293 transfection reagent (Mirus, Madison, WI). 24 hours post-transfection, cells were stimulated with MSP (100 ng/mL), for 20 hours and luciferase activity was measured using the Dual Luciferase reporter system (Promega, Madison, WI). Renilla luciferase activity was used to normalize for transfection efficiency. For Arg1 promoter deletion constructs, equimolar amounts of the plasmids were added and balanced to the 3.29 construct with PCI-Neo. For experiments with the Src constructs, 100 ng/well of plasmid was added.
Western blot analysis
1×106 resident peritoneal macrophages or 3×106 thioglycollate-elicited macrophages were stimulated with MSP (100 ng/mL) or IL-4 (10 ng/mL). Cells were washed in PBS and lysed for 30 minutes at 4°C in RIPA buffer containing protease and phosphatase inhibitors (10 mM Tris-HCL, pH 7.5, 140 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, 1% Triton X-100, 0.1% SDS, 0.1% Na-deoxycholate, NaF (10 mM), Na3VO4 (4 mM), PMSF (1 mM), Aprotinin (10 µg/mL), Leupeptin (10 µg/mL), and Pepstatin A (1 µg/mL). Lysates were cleared by centrifugation at 14,000×g, separated by SDS-PAGE and transferred onto Immobilon-P PVDF membranes (Millipore, Bedford, MA). Blots were incubated overnight with primary antibody at 4°C. Anti-rabbit and anti-mouse HRP conjugated secondary antibodies were added for 45 minutes at room temperature. ECL Plus (Amersham, Piscataway, NJ) was used to develop the blots.
Quantitative RT-PCR
For mRNA expression analysis in resident peritoneal macrophages, 1×106 cells were stimulated with MSP (100 ng/mL). RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA). Reverse transcription was carried out using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). 100 ng of cDNA, 300 nM of each primer and 20 nM of probe were used per qPCR reaction with TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA), and reactions were run and analyzed using an ABI7300. Gapdh (Applied Biosystems, Foster City, CA) was used as an internal control. Arg1 primers: forward, 5’-TTGGGTGGATGCTCACACTG-3’, reverse 5’-TTGCCCATGCAGATTCCC-3’; probe: 5’-CATCAACACTCCCCTGACAACCAGCTC-3’. Gene-specific primer and probe sets for Mrc1, Clec7a, Chi3l3, Retnia, Tnf, Il1b, Il6, Il12b and Inos were purchased from Applied Biosystems.
For Ron and ArgI comparative gene expression in TEMs, inflammatory TAMs and resident peritoneal macrophages, mRNA from at least 2.5–10×104 cells was purified following the RNeasy Micro kit guidelines (Qiagen). RNA was retrotranscribed with SuperScript III (SuperScript® VILO™ cDNA Synthesis Kit, Invitrogen). qPCR analyses were performed with TaqMan probes from Applied Biosystems using an ABI7900HT (Applied Biosystems). To calculate differences in mRNA levels of Ron and Arg1 in TEMs and inflammatory TAMs, β2m and Gapdh were used as reference genes, respectively. Statistical analysis of the data was performed on actual ΔCt values by Student’s t-test on two biological replicates. To compare mRNA levels of Ron and Arg1 in TEMs, inflammatory TAMs and resident peritoneal macrophages, Ron and ArgI mRNA copies are expressed as relative to b2m mRNA copies. Statistical analysis of the data was performed on actual ΔCt values by Student’s t-test on three (TEMs and inflammatory TAMs) or two (resident peritoneal macrophages) biological replicates.
Arginase activity assay
Primary resident peritoneal macrophages were treated with the indicated inhibitors for 2 hours followed by MSP or IL4 treatment for 4 hours. Cells were lysed with 100 µl of 0.1% Triton X-100. After 30 min on a shaker, 100 µl of 25 mM Tris-HCl was added. To 100 µl of this lysate, 10 µl of 10 mM MnCl2 was added, and the enzyme was activated by heating for 10 min at 55°C. Arginine hydrolysis was conducted by incubating the lysates with 100 µl of 0.5 M L-arginine (pH 9.7) at 37°C for 60 min. The reaction was stopped with 800 µl of H2SO4 (96%)/H3PO4 (85%)/H2O (1/3/7, v/v/v). The urea concentration of samples and standards was measured at 550 nm after addition of 40 µl of α-isonitrosopropiophenone (sigma-Aldrich) (dissolved in 100% ethanol), followed by heating at 100°C for 30 min.
Chromatin Immunoprecipitation
Resident or thioglycollate-elicited peritoneal macrophages stimulated with 100 ng/mL MSP or 10 ng/mL IL-4 were fixed with 1% formaldehyde for 10 minutes at room temperature and quenched with 0.125 M glycine for 5 minutes. 10×106 cells/sample were spun at 2000×g at 4°C for 5 minutes, and resuspended in 300 µL cell lysis buffer (50 mM Tris-HCL pH 8.0, 10 mM EDTA, 1% SDS and protease inhibitors (Aprotinin (10 µg/mL), Leupeptin (10 µg/mL) and Pepstatin A (1 µg/mL)). Chromatin was sonicated using the Diagenode Bioruptor (power setting high, 20 cycles of 30 seconds on, 60 seconds off) yielding DNA of 200–600 bp in length. Samples were centrifuged at 14,000×g for 15 minutes at 4°C to remove debris. The volume equivalent of 1.5×106 resident or 4×106 thioglycollate-elicited macrophages was aliquoted into 10× ChIP dilution buffer (0.5% Triton-X 100, 2.2 mM EDTA, 22 mM Tris-HCL pH8.0, 150 mM NaCl + protease inhibitors), and incubated overnight at 4°C with primary antibody (3 µg/mL). The next morning, 20 µL of protein A or G magnetic beads (New England Biolabs, Beverly, MA) was added for 2 hours at 4°C. Beads were collected by magnetic isolation and washed three times with 1 mL of low salt wash buffer (0.1% SDS, 1% Triton-X 100, 2 mM EDTA, 20 mM Tris-HCL pH 8.1, 150 mM NaCl) and twice with TE. Complexes were reverse-crosslinked in 140 µL elution buffer (20 mM Tris-HCl pH 8.0, 5 mM EDTA, 50 mM NaCl, 1% SDS, 125 µg/mL proteinase K) at 65°C for 3 hours. DNA was collected using DNA Clean and Concentrator (Zymo Research, Orange, CA). 2 µL of DNA per qPCR reaction using SensiMix SYBR (Bioline, Taunton, MA) was analyzed on an ABI7900HT. Primers: AP1-site: forward, 5’-GCCCCATGCTTTCCTAGACA-3’, reverse, 5’-GAGCATCCTGAGTCAAGGCTTC-3’; Stat6-site: forward, 5’-GCATTGTTCAGACTTCCTTATGCTT-3’, reverse, 5’-TGTTGGCTAATACAGCCTGTTCAT-3’.
Tumor measurements
The indicated tumor cells were grown to confluence, trypsinized, and resuspended in PBS. 100 µL containing 5×105 cells was injected subcutaneously into the shaved right flank of wild-type or Ron−/− mice. Upon onset of palpable tumor growth, mice were examined every other day by caliper for tumor volume as determined by the formula ab2×0.4, where a is the length and b is the width.
Mixed Lymphocyte Reaction
Splenocytes from tumor free BALB/c mice were γ-irradiated and 2–4×105 cells per well were plated as stimulators. 2–8×105 C57BL/6 splenocytes from wild-type or Ron−/− tumor-bearing mice were plated as responders. Cells were cultured for 5 days at 37°C, and for the final 24 hours cells were pulsed with 0.5 µCi per well of [3H]-thymidine. Cells were collected with a semi-automated cell harvester and analyzed on a scintillation counter for [3H]-thymidine content.
Antigen specific challenge of splenocytes and ELISA
Splenocytes were collected from day 15 EG.7 mice and lysed with ACK lysis buffer as above. 5×106 splenocytes were added per well of a 24 well plate in 600 µL of complete DMEM. 1 mL containing 4×105 γ-irradiated EG.7 cells (5000 RADS) were added to each well of the co-culture. Cells were incubated at 37°C for 3 days at which time an additional 400 µL containing 6×105 γ-irradiated EG.7 cells was added. On day 4, plates were centrifuged at 250×g for 5 minutes, and 1 mL of supernatant was collected for ELISA analysis using BD OptEIA kits (BD-Pharmingen, San Diego, CA).
Multiplex cytokine assay
Serum was collected from euthanized mice by cardiac puncture on day 7, 11, and 15 from EG.7 wild-type and Ron−/− TBM. Serum was analyzed for IP10 using the LincoPlex assay kit (Millipore).
Statistical analysis
Minitab software was used for statistical analysis. Students T test, ANOVA and MANOVA were used as indicated. Differences were considered significant at p<0.05.
Results
Ron inhibits expression of inflammatory mediators and promotes expression of Arg1 in primary macrophages
M1 and M2 polarized macrophages, induced by IFNγ and TLR agonists vs. IL-4 and IL-13, respectively, represent the endpoints of a continuum of macrophage phenotypes responsible for such diverse functions as sensing the presence of microbes and promoting immunity to infection, phagocytosis of a wide array of pathogen- and host-derived factors, and resolution of inflammation and tissue repair. We have shown previously that Ron inhibits the induction of iNOS and IL-12p40 in response to M1 polarizing signals while promoting the upregulation of Arg1. In order to further assess the role of Ron in the regulation of these polarized phenotypes, we stimulated primary peritoneal macrophages with MSP, and assessed the expression of a panel of M1 and M2 associated genes by real-time PCR. MSP alone induced expression of Il1b and Il6, markers of M1 macrophage activation, and Arg1 and Clec7a (the gene encoding Dectin-1), markers of M2 macrophage activation (Figure 1A). Primary macrophages were also stimulated with LPS or IL-4, to polarize cells toward an M1 or M2 phenotype, respectively, following overnight stimulation with MSP. As demonstrated previously, MSP inhibited expression of the M1 markers, Inos, Il12b (the gene encoding IL-12p40), Tnf, and Il1b, but not Il6, in the presence of LPS (Figure 1B). The expression of the M2 markers Mrc1,Chi3l3 (the gene encoding Ym-1) and Retnla (the gene encoding Fizz-1), by IL-4 was also significantly reduced in the presence of MSP (Figure 1C), however expression of Arg1 was maintained or slightly increased under these conditions. This observation suggests the possibility that, in addition to Th1-mediated inflammation, Ron could also play a role in the inhibition of Th2-mediated inflammation, during which macrophage-derived Arg1 plays a protective role.
Figure 1. Ron regulates M1 and M2 macrophage activation.
A) Primary resident peritoneal macrophages were stimulated for 4 hours with 100 ng/ml MSP and expression of genes associated with M1 and M2 macrophage activation were assessed by real-time PCR. B) Primary peritoneal macrophages were cultured overnight with or without MSP followed by stimulation with 100 ng/ml LPS for four hours and the expression of genes associated with M1 macrophage activation was assessed by real-time PCR. C) Primary peritoneal macrophages were cultured overnight with or without MSP followed by stimulation with 5ng/ml IL-4 for four hours and expression of genes associated with M2 macrophage activation was assessed by real-time PCR. Results are the average of two independent experiments. *p<0.05, **p<0.01, ***p<0.001.
Ron induces Arg1 promoter activity
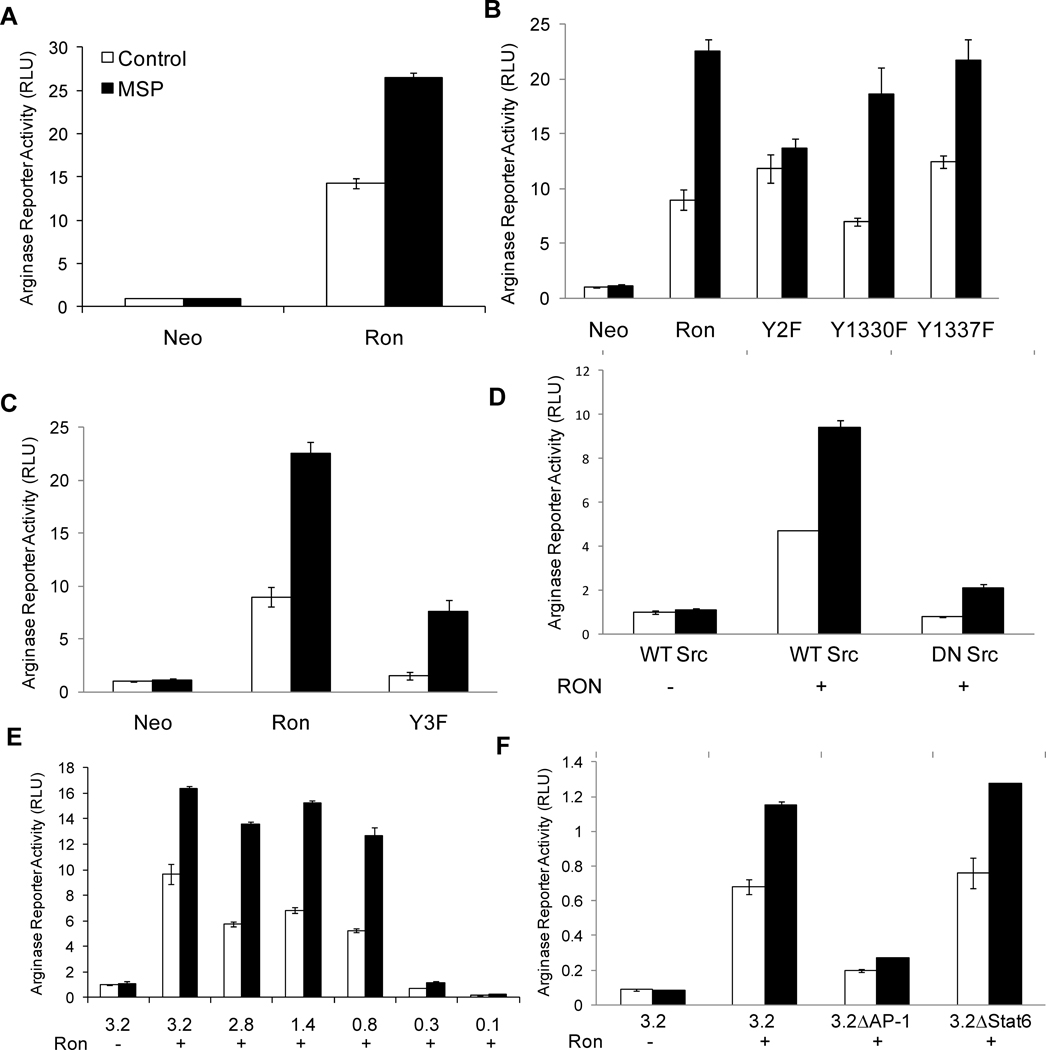
In order to determine the mechanism underlying the induction of Arg1 by Ron, we utilized a previously described Arg1 luciferase reporter containing 3.29 kb DNA upstream of the transcription start site (TSS) of the Arg1 promoter(31). The Arg1 reporter was transiently transfected with or without a cDNA encoding Ron in 293T cells and luciferase activity was measured. Reporter activity increased markedly in Ron-expressing cells, and was further induced by stimulation with MSP (Figure 2A). This confirms that Ron induces Arg1 expression by regulating Arg1 promoter activity. Two docking site tyrosines (Y1330 and Y1337) in the c-terminal tail of Ron mediate cell signaling and phenotypic events in response to MSP(32). To determine whether the docking site tyrosines are required for induction of Arg1 promoter activity by Ron, we co-transfected Ron-Y2F (Y1330/37F), Ron-Y1330F or RonY1337F with the Arg1 reporter plasmid and examined luciferase activity in the presence and absence of MSP. As expected, Ron-Y2F failed to induce Arg1 reporter activity in response to MSP stimulation (Figure 2B). However, MSP retained its ability to induce Arg1 promoter activity upon stimulation of the single docking site tyrosine mutants RonY1330F or Ron-1337F, indicating that these docking site tyrosines induce overlapping signaling pathways that are required for the induction of Arg1 promoter activity in response to MSP stimulation.
Figure 2. Ron induction of Arg1 promoter activity is mediated by an AP-1 binding site.
A–C) 293T cells were co-transfected with the indicated Ron constructs or Neo control, Renilla, and a luciferase reporter driven by a 3.29 kb fragment of the Arg1 promoter. Following transfection, cells were left unstimulated, or stimulated with MSP for 20 hours, and luciferase reporter activity was measured and normalized to Renilla. D) 293T cells were co-transfected with Ron or Neo, Renilla and wild-type or dominant negative Src as indicated. Arg1 reporter activity was measured and normalized to Renilla. Equivalent expression of Ron was confirmed in all experiments by Western blot analysis (data not shown). Data are presented as mean ± S.D., and are representative of three or more independent experiments. E) 293T cells were co-transfected with Ron or Neo control, Renilla, and the indicated series of 5’-terminally deleted Arg1 promoter constructs. Following transfection, cells were left unstimulated, or stimulated with 100 ng/mL MSP for 20 hours, and luciferase reporter activity was measured and normalized to Renilla. F) 293T cells were co-transfected with Ron or Neo, Renilla, and the 3.29 kb Arg1 promoter construct with or without mutations in the AP-1 binding site or the Stat6 binding site as indicated. Arg1 reporter activity was measured and normalized to Renilla. Data are presented as mean ± S.D., and are representative of five independent experiments.
Ron also induces Arg1 promoter activity in these cells in the absence of ligand (Figure 2A). Previously, we found that ligand-independent induction of cell signaling by Ron is independent of the docking site tyrosines, but dependent on tyrosines 1175, 1265 and 1294 in the kinase domain of Ron, in a Src kinase-dependent manner(32). To determine whether these tyrosines are responsible for ligand-independent induction of Arg1 promoter activity by Ron, we co-transfected the Arg1 promoter with a Ron mutant harboring tyrosine-to-phenylalanine mutations at positions 1175, 1265 and 1294 (Y3F), and compared reporter activity with that induced by the WT receptor. Although the Y3F mutant was unable to induce reporter activity in a ligand-independent manner, it retained its ability to induce Arg1 reporter activity in response to MSP stimulation (Figure 2C). To determine whether the ligand-independent induction of Arg1 is mediated by Src, we co-transfected Ron with either WT or dominant-negative Src (Src-DN) and assessed Arg1 reporter activity. Co-transfection with Src-DN inhibited Ron-mediated Arg1 reporter activity to control levels, but MSP-induced activity was retained (Figure 2D).
Ron regulates Arg1 expression through an AP-1 site in the promoter proximal region
In order to identify the region of the Arg1 promoter responsible for mediating its induction by Ron, we utilized a previously described series of 5’-terminally deleted promoter constructs(31). A Stat6 binding element approximately 2.9 kb upstream of the TSS is critical for the induction of Arg1 promoter activity by IL-4 and IL-13(31, 34). However, deletion of this region did not result in a significant loss in promoter activity induced by Ron in the presence or absence of MSP. Alternatively, the region between −0.8 and −0.3 kb of the promoter was critical for the induction of Arg1 promoter activity by Ron in the presence or absence of ligand (Figure 2E). Our results indicate that induction of the Arg1 promoter by Ron occurs via a mechanism distinct from that previously observed for Th2 cytokines IL-4 and IL-13.
We employed MatInspector software to search the region between −0.8 kb and −0.3 kb of the Arg1 promoter for potential transcription factor binding sites. Of the potential sites identified, we focused on a match to the consensus binding site for AP-1 (TGAc/gTCA), a MAP kinase-responsive element, located 433 bp upstream of the TSS. To determine whether this binding site contributes to induction of Arg1 promoter activity by Ron, we mutated the AP-1 site in the context of the −3.29 kb and −0.8 kb Arg1 reporter constructs, co-transfected the reporter constructs with Ron in 293T cells, and measured luciferase activity. In the context of both the 3.29 kb (Figure 2F) and the 0.8 kb (data not shown) promoter fragments, the AP-1 mutation abolished Ron-induced reporter activity, whereas mutation of the upstream Stat6 element, previously identified as an IL-4-responsive site, did not decrease Ron-induced activity. Specificity of the AP-1 mutations was confirmed by testing mutations which flank the AP-1 site. These mutants did not result in the loss of activity (data not shown).
MSP induces MAP kinase signaling in primary macrophages
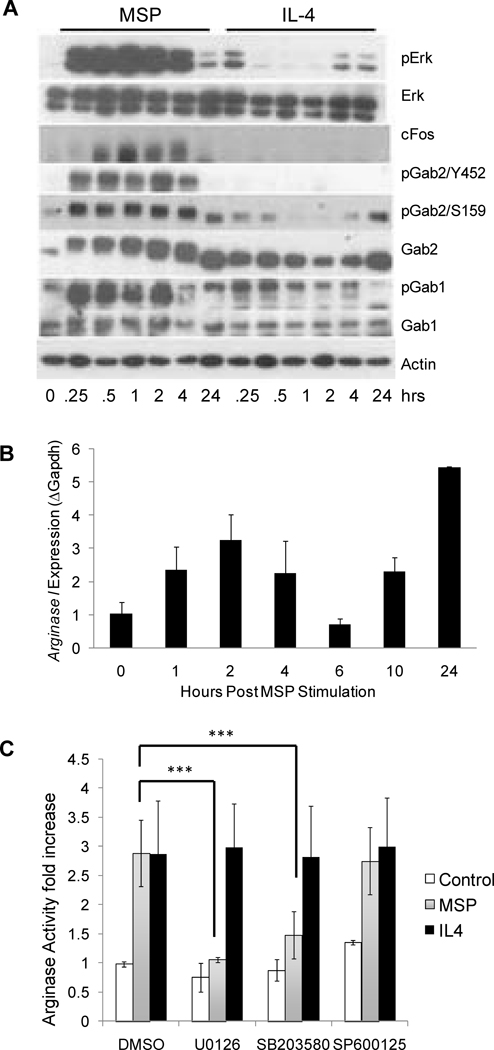
Consistent with the ability of Ron to induce Arg1 activity in an AP1-dependent manner, MSP stimulation of primary peritoneal macrophages induced strong and sustained activation of the MAP kinase, Erk, compared to weak and transient induction of Erk in these cells by the Th2 cytokine, IL-4 (Figure 3A). Consistent with these results, MSP, but not IL-4, induced robust expression of c-Fos in these cells. Activation of MAP kinase signaling downstream of Ron is mediated by the IRS-related adaptor proteins, Gab1 and Gab2. Docking site Y1330 binds directly to Gab1 via a Met binding domain (MBD)(35), whereas Grb2 binds to the second docking site Y1337 and thereby indirectly recruits either Gab1 or Gab2(36, 37). MSP induced strong phosphorylation of both Gab1 and Gab2 in primary peritoneal macrophages. This induction was not observed in macrophages stimulated with IL-4.
Figure 3. MSP induces arginase I expression in primary macrophages in a Map kinase-dependent manner.
A) Resident peritoneal macrophages were either unstimulated, or stimulated with 100 ng/mL MSP or 10 ng/mL IL-4 for the indicated times. Cell lysates were isolated and levels of phosphorylated Erk (pErk), total Erk, cFos, phosphorylated Gab2 (pGab2Y452 and pGab2S159), total Gab2, phosphorylated Gab1 (pGab1Y307), total Gab1, and actin were assessed by Western blot analysis. Data are representative of two or more independent experiments. B) Resident peritoneal macrophages stimulated with 100 ng/mL MSP for the indicated times and RNA was collected for quantitative RT-PCR analysis. Data are presented as mean ± S.D. and are representative of four independent experiments. C) Resident peritoneal macrophages were stimulated with DMSO, 10 µM U0126, 10 µM SB203580 or 20 µM SP600125 for 2 hours followed by stimulation for 24 hours with 100 ng/ml MSP or 5 ng/ml IL-4 and arginase activity was assessed. Results are the average of two independent experiments. ***p<0.001.
In order to determine the timeframe during which Arg1 transcription is induced by MSP in primary macrophages, Arg1 mRNA levels were quantified by qRT-PCR. While MSP induction of Arg1 expression was observed as early as 1 hour post-stimulation, the induction pattern was biphasic (Figure 3B). The timing of the early phase (1–4 hours post stimulation) of Arg1 induction by MSP mirrored that observed for the upregulation of c-Fos expression and the phosphorylation of Gab1, Gab2 and Erk in these cells (Figure 3A), suggesting that this early wave of expression could be regulated by AP-1. To determine whether the induction of Arg1 by MSP is Map kinase-dependent, we stimulated primary peritoneal macrophages with MSP or IL-4 in the presence and absence of the Map kinase inhibitors U0126 (MEK inhibitor), SB203580 (p38 inhibitor), and SP600125 (Jnk inhibitor), and assessed Arg1 activity (Figure 3C). The induction of Arg1 by MSP was completely inhibited by Mek inhibitor, suggesting that the upregulation of c-Fos expression by MSP could play a critical role in the induction of Arg1. None of these inhibitors had an adverse effect on the induction of Arg1 activity by IL-4.
MSP induces binding of c-Fos to the Arg1 promoter
To determine whether MSP induces AP-1 binding to the Arg1 promoter in primary macrophages at this early timepoint, we performed chromatin immunoprecipitation (ChIP) two hours following MSP stimulation. qPCR for the AP-1 site was performed on chromatin immunoprecipitates to detect binding of the AP-1 family member, Fos. Consistent with our in vitro results, we observed a 4-fold enrichment of Arg1 DNA by Fos ChIP in response to MSP, while IL-4, a weak MAP kinase activator, failed to induce significant Fos binding at this site (Figure 4A). Conversely, while IL-4 induced 10-fold enrichment of DNA by immunoprecipitation of Stat6 at the IL-4-response element (Figure 4B) as previously described(34), MSP induced little or no binding of Stat6 to the IL-4-response element. This is consistent with results in Figure 2 showing that the Stat6 site plays no role in the induction of Arg1 by MSP. In order to confirm these results, we performed similar studies in thioglycollate-elicited macrophages. We confirmed Ron expression in thioglycollate-elicited macrophages by flow cytometry (Figure 4C), and demonstrated that stimulation of these cells with MSP promoted Erk phosphorylation (Figure 4D), and induced the upregulation of Arg1 mRNA (Figure 4E). ChIP analysis in these cells confirmed binding of Fos to the AP-1 site in response to MSP (Figure 4F). Taken together, our results suggest that MSP initiates Arg1 transcription in primary macrophages through induction of AP-1 binding to the Arg1 promoter.
Figure 4. MSP stimulation of primary macrophages induced Fos binding to the AP-1 site in the Arg1 promoter.
Primary peritoneal macrophages were stimulated with 100 ng/mL MSP or 10 ng/mL IL-4 and chromatin immunoprecipitation (ChIP) and subsequent qPCR was performed for A) Fos and B) Stat6. Data are presented as mean ± S.D., normalized to unstimulated macrophages, and are representative of three independent experiments. C) Day 4 thioglycollate-elicited macrophages and resident peritoneal macrophages from wild-type or Ron−/− mice were analyzed by flow cytometry for Ron expression. D) Thioglycollate-elicited macrophages were stimulated with 100 ng/ml MSP for the indicated times and lysates were immunoblotted for phosphorylated Erk. E) Thioglycollate-elicited macrophages were stimulated with 100 ng/mL MSP for 2 hours and assessed for Arg1 expression by qRT-PCR. F) Thioglycollate-elicited macrophages were stimulated with 100 ng/mL MSP for 2 hours and Fos binding to the AP1 site in the Arg1 promoter was assessed by ChIP and subsequent qPCR. Data are presented as mean ± S.D., normalized to unstimulated macrophages, and representative of two independent experiments.
Ron promotes expression of Arg1 in tumor-associated macrophages in vivo
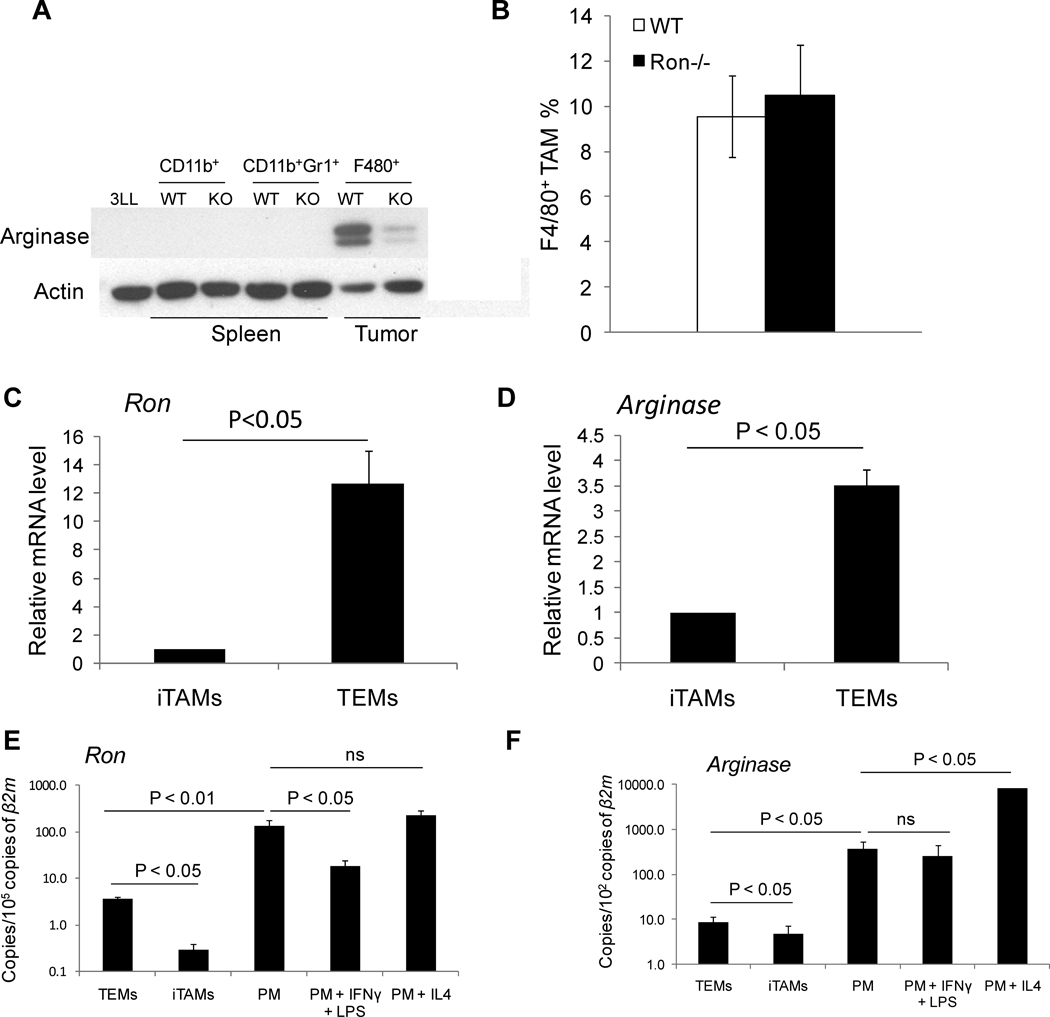
Tumor-associated macrophages (TAMs) express high levels of Arg1(15). In order to determine whether Ron regulates Arg1 expression in vivo, we examined Arg1 expression in CD11b+/F480+ TAMs from wild-type and Ron−/− animals. Consistent with the ability of Ron to induce Arg1 expression in primary macrophages in vitro, TAMs isolated from Ron−/− tumor-bearing mice (TBM) exhibited reduced Arg1 expression compared with wild-type controls (Figure 5A). Arg1 regulation was independent of TAM recruitment to the tumor microenvironment as the percentage of TAMs in wild-type and Ron−/− mice did not differ significantly (Figure 5B).
Figure 5. Reduced Arg1 expression in TAMs isolated from Ron−/− mice.
A) Tumors and spleens were isolated from day 15 3LL-injected wild-type and Ron−/− TBM. TAMs and MDSCs were isolated by FACS as CD11b+/F480+ and CD11b+/Gr1+ cells, respectively. Cell lysates were pooled from 4 animals and immunoblotted for Arg1 expression. Data are representative of three independent experiments. B) Tumors from day 15 EG.7-injected wild-type and Ron−/− TBM were collected and analyzed for percentage of F4/80+ TAMs by flow cytometry. WT (n=9), Ron−/− (n=10). Data are presented as ± S.E.M. C and D) TEMs (7-AADCD11b+GR1-CD31low/−MRC1+) and inflammatory TAMs (iTAMs; -AADCD11b+GR1CD31low/−CD11c+) were isolated by FACS from MMTV-PyMT spontaneous mammary tumors. Expression of Ron and Arg1 in these macrophage subsets was assessed by qPCR and is expressed as relative to expression in iTAMs. To calculate differences in mRNA levels, β2m and Gapdh were used as reference genes for Ron and Arg1, respectively. Statistical analysis of the data was performed on actual ΔCt values by Student’s t-test on two biological replicates. E and F) Expression Ron and Arg1 in TEMs, iTAMs and unstimulated peritoneal macrophages or macrophages stimulated with IFNγ and LPS or IL-4 was assessed by qPCR and normalized to β2m. To compare mRNA levels, Ron and Arg1 mRNA copies are expressed as relative to β2m mRNA copies. Statistical analysis of the data was performed on actual ΔCt values by Student’s t-test on three (TEMs and iTAMs) or two (resident peritoneal macrophages) biological replicates.
F480+ TAMs can be further divided into two main, non-overlapping subsets based on the expression of CD11c and the mannose receptor (MRC1)(16). F480+/CD11c+ TAMs express high levels of pro-inflammatory and anti-angiogenic cytokines and have been termed “inflammatory TAMs”(16). Conversely, F480+/MRC1+ TAMs express lower levels of pro-inflammatory molecules and higher levels of pro-angiogenic and tissue-remodeling molecules than inflammatory TAMs. These M2-skewed TAMs express Tie2 and are also known as TEMs(16). These two TAM subsets co-exist in tumors, where they exert distinct and perhaps opposing functions(16). In contrast to barely detectable expression of Ron in CD11c+ inflammatory TAMs, expression of Ron was increased by an average of 12-fold in MRC1+ TEMs (Figure 5C). In addition, MRC1+ TEMs express elevated levels of Arg1 compared with CD11c+ inflammatory TAMs (Figure 5D). These results suggest that regulation of Arg1 expression by Ron occurs within the MRC1+ subset of TAMs. Resident peritoneal macrophages expressed higher Ron mRNA levels than tumor-derived macrophages (Figure 5E). However, there was a clear correlation between the expression levels of Ron and Arg1 in each population analyzed (Figure 5F).
Reduced syngeneic tumor growth in Ron−/− mice
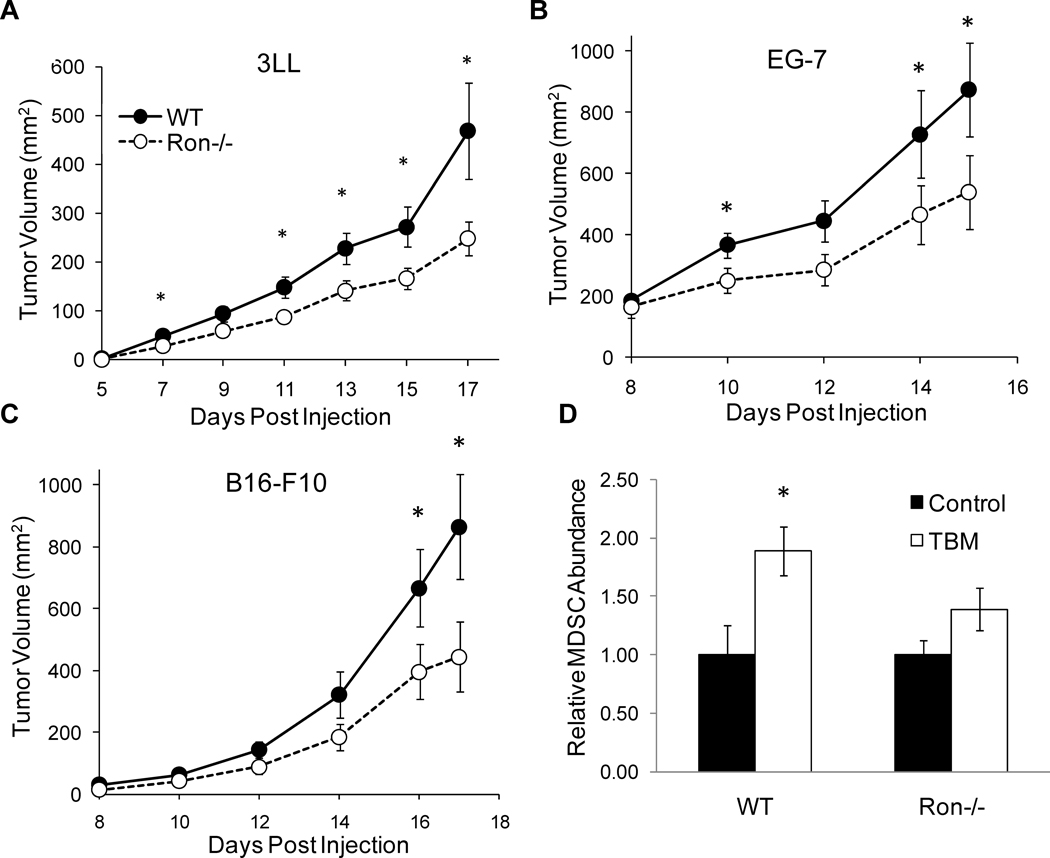
Arg1 expression in TAMs has been implicated in promoting tumor growth. In order to determine whether decreased Arg1 expression in Ron−/− TAMs correlates with decreased tumor growth, wild-type and Ron−/− animals were injected subcutaneously with 3LL (non-small cell Lewis lung carcinoma), B16-F10 (melanoma) or EG.7 (lymphoma) tumor cell lines and tumor growth was assessed. As shown in Figure 6A–C, Ron−/− mice develop smaller tumors than their wild-type counterparts. In all cases, differences in tumor growth are observable as early as can be reasonably assessed by caliper measurement and persist throughout the duration of the experiment. CD11b+/Gr1+ MDSCs increase in numbers within secondary lymphoid organs upon tumor onset. While MDSC expansion was increased in the spleens of wild-type TBM, expansion of MDSCs was attenuated in Ron−/− animals (Figure 6D). We also observed a reduction in MCP-1, G-CSF, IL-6 and IL-1, which participate in the recruitment of MDSCs, in the sera of Ron−/− TBM (Figure 7A–D).
Figure 6. Reduced syngeneic tumor growth in Ron−/− mice.
Wild-type and Ron−/− mice were injected subcutaneously with A) 3LL, B) B16-F10 or C) EG.7 cells. Upon onset, tumors were measured every other day by caliper. 3LL: WT (n=22), Ron−/− (n=23); B16-F10: WT (n=12), Ron−/− (n=13); EG.7: WT (n=9), Ron−/− (n=10). Data are presented as ± S.E.M. *p<0.05. D) Splenocytes from day 17 B16-F10 TBM were collected and analyzed by flow cytometry for CD11b and Gr1 expression. WT (n=12), Ron−/− (n=13). Data are presented as ± S.E.M. *p<0.05.
Figure 7. Decreased production of tumor-promoting cytokines in Ron−/− mice.
EG.7-injected wild-type and Ron−/− TBM were euthanized on days 7, 11 and 15 and serum was collected by cardiac puncture. Serum was analyzed using a lincoplex assay for A) MCP-1, B) G-CSF, C) IL-6, and D) IL-1α. Day 7: WT (n=7), Ron−/− (n=8); Day 11: WT (n=8), Ron−/− (n=8); Day 15: WT (n=9), Ron−/− (n=10). Data are ± S.E.M. *p<0.05, †p=0.07, n.d.=none detected.
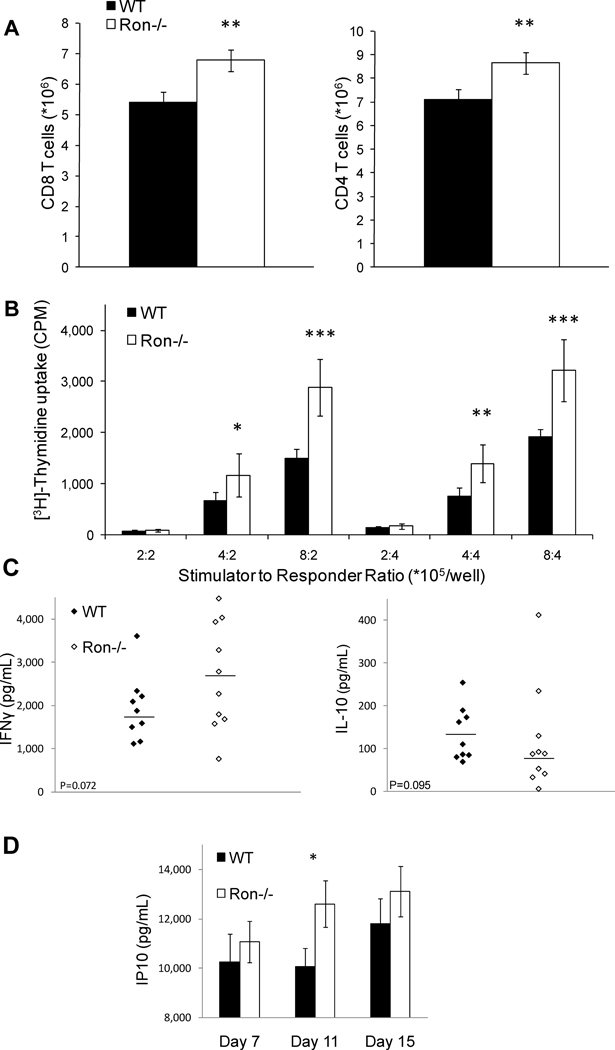
MDSCs inhibit both antigen specific and alloreactive T cell activation. Consistent with the decrease in splenic MDSCs in Ron−/− mice, the numbers of CD4+ and CD8+ T cells in the spleens of Ron−/− TBM were higher than those from wild-type TBM (Figure 8A). To determine whether these cells exhibit increased proliferation in response to allogeneic stimulation, we performed a mixed lymphocyte reaction (MLR). When splenocytes from C57BL/6 TBM were used as responders to γ-irradiated BALB/c splenocytes, a greater proliferative response was observed by splenocytes from Ron−/− TBM compared with control animals (Figure 8B). Antigen specific re-challenge with γ-irradiated tumor cells elicited a trend toward increased production of IFNγ and decreased production of IL-10 (Figure 8C) by T cells from Ron−/− compared with wild-type TBM. Furthermore, the IFNγ responsive gene, IP10, was increased in the sera of Ron−/− TBM (Figure 8D). Together, these results demonstrate that expression of Ron in cells of the tumor microenvironment promotes tumor growth, and that the decrease in tumor growth in the absence of Ron correlates with decreased expansion of MDSCs and increased T cell activity in the spleens of Ron−/− TBM.
Figure 8. Enhanced splenic T cell activity in Ron−/− tumor-bearing mice.
A) Splenocytes were collected from day 17 B16-F10-injected wild-type and Ron−/− TBM and analyzed for total numbers of CD4+ and CD8+ T cells by flow cytometry. B) Splenocytes were isolated from day 17 B16-F10 wild-type or Ron−/− TBM and co-cultured with γ-irradiated BALB/c allogeneic splenocytes. Splenocytes from 12 wild-type and 12 Ron−/− mice were isolated and pooled in groups of 4 for analysis. Data are presented as ± S.E.M. *p<0.05; **p<0.01, ***p<0.001. Splenocytes from EG.7-injected wild-type and Ron−/− TBM were collected on day 15 and re-challenged with γ-irradiated EG.7 cells. Supernatants were analyzed by ELISA for C) IFNγ (p=0.072) and IL-10 (p=0.095). D) Serum was isolated from day 7, 11 and 15 EG.7-injected wild-type and Ron−/− TBM. IP10 was assessed using a lincoplex assay. Data are presented as ± S.E.M. *p<0.02
Discussion
Recent studies using Arg1 deficient bone marrow or macrophage-specific Arg1 knockouts have found that immune cell/macrophage-derived Arg1 is critical for suppressing inflammation and fibrosis during infection with Th2-inducing pathogens(5). However, Arg1 expression is also upregulated in the absence of Th2-mediated inflammation(38), suggesting that IL-4/IL-13-independent mechanisms governing Arg1 expression exist in vivo. Previously we demonstrated that MSP, the ligand for the Ron receptor tyrosine kinase, induces Arg1 expression in resident peritoneal macrophages in the absence of Th2 cytokines(30). Here, we show that this induction is mediated by an AP-1 site in the Arg1 promoter. Furthermore, we demonstrate that Ron promotes Arg1 expression in TAMs, associated with increased tumor growth and decreased T cell-mediated immunity in the presence of Ron. These findings highlight the importance of Ron in the induction of Arg1 expression in vivo. The lower expression levels of Ron in TEMs compared with peritoneal macrophages raises the possibility that the ability of Ron to promote Arg1 expression in vivo could be due to both cell autonomous and non-autonomous regulation.
The AP-1 site identified in this study is not the only functional AP-1 site in the murine Arg1 promoter. An AP-1 site located 3157 bp upstream of the TSS was recently shown to be required for induction of Arg1 in endothelial cells by thrombin(39). Promoter fragments containing the AP-1 site at −433 were not inducible by thrombin. In contrast to MSP induction of Fos binding to the −433 AP-1 site, thrombin activation of the Arg1 promoter involved binding of c-Jun and activating transcription factor-2 (ATF-2) to the −3157 AP-1 site(39). AP-1 is comprised of homo- and heterodimers of various Jun, Fos and ATF family members. Thus, activation of different AP-1 elements within the Arg1 promoter may reflect tissue-specific differences in expression of various AP-1 family members and/or specific sequence contexts in which the AP-1 sites reside.
Although MSP induces robust phosphorylation of Stat6 in primary macrophages (data not shown), we observed little or no binding of Stat6 to the Arg1 promoter following MSP stimulation, and mutation or deletion of the Stat6 binding site in the Arg1 promoter did not affect induction of the promoter by MSP. This is consistent with the observation that MSP does not induce expression of the Stat6-dependent genes Chi3l3 (Ym1) and Retnla (Fizz1). Moreover, MSP inhibits the expression of Chi3l3 and Retnla in response to IL-4, suggesting that MSP likely inhibits Stat6 transcriptional activity. However, induction of Arg1 by IL-4 is maintained in the presence of MSP, highlighting the differential regulation of Arg1 and other M2 markers. These results are consistent with the observation that Ron−/− mice express normal levels of Arg1 in a mouse model of schistosomiasis (T. Wynn, unpublished observations), suggesting that Ron may play a less important role in regulating Arg1 expression in the context of a robust Th2 response.
Ron is expressed on tissue-resident macrophages, including Kupffer cells, mesangial cells, Langerhans cells, microglia, alveolar macrophages and resident peritoneal macrophages, but not on inflammatory macrophages. Recent studies suggest that inflammatory and tissue-resident macrophages derive from two distinct populations of circulating monocytes that can be distinguished based on their expression of Gr1, CCR2, L-selectin, Cx3CR1 and CD43(40, 41). The so-called “resident” population of circulating monocytes may provide a source of tissue-infiltrating macrophages, which tend to be more M2-polarized than inflammatory macrophages and play an important trophic role by promoting tissue healing following inflammatory damage(42). Our observation that Ron is expressed predominantly by the MRC1+ subpopulation of TAMs, which are hypothesized to derive from circulating “resident” monocytes and promote tumor growth(16), is consistent with the restricted expression of Ron on resident macrophages and its role in protecting tissues from inflammatory damage(25).
Regulating the balance of M1 vs. M2 activation of TAMs has a major impact on tumor growth, and expression of Arg1 by TAMs plays a key role in this regulation. Ship−/− TAMs are M2 in nature and express elevated levels of Arg1. Consequently, tumor growth in Ship−/− mice is enhanced(43). Conversely, reduced Arg1 expression and increased iNOS expression in Stat6−/− TAMs has been implicated in rejection of established tumors in Stat6−/− mice(44). Furthermore, direct inhibition of Arg1 activity in TBM reduces tumor growth, associated with increased antigen-specific T cell responses. This inhibition is lost in scid mice, indicating that Arg1 mediates its effects by reducing T cell-mediated immunity(45). Thus, while the enhanced T cell activity in the spleens of Ron−/− TBM correlates with a decrease in splenic MDSCs, the induction of Arg1 by Ron in TAMs could also contribute to the inhibition of T cell-mediated immunity within the tumor microenvironment, which would cooperate with the proangiogenic and tissue-remodeling programs of TAMs to favor tumor growth.
Recent studies demonstrate that the multi-target RTK inhibitor, sunitinib malate, inhibits tumor cell growth and also promotes tumor immunity in both a mouse model and human clinical trials(46, 47). While the effect of sunitinib on TAM activity is currently unknown, these studies provide proof of principle that targeting RTK activity in the tumor microenvironment could be therapeutically beneficial. Our studies indicate that the absence of Ron in the tumor microenvironment results in reduced Arg1 expression by TAMs, potentially contributing to the decreased tumor growth observed in Ron−/− mice. Thus, understanding the molecular mechanism by which Ron promotes Arg1 expression is an important step in the development of therapies aimed at targeting macrophage regulation of tumor growth. The widespread overexpression of Ron in epithelial carcinomas, as well as its ability to induce tumor-promoting activities in infiltrating TAMs, renders Ron a promising therapeutic target for a wide range of malignancies.
Footnotes
This work was supported by Grant # 09GRNT2390164 from the American Heart Association and Grant # R01 GM57384 from the National Institutes of Health.
REFERENCES
- 1.Wu G, Morris S. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–7. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris S, Kepka-Lenhart D, Chen L-C. Differential regulation of arginases and inducible nitric oxide synthase in murine macrophage cells. Am J Physiol Endocrinol Metab. 1998;275:E740–E747. doi: 10.1152/ajpendo.1998.275.5.E740. [DOI] [PubMed] [Google Scholar]
- 3.Gordon S. Alternative Activation of Macrophages. Nature Revews Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 4.Hesse M, Modolell M, La Flamme A, Schito M, Fuentes J, Cheever A, Pearce E, Wynn T. Differential regulation of nitric oxide synthase-2 and arginase-1 by type1/type2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 5.Pesce J, Ramalingam T, Mentink-Kane M, Wilson M, El Kasmi K, Smith A, Thompson R, Cheever A, Murray P, Wynn T. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000393. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas AC, Sala-Newby GB, Ismail Y, Johnson JL, Pasterkamp G, Newby AC. Genomics of Foam Cells and Nonfoamy Macrophages From Rabbits Identifies Arginase-I as a Differential Regulator of Nitric Oxide Production. Arterioscler Thromb Vasc Biol. 2007;27:571–577. doi: 10.1161/01.ATV.0000256470.23842.94. [DOI] [PubMed] [Google Scholar]
- 7.Teupser D, Burkhardt R, Wilfert W, Haffner I, Nebendahl K, Thiery J. Identification of Macrophage Arginase I as a New Candidate Gene of Atherosclerosis Resistance. Arterioscler Thromb Vasc Biol. 2006;26:365–371. doi: 10.1161/01.ATV.0000195791.83380.4c. [DOI] [PubMed] [Google Scholar]
- 8.El Kasmi K, Qualls J, Pesce J, Smith A, Thompson R, Henao-Tamayo M, Basaraba R, Konig T, Schleicher U, Koo M, Kaplan G, Fitzgerald K, Tuomanen E, Orme I, Kanneganti T, Bogdan C, Wynn T, Murray P. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol. 2008;9:1399–1406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A. Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol. 2000;17:445–451. doi: 10.3892/ijo.17.3.445. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, Antonia S, Ochoa JB, Ochoa AC. Arginase I Production in the Tumor Microenvironment by Mature Myeloid Cells Inhibits T-Cell Receptor Expression and Antigen-Specific T-Cell Responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 11.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O'Neill A, Mier J, Ochoa AC. Arginase-Producing Myeloid Suppressor Cells in Renal Cell Carcinoma Patients: A Mechanism of Tumor Evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 12.Balkwill F, Charles K, Mantovani A. A Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Bingle L, Brown N, Lewis C. The role of tumor-associated macrophages in tumor progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 14.Pollard J. Tumor-educated macrophages promote tumor progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigez P, Quiceno D, Zabaleta J, Ortiz B, Zea A, Piazuelo M, Delgado A, Correa P, Brayer J, Sotomayor E, Antonia S, Ochoa J, Ochoa A. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 16.Pucci F, Venneri M, Biziato D, Nonis A, Boi D, Sica A, Di Serio C, Naldini L, De Palma M. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood "resident" monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–914. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- 17.De Palma M, Venneri M, Galli R, Sergi L, Politi L, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Wang M, Lee W, Luo Y, Weis M, Hao H. Altered expression of the RON receptor tyrosine kinase in various epithelial cancers and its contribution to tumourigenic phenotypes in thyroid cancer cells. J Pathol. 2007;213:402–411. doi: 10.1002/path.2245. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, He C, Chen Y, Wang D, Wang M. Altered expression of the Ron receptor tyrosine kinase in primary human colorectal adenocarcinomas: generation of differential splicing RON variants and their oncogenic potential. Oncogene. 2003;22:186–197. doi: 10.1038/sj.onc.1206075. [DOI] [PubMed] [Google Scholar]
- 20.Maggiora P, Marchio S, Stella M, Giai M, Belfiore A, De Borotli M, Di Renzo M, Constantino A, Sismondi P, Comoglio P. Overexpression of the Ron gene in human breast carcinoma. Oncogene. 1998;16:2927–2933. doi: 10.1038/sj.onc.1201812. [DOI] [PubMed] [Google Scholar]
- 21.Welm A, Sneddon J, Taylor C, Nuyten D, van de Vijver M, Hasegawa B, Bishop J. The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis. Proc Natl Acad Sci USA. 2007;104:7570–7575. doi: 10.1073/pnas.0702095104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kretschmann K, Eyob H, Buys S, Welm A. The macrophage stimulating protein/Ron pathway as a potential therapeutic target to impede multiple mechanisms involved in breast cancer progression. Curr Drug Targets. 2010;11(9):1157–1168. doi: 10.2174/138945010792006825. [DOI] [PubMed] [Google Scholar]
- 23.Morrison A, Wilson C, Ray M, Correll P. Macrophage-stimulating protein, the ligand for the stem cell-derived tyrosine kinase/RON receptor tyrosine kinse, inhibits IL-12 production by primary peritoneal macrophages stimulated with IFN-gamma and lipopolysaccharide. J Immunol. 2004;172:1825–1832. doi: 10.4049/jimmunol.172.3.1825. [DOI] [PubMed] [Google Scholar]
- 24.Liu Q, Fruit K, Ward J, Correll P. Negative regulation of macrophage activation in response to IFN-gamma and lipopolysaccharide by the Stk/Ron receptor tyrosine kinase. J Immunol. 1999;163:6606–6613. [PubMed] [Google Scholar]
- 25.Wilson C, Ray M, Lutz M, Sharda D, Xu J, Hankey P. The Ron receptor tyrosine kinase regulates IFN-gamma production and responses in innate immunity. J Immunol. 2008;181:2303–2310. doi: 10.4049/jimmunol.181.4.2303. [DOI] [PubMed] [Google Scholar]
- 26.Ray M, Yu S, Sharda D, Wilson C, Liu Q, Kaushal N, Prabhu K, Hankey P. Regulation of TLR4 signaling in macrophages by the Ron receptor tyrosine kinase and its ligand, MSP. J Immunol. 185(12):7309–7316. doi: 10.4049/jimmunol.1000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsutsui S, Noorbakhsh F, Sullivan A, Henderson A, Warren K, Toney-Earley K, Waltz S, Power C. Ron-regulated innate immunity is protective in an animal model of multiple sclerosis. Ann Neurol. 2005;57:883–895. doi: 10.1002/ana.20502. [DOI] [PubMed] [Google Scholar]
- 28.Lentsch A, Pathrose P, Kader S, Kuboki S, Collins M, Waltz S. The Ron receptor tyrosine kinase regulates acute lung injury and suppresses nuclear factor kappaB activation. Shock. 2007;27:274–280. doi: 10.1097/01.shk.0000239755.82711.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Correll P, Iwama A, Tondat S, Mayrhofer G, Suda T, Bernstein A. Deregulated inflammatory response in mice lacking the Stk/Ron receptor tyrosine kinase. Genes Funct. 1997;1:69–83. doi: 10.1046/j.1365-4624.1997.00009.x. [DOI] [PubMed] [Google Scholar]
- 30.Morrison A, Correll P. Activation of the stem cell-derived tyrosine kinase/RON receptor tyrosine kinase by macrophage-stimulating protein results in the induction of arginase activity in murine peritoneal macrophages. J Immunol. 2002;168:853–860. doi: 10.4049/jimmunol.168.2.853. [DOI] [PubMed] [Google Scholar]
- 31.Gray M, Poljakovic M, Kepka-Lenhart D, Morris S. Induction of arginase I transcription by IL-4 requires a composite DNA response element for Stat6 and C/EBPbeta. Gene. 2005;353:98–106. doi: 10.1016/j.gene.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Wei X, Ni S, Correll P. Uncoupling ligand-dependent and -independent mechanisms for mitogen-activated protein kinase activation by the murine Ron receptor tyrosine kinase. J Biol Chem. 2005;280:35098–35107. doi: 10.1074/jbc.M505737200. [DOI] [PubMed] [Google Scholar]
- 33.Wei X, Hao L, Ni S, Liu Q, Xu J, Correll P. Altered exon usage in the juxtamembrane domain of mouse and human Ron regulates receptor activity and signaling specificity. J Biol Chem. 2005;280:40241–40251. doi: 10.1074/jbc.M506806200. [DOI] [PubMed] [Google Scholar]
- 34.Pauleau A, Rutschman R, Lang R, Pernis A, Watowich S, Murray P. Enhancer-mediated control of macrophage-specific arginase I expression. J Immunol. 2004;172:7565–7573. doi: 10.4049/jimmunol.172.12.7565. [DOI] [PubMed] [Google Scholar]
- 35.Lock L, Frigault M, Saucier C, Park M. Grb2-independent recruitment of Gab1 requires the c-terminal lobe and strucutral integrity of the Met receptor kinase domain. J Biol Chem. 2003;278:30083–30090. doi: 10.1074/jbc.M302675200. [DOI] [PubMed] [Google Scholar]
- 36.Lock L, Royal I, Naujokas M, Park M. Identification of an atypical Grb2 carboxyl-terminal SH3 domain binding site in Gab2 docking proteins reveals Grb2-dependent and Grb2-independent recruitment of Gab1 to receptor tyrosine kinases. J Biol Chem. 2000;275:31536–31545. doi: 10.1074/jbc.M003597200. [DOI] [PubMed] [Google Scholar]
- 37.Teal H, Ni S, Xu J, Finkelstein L, Cheng A, Paulson R, Feng G, Correll P. Grb2-mediated recruitment of Gab2, but not Gab1, to Sf-Stk supports the expansion of Friend virus-infected erythroid progenitor cells. Oncogene. 2006;25(17):2433–2443. doi: 10.1038/sj.onc.1209288. [DOI] [PubMed] [Google Scholar]
- 38.Daley J, Brancato S, Thomay A, Reichner J, Albina J. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu W, Chandrasekharan U, Bandyopadhyay S, Morris S, DiCorleto P, Kashyap V. Thrombin induces endothelial arginase through AP-1 activation. Am J Physiol Cell Physiol. 2010;298:C952–C960. doi: 10.1152/ajpcell.00466.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geissman F, Manz M, Jung S, Sieweke M, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Auffrey C, Sieweke M, Geismann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 42.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geismann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 43.Rauh M, Ho V, Pereira C, Sham A, Sly L, Lam V, Huxham L, Minchinton A, Mui A, Krystal G. SHIP represses the generation of alternatively activated macrophages. Immunity. 2005;23:361–374. doi: 10.1016/j.immuni.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Sinha P, Clements V, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of establiblished metastatic disease. J Immunol. 2005;174:636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez P, Quiceno D, Zabaleta J, Ortiz B, Zea A, Piazuelo M, Delgado A, Correa P, Brayer J, Sotomayor E, Antonia S, Ochoa J, Ochoa A. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T cell responses. Cancer Res. 2004;64(16):5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 46.Ozao-Choy J, Ma G, Kao J, Wang G, Meseck M, Sung M, Schwartz C, Divino M, Pan P, Chen S. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69(6):2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xin H, Zhang C, Hermann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–2513. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]