Abstract
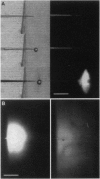
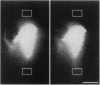
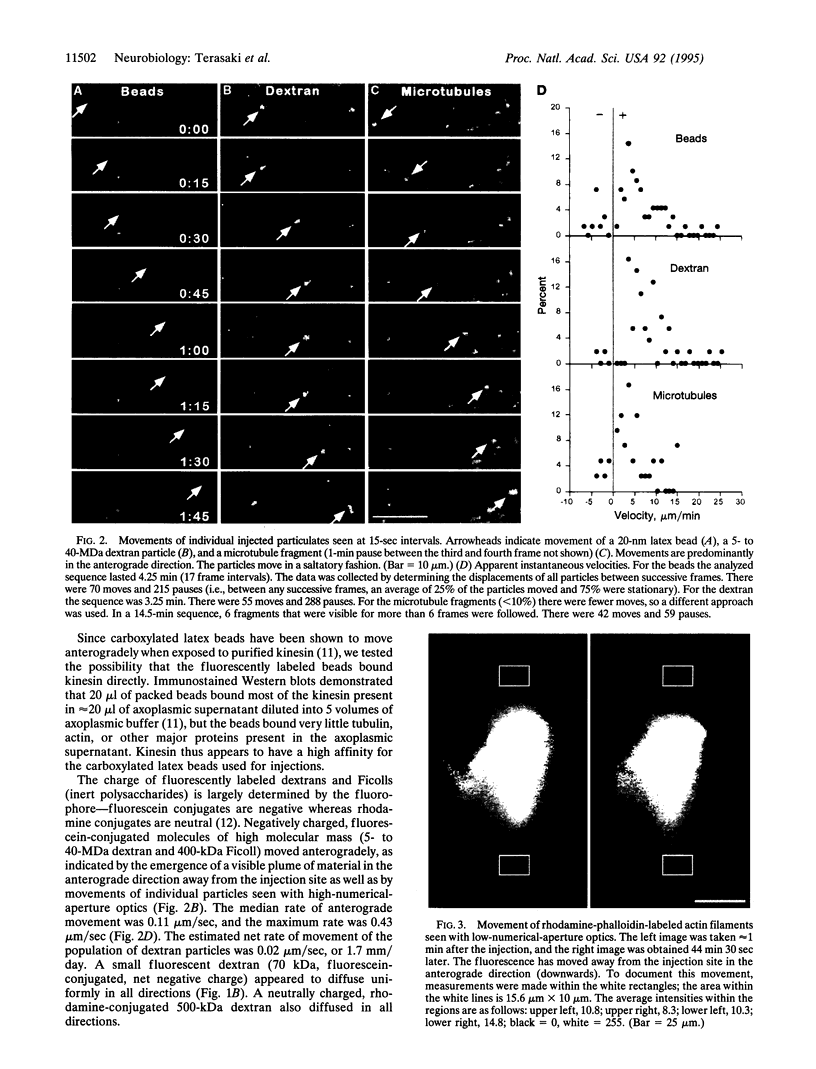
In order to explore how cytoskeletal proteins are moved by axonal transport, we injected fluorescent microtubules and actin filaments as well as exogenous particulates into squid giant axons and observed their movements by confocal microscopy. The squid giant axon is large enough to allow even cytoskeletal assemblies to be injected without damaging the axon or its transport mechanisms. Negatively charged, 10- to 500-nm beads and large dextrans moved down the axon, whereas small (70 kDa) dextrans diffused in all directions and 1000-nm beads did not move. Only particles with negative charge were transported. Microtubules and actin filaments, which have net negative charges, made saltatory movements down the axon, resulting in a net rate approximating that previously shown for slow transport of cytoskeletal elements. The present observations suggest that particle size and charge determine which materials are transported down the axon.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. J., Bray D. Rapid transport of foreign particles microinjected into crab axons. Nature. 1983 Jun 23;303(5919):718–720. doi: 10.1038/303718a0. [DOI] [PubMed] [Google Scholar]
- Andrews S. B., Gallant P. E., Leapman R. D., Schnapp B. J., Reese T. S. Single kinesin molecules crossbridge microtubules in vitro. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6503–6507. doi: 10.1073/pnas.90.14.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer E. L., DeGiorgis J. A., Bodner R. A., Kao A. W., Reese T. S. Evidence for myosin motors on organelles in squid axoplasm. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11252–11256. doi: 10.1073/pnas.90.23.11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L. G., Wang Y. L. Mechanism of the formation of contractile ring in dividing cultured animal cells. II. Cortical movement of microinjected actin filaments. J Cell Biol. 1990 Nov;111(5 Pt 1):1905–1911. doi: 10.1083/jcb.111.5.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant P. E. Effects of the external ions and metabolic poisoning on the constriction of the squid giant axon after axotomy. J Neurosci. 1988 May;8(5):1479–1484. doi: 10.1523/JNEUROSCI.08-05-01479.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant P. E. The direct effects of graded axonal compression on axoplasm and fast axoplasmic transport. J Neuropathol Exp Neurol. 1992 Mar;51(2):220–230. doi: 10.1097/00005072-199203000-00011. [DOI] [PubMed] [Google Scholar]
- Grafstein B., Forman D. S. Intracellular transport in neurons. Physiol Rev. 1980 Oct;60(4):1167–1283. doi: 10.1152/physrev.1980.60.4.1167. [DOI] [PubMed] [Google Scholar]
- Keith C. H. Slow transport of tubulin in the neurites of differentiated PC12 cells. Science. 1987 Jan 16;235(4786):337–339. doi: 10.1126/science.2432662. [DOI] [PubMed] [Google Scholar]
- Kiehart D. P. Microinjection of echinoderm eggs: apparatus and procedures. Methods Cell Biol. 1982;25(Pt B):13–31. doi: 10.1016/s0091-679x(08)61418-1. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Schnapp B., Inouye H., Neve R. L. The primary structure and analysis of the squid kinesin heavy chain. J Biol Chem. 1990 Feb 25;265(6):3278–3283. [PubMed] [Google Scholar]
- Kuznetsov S. A., Langford G. M., Weiss D. G. Actin-dependent organelle movement in squid axoplasm. Nature. 1992 Apr 23;356(6371):722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S. A., Rivera D. T., Severin F. F., Weiss D. G., Langford G. M. Movement of axoplasmic organelles on actin filaments from skeletal muscle. Cell Motil Cytoskeleton. 1994;28(3):231–242. doi: 10.1002/cm.970280306. [DOI] [PubMed] [Google Scholar]
- Langford G. M., Kuznetsov S. A., Johnson D., Cohen D. L., Weiss D. G. Movement of axoplasmic organelles on actin filaments assembled on acrosomal processes: evidence for a barbed-end-directed organelle motor. J Cell Sci. 1994 Aug;107(Pt 8):2291–2298. doi: 10.1242/jcs.107.8.2291. [DOI] [PubMed] [Google Scholar]
- Lasek R. J. Polymer sliding in axons. J Cell Sci Suppl. 1986;5:161–179. doi: 10.1242/jcs.1986.supplement_5.10. [DOI] [PubMed] [Google Scholar]
- Navone F., Niclas J., Hom-Booher N., Sparks L., Bernstein H. D., McCaffrey G., Vale R. D. Cloning and expression of a human kinesin heavy chain gene: interaction of the COOH-terminal domain with cytoplasmic microtubules in transfected CV-1 cells. J Cell Biol. 1992 Jun;117(6):1263–1275. doi: 10.1083/jcb.117.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S., Hirokawa N. Axonal transport. Curr Opin Cell Biol. 1989 Feb;1(1):91–97. doi: 10.1016/s0955-0674(89)80043-2. [DOI] [PubMed] [Google Scholar]
- Reinsch S. S., Mitchison T. J., Kirschner M. Microtubule polymer assembly and transport during axonal elongation. J Cell Biol. 1991 Oct;115(2):365–379. doi: 10.1083/jcb.115.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings H., Hunt C. The nature of the clear zone around microtubules. Cell Tissue Res. 1982;227(3):609–617. doi: 10.1007/BF00204791. [DOI] [PubMed] [Google Scholar]
- Urrutia R., McNiven M. A., Albanesi J. P., Murphy D. B., Kachar B. Purified kinesin promotes vesicle motility and induces active sliding between microtubules in vitro. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6701–6705. doi: 10.1073/pnas.88.15.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Schnapp B. J., Reese T. S., Sheetz M. P. Organelle, bead, and microtubule translocations promoted by soluble factors from the squid giant axon. Cell. 1985 Mar;40(3):559–569. doi: 10.1016/0092-8674(85)90204-1. [DOI] [PubMed] [Google Scholar]