Abstract
Electroencephalography (EEG) is an ideal neuroscientific approach, providing a direct measurement of neural activity that demonstrates reliability, developmental stability and high heritability. This systematic review of a subset of domains evaluates the utility of electrophysiological measures as potential intermediate phenotypes for ADHD in the domains of quantitative EEG indices of arousal and intra-individual variability, and functional investigations of inhibitory and error processing using the event-related potential (ERP) technique. Each domain demonstrates consistent and meaningful associations with ADHD, a degree of genetic overlap with ADHD and potential links to specific genetic variants. Investigations of the genetic and environmental contributions to EEG/ERP and shared genetic overlap with ADHD may enhance molecular genetic studies and provide novel insights into aetiology. Such research will aid in the precise characterisation of the clinical deficits seen in ADHD and guide the development of novel intervention and prevention strategies for those at risk.
Keywords: ADHD, arousal, default-mode, electrophysiology, endophenotype, executive function, genetics, heritability
Attention deficit hyperactivity disorder (ADHD) is a developmental condition characterised by impairing levels of inattentive, impulsive and hyperactive symptoms (Ref. 1), with prevalence in school-aged children around 5% (Ref. 2). The disorder frequently persists into adult life, with approximately 15% of children with ADHD retaining the diagnosis by the age of 25 years and a further 50% showing persistence of some symptoms giving rise to continued impairments (Ref. 3).
ADHD tends to run in families, with a risk of ADHD to first-degree relatives of an affected proband around four to ten times the general population rate (Ref. 4). Twin studies suggest that around 70-80% of the phenotypic variance is explained by genetic factors (Ref. 5). Such quantitative genetic studies suggest that ADHD represents the extreme of one or more continuously distributed traits, rather than a distinct categorical disorder (Ref. 6, 7, 8, 9, 10).The conceptualisation of ADHD symptoms as continuous traits (Ref. 10) seems to better reflect the underlying aetiological processes involved, in which risk factors for ADHD influence levels of ADHD symptoms throughout the population (Ref. 8). Overall, quantitative genetic studies support the use of both categorical and quantitative trait locus (QTL) approaches in the investigation of genetic risk factors for ADHD (Ref. 11) and the underlying neurobiological processes involved.
Candidate gene studies implicate genetic variants involved in the regulation of dopamine and related neurotransmitter systems, predicted by the effects of stimulant medications that increase the amount of synaptic dopamine (Ref. 12). The most consistent evidence of genetic associations with ADHD are for variants within or near the dopamine D4 and D5 receptor genes (Ref. 13). There are numerous, yet inconsistent, reports of association with the dopamine transporter gene, which nevertheless seem to implicate this gene with associated polymorphisms found in two distinct regions (Ref. 13, 14, 15, 16, 17, 18, 19). Other neurotransmitter systems are also likely to be involved. For example, serotonin is linked to poor impulse regulation (Ref. 20), low platelet and whole blood levels of serotonin have been reported in ADHD (Ref. 21) and several studies report association between ADHD and the serotonin transporter and serotonin 1B receptor genes (Ref. 19).
Such studies have, however, only had limited success in identifying risk alleles for ADHD, major limitations being the low risk conferred by individual genetic variants and insufficient sample size (Ref. 22). Recent genomewide association scans found no genetic variants that passed genomewide levels of significance, although there was evidence for association in a group analysis of 51 nominated candidate genes (Ref. 23, 24, 25). A potential novel finding is association with the Cadherin gene (CDH13), which was implicated in more than one GWAS of ADHD (Ref. 26, 27, 28) and lies within the only region that reached genome-wide significance in a meta-analysis of ADHD linkage studies ADHD (Ref. 29). This finding and other hints from GWAS indicate that genes involved in cell division, cell adhesion, neuronal migration and neuronal plasticity may also be implicated in ADHD (Ref. 28).
Despite some advances, it is necessary to consider the reasons for the overall lack of progress. The most likely reasons are the presence of multiple genes of very small effect, heterogeneity of aetiological influences, and interactions between genes and environment (Ref. 22). In addition, we do not yet understand the contribution made to ADHD from rare copy number variants (CNVs), which confer moderate to large effects in some cases (Ref. 30, 31).
One approach to these problems is to gather very large sample sizes needed for sufficient power to detect genes of very small effect. Yet there are complementary strategies that posit that molecular genetic research should not be restricted to the clinical phenotype alone, but should also investigate genetic factors that account for neurobiological processes that underlie the heterogeneity of ADHD.
The intermediate phenotype (endophenotype) concept
Intermediate phenotype research aims to identify neurobiological processes that mediate between genes and behaviour and might therefore be more proximal to gene function (Ref. 32). Key criteria for endophenotypes are listed in Box 1. Intermediate phenotypes may be less heterogeneous and genetically less complex than behavioural phenotypes, and potentially associated with greater effect sizes from individual genes. For example, some risk alleles may explain up to 10% of phenotypic variance for certain functional magnetic resonance imaging (fMRI) phenotypes (Ref. 33, 34). Furthermore, investigation of measures related directly to brain function are required if we wish to elucidate the neurobiological processes that underlie risk for ADHD.
BOX 1. Criteria for intermediate-phenotypes (Ref. 32, 230).
Intermediate-phenotypes should:
be associated with the clinical disorder;
be reliable, as reliability sets an upper limit on the estimates of heritability. Any deviations from perfect reliability will increase measurement error and therefore nonshared environmental influences (Ref. 40);
be heritable;
be stable over time and state-independent such that it manifests in an individual whether or not the disorder is active (this criterion has greatest relevance to fluctuating state-like conditions such as schizophrenia or major depression than the trait-like condition of ADHD);
co-segregate with the disorder within families;
for disorders with complex inheritance patterns such as ADHD, found in non-affected family members at a higher rate than the general population;
be associated with a candidate gene or region of a gene;
share genetic influences with the disorder;
mediate genetic effects between phenotype and genotype rather than reflect pleiotropic influences (multiple outcomes of individual genes (Ref. 227, 231). For example, shared genetic effects between ADHD and autism (Ref. 232) or reading ability (Ref. 233) reflect pleiotropic effects of genes rather than processes that mediate between genetic risk factors and ADHD. In a similar way, cognitive performance and other neurobiological measures that share genetic influences with ADHD may reflect the multiple outcomes of the genes involved, rather than necessarily representing processes that mediate between genes and ADHD behaviours. Tests of mediation versus pleiotropy can be used to specifically infer the causal role of a neurobiological process once specific genetic risk factors are identified that are associated with both ADHD and associated neurobiological measure. One other approach would be to test for co-variation of ADHD and neurobiological measures during the treatment response (Ref. 229).
Several potential intermediate phenotypes have been identified in ADHD (for reviews see (Ref. 35, 36, 37, 38). Here we focus on electrophysiological approaches using electroencephalography (EEG), which records the ongoing electrical activity generated by underlying brain structures, recorded from electrodes placed on the scalp. Electrophysiological parameters are ideal for intermediate-phenotype research in ADHD because of the supreme temporal resolution that enables investigation of the stages of information processing that are impaired and abnormal state processes such as arousal or default mode network impairments, and the high reliability and heritability of many electrophysiological measures (Ref. 39). Furthermore, there are consistent findings across studies suggesting abnormal electrophysiological processes in ADHD (Ref. 39, 40) and evidence that some of the impaired processes are developmentally stable (Ref. 41, 42). Finally, the non-invasive and cost-effective nature of EEG helps to generate the relatively large sample sizes required for molecular genetic studies.
This systematic review evaluates the use of a subset of candidate electrophysiological measures as potential intermediate-phenotypes for ADHD, assessing the following: association between the measure and ADHD; heritability of the measure; the extent to which ADHD and the measures share familial/genetic influences; associations between the measure and genetic variants.
Quantitative EEG
EEG Power
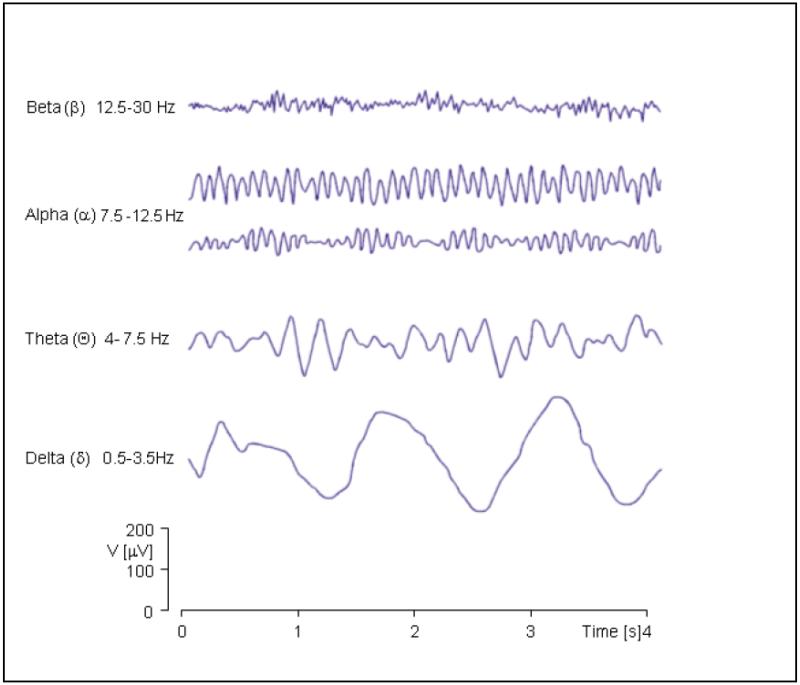
EEG power is quantified into certain frequency bands of interest (defined in Figure 1) and demonstrates high test-retest reliability (0.71-0.95), particularly for theta and delta frequency bands (Ref. 43).
FIGURE 1. EEG frequency bands investigated in ADHD (adapted from (Ref. 234)).
In quantitative EEG, recordings of brain electrical activity at the scalp are quantified in the frequency range of interest, which usually extends between 1 and 70 cycles per second (Hz). In ADHD research, this frequency range is traditionally separated into four frequency bands Recently ADHD research has further extended to very low-frequency oscillations below 0.5Hz. Figure adapted from Malmivuo and Plonsey (Ref. 49; © 1995 Oxford University Press) by permission of Oxford University Press, Inc. (http://www.oup.com). Abbreviations: ADHD, attention deficit hyperactivity disorder; EEG, electroencephalography; VLF, very low frequency.
Association with ADHD
Associations between quantitative EEG parameters and ADHD are widely documented (Ref. 44). Children, adolescents and adults with ADHD were found to exhibit increased theta activity and decreased alpha and beta activity during rest, compared to typical controls (Ref. 45, 46, 47, 48), although not all data is consistent with these findings (Ref. 50, 51). This is widely interpreted to indicate the presence of cortical underactivation in ADHD due to the association of theta activity with drowsiness (Ref. 52). Additional ADHD-control differences have been reported in evoked gamma oscillations, suggesting neuronal hyperexcitability (Ref. 53).
Generally EEG studies indicate a predominance of slow-wave delta and theta in infancy that increases in frequency during childhood (Ref. 54); and is therefore thought to reflect brain immaturity (Ref. 55). In addition, children and adolescents with ADHD exhibit a higher ratio of theta activity, particularly in comparison to faster beta activity, which has led to the suggestion that the disorder is a product of maturational delay (Ref. 56). However, there is evidence that the theta-beta ratio is abnormal in adults with ADHD, suggesting that neuronal inefficiency may span across the lifetime (Ref. 45).
Heritability and genetic overlap with ADHD
It is well established that EEG parameters are largely determined by genetic factors. Higher twin concordance rates in the spectral characteristics of resting eyes-closed EEG have been reported for monozygotic (MZ) compared to dizygotic (DZ) pairs (Ref. 57, 58, 59). The first large twin study of resting EEG found high heritability across all frequency bands (delta 76%, theta 89%, alpha 89%, beta 86%), with heritability ranging from 55-90% in 5-year-old twins and from 70-90% in 16-year-old twins (Ref. 60, 61). Meta-analysis estimated an average heritability of 79% for alpha power (Ref. 62). Frontal areas tend to exhibit more unique genetic influences for the individual frequency bands, compared to occipital sites where genetic influences are largely shared across frequency bands (Ref. 63); highlighting the complexity of genetic influences on EEG across frequency bands and scalp locations additionally reported using bipolar electrode derivations (Ref. 64). The specificity of genetic influences in frontal regions suggests that different neurobiological pathways may be responsible for different frequency bands of the EEG (Ref. 63). These findings may link with studies indicating band specificity in ADHD (i.e. reduced theta, increased beta) and findings of alpha asymmetry (see EEG Coherence and Connectivity).
EEG frequency bands were found to correlate between siblings in families multiply affected with ADHD. At rest siblings were more similar for lower frequency band theta (0.36-0.59) compared to the higher frequency bands (alpha: 0.42-0.49; beta1: 0.45-0.57; beta2: 0.28-0.52), suggesting the reduced theta power observed at rest is familial. (Ref. 65), In contrast, for cognitive activation conditions (resting eyes open and completion of the continuous performance test (CPT)) higher sibling correlations were reported for beta1 (0.45-0.61), which suggests familial influences underlie reduced beta power and lack of typical beta increase during cognitive activation conditions. In addition, highly significant parent-offspring correlations for alpha power were reported under resting eyes open (0.47-0.56) and CPT (0.46-0.50), similar to a previous preliminary study (Ref. 66).
EEG further demonstrated familial clustering with ADHD subtypes and symptoms (Ref. 65). In children increased theta was found in ADHD regardless of subtype, whereas in adults EEG, theta, alpha and beta varied according to ADHD subtype. Parents with the predominantly inattentive subtype displayed significantly elevated theta compared to parents with the combined subtype and unaffected parents, suggesting a potential link between ADHD that persists into adulthood, inattention and elevated theta. Some of the familial correlations for the EEG parameters are higher than expected for the action of genetic influences alone, suggesting the influence of the familial environment. However the selection of affected sibling pairs may inflate the familial correlations since they may reflect in part state effects (i.e. both having ADHD).
Genetic association studies
A recent review of the relationship between neurotransmitters and brain oscillations highlights the role of dopamine in brain oscillatory activity (Ref. 67). Theta and beta2 (16-20Hz) have been associated with DRD4 (Ref. 65); children with the 7-repeat allele (DRD4-7R: the risk allele associated with ADHD) had reduced beta2 power across all conditions (likely indicating reduced cortical activation) compared to children without DRD4-7R. Parents also demonstrated the same association between DRD4-7R and reduced beta2 power under resting eyes open and CPT performance, but not under resting eyes closed condition (Ref. 65), suggesting possible developmental effects.
The association between the dopamine transporter gene (SLC6A3/DAT1) and EEG patterns was investigated in a double-blind placebo-controlled methylphenidate (MPH) study in a small sample of 27 children with ADHD (Ref. 68). Findings indicated poor performance on the CPT (increased reaction time variability and error rate) in children with two copies of the 10-repeat allele (DAT1-10R: risk allele associated with ADHD in children) compared to those with one or two copies of the 9-repeat allele (DAT1-9R). MPH treatment led to decreased theta activity and lower theta/beta ratio in children with DAT1-10R, whereas those with DAT1-9R showed the opposite pattern. Genetic variation of SLC6A3 may therefore mediate medication-related changes to EEG patterns; and response variability shown in the DAT1-10R group might reflect underarousal. Such medication-related changes are reported elsewhere in the literature (Ref. 69, 70), highlighting the potential for combining genetic and electrophysiological data when considering treatment response. Taken together, these findings suggest that variation in dopaminergic genes may mediate susceptibility to ADHD through effects on cortical activation.
Intermediate-phenotype studies also provide information on potential mechanisms of gene function. In classical auditory target detection paradigms, target stimuli evoke increased gamma activity compared to standard stimuli in typical controls (Ref. 71, 72), and when compared to typical controls, individuals with ADHD display higher amplitude gamma regardless of whether evoked by target or standard stimuli (Ref. 53). Using the same task in typical controls, DRD4-7R was associated with an increase in gamma responses to both target and standard stimuli, whereas the SLC6A3-10R/10R genotype was associated with an increase in gamma response specifically to target stimuli (Ref. 73). This suggested that the pattern of the evoked gamma response associated with DRD4 relates to reduced inhibition, whereas the SLC6A3 effect is related to the target detection mechanism, indicating the role of dopamine in the modulation of such activity in more than one way.
Very low-frequency activity and intra-individual variability
One of the most replicated findings in ADHD research is the increased rate of variability in reaction time (RT) on speeded RT tasks (Ref. 74). Such variability was identified as the best discriminator of ADHD compared to controls out of several variables, demonstrating substantially larger group effect sizes than those found for commission and omission errors (Ref. 74). RT variability (RTV) is heritable (Ref. 36) and shares familial (Ref. 75, 76, 77) and genetic influences with ADHD (Ref. 75, 78, 79) and ‘hyperactivity’ (Ref. 80). Furthermore, multivariate genetic modelling suggests RTV in ADHD forms a distinct familial cognitive factor separate from commission and omission errors (Ref. 35) and the effect of IQ (Ref. 81, 82); and may account for as much as 85% of the familial effects on ADHD (Ref. 35).
There are several theories postulated to explain increased RTV in ADHD including inefficient executive control (Ref. 83) and temporal processing deficits (Ref. 38). A prominent hypothesis posits that RTV in ADHD is related to fluctuations in arousal/activation (Ref. 39, 84, 85, 86, 87, 88) which can also be measured using EEG. Increased theta, that is hypothesised to indicate underarousal, was linked to RTV in a family study of ADHD, which found a higher familial contribution to EEG power in a cognitive activation condition than in a resting state condition (Ref. 66), suggesting that the familial risk for ADHD is associated with a requirement for greater neural activation to achieve a typical level of performance.
A relatively novel approach to investigate the source of RTV in ADHD, is the measurement of very low-frequency activity (VLF; <.05Hz). VLF fluctuations may be associated with the brains default-mode network (DMN; that itself is characterised by slow fluctuations in hemodynamic signal (Ref. 89)), reflect cognitive resource allocation (Ref. 90), modulation of gross cortical excitability (Ref. 91) or an index of conscious perception (Ref. 92). Infraslow EEG corresponds to regional correlations in the infraslow BOLD signal (Ref. 92) and might modulate higher frequency activity (Ref. 91). Further research is required to clarify the precise relationship between the DMN and VLFOs before direct comparisons can be made, but current findings suggest VLF activity can be taken as a novel measure of arousal levels.
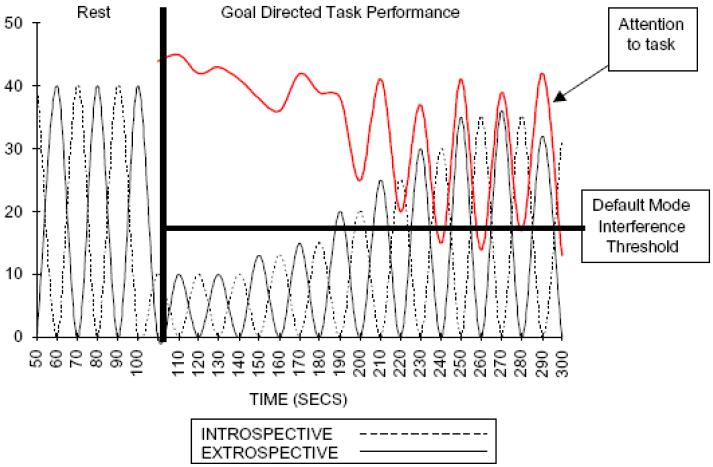
VLF fluctuations are ultra-slow multi-second oscillations that have a duration of 20 seconds for a single wave, and as such might influence RTV that peaks every 20 seconds in ADHD (Ref. 93). In support of this, greater RTV in ADHD is reported specifically in the 0.27-0.72Hz range by conducting a Fast Fourier Transform analysis on the RT spectrum (Ref. 94). VLF fluctuations are postulated to intrude on active processing where higher frequency oscillations are involved, due to a failure to effectively transition from default mode to processing mode. This has become known as the default-mode interference (DMI) hypothesis (Figure 2 (Ref. 95, 96, 97)).
FIGURE 2. Emergent default-mode interference following initial attenuation of task negative introspection by goal-directed focused attention (Ref. 95).
The figure illustrates attenuation of task negative default-mode activity associated with a shift from rest to goal-directed performance and the gradual reemergence of activity within this network as the power within task negative networks returns. The red line represents the hypothetical effect on the emergence of default mode activity during goal-directed tasks on performance. Units on the y axis are arbitrary. Figure reproduced from Ref. 102 (© 2007 Elsevier Ltd), with permission from Elsevier.
Association with ADHD
fMRI studies show associations between the brain regions involved in the DMN and ADHD (Ref. 98, 99, 100, 101), as well as the DMN and slower RT, increased RTV and error rates (Ref. 102, 103, 104, 105). Although fMRI provides excellent spatial resolution, high temporal resolution is critical to evaluate the relationship between fast-occurring cognitive processes, task performance and brain processing (Ref. 106). A preliminary study measured VLF activity at rest in adults with attentional problems and found that reduced power in the frequency range slow-3 (.06-.2Hz) was associated with a higher number of inattentive symptoms, and resembled oscillatory patterns implicated in the DMN (Ref. 107). In the same sample, individuals with increased ADHD symptoms had reduced rest-task VLFO attenuation (or increased DMI during the cognitive task), and there was a small but significant synchrony between VLF brain activity and fluctuations in RT (Ref. 108). The authors further demonstrated that adolescents with ADHD also showed reduced VLF activity at rest and reduced rest-task attenuation of VLF compared to controls (Ref. 109). Although reduced attenuation (or increased DMI) was associated with a higher number of errors and increased RTV, the small sample size limits firm conclusions at this stage. Nevertheless, such findings emphasise the potential importance of the link between attentional control and the DMN and the potential link between attenuation of the default resting state and theories highlighting altered arousal states in ADHD.
Heritability and genetic overlap with ADHD
As discussed, EEG frequency bands show moderate to high heritability and overlap with ADHD (see EEG power; Heritability section above). In addition, we know that RTV is also heritable and shares familial influences with ADHD (see Very low-frequency activity and intraindividual variability section above). Functional connectivity of the DMN is reported to be heritable with estimates at 0.42 (Ref. 110). However, as yet there is no information on the heritability of VLF activity or the extent to which shared genetic influences explain the phenotypic associations between ADHD, RTV and measures of the DMN.
Genetic association studies
To date no studies report direct genetic associations for VLF activity, although there are some promising links with the DMN; systems-level connectivity has been associated with serotonergic and dopaminergic (5HTTLPR/SLC6A4; MAOA; DARPP-32/PPP1R1B) genetic variants (Ref. 111), and healthy subjects homozygous for the functional COMT (catechol-O-methyltransferase) val allele exhibit reduced DMN connectivities at prefrontal regions (Ref. 112). This is a fruitful area for future research.
EEG coherence and connectivity
EEG coherence is calculated as the cross-correlation in the frequency domain between two EEG time points, measured simultaneously at different scalp locations. EEG coherence is regarded as an index of both structural and functional brain characteristics and a description of how different parts of the brain relate during task performance (Ref. 113). EEG coherence is also referred to as ‘asymmetry’ which refers to the relative ratio of power between two electrode points generally in the two hemispheres. Test-retest reliability for coherence measures suggest that only 60% of the variance can be explained by stable individual differences (Ref. 114).
Association with ADHD
EEG studies indicate that both intra- and inter-hemispheric coherence is elevated in ADHD, predominantly in the frontal areas of the brain (Ref. 115, 116, 117) and relating to slow-wave (delta and theta) activity in particular (Ref. 115, 118), thought to indicate reduced cortical differentiation (Ref. 119), although reduced interhemispheric coherence (Ref. 120) and intra- and interhemispheric asymmetry (Ref. 46) have also been reported. In addition, increased rightward alpha asymmetry has been demonstrated in children (Ref. 46, 121) and adults (Ref. 122) with ADHD compared to typical controls, suggesting that the ratio of alpha power is greater in the right compared to the left hemisphere.
Heritability and genetic overlap with ADHD
EEG coherence is reported to be moderately heritable, with estimates between 50% and 70% across typical children, adolescents and adults in twin populations (Ref. 123, 124, 125, 126). There are differences between frequency bands in the genetic influences on inter-hemispheric coherence, with estimates between 40% and 60% for coherences in theta and alpha bands in a large sibling sample (Ref. 127). One other study (Ref. 128) reported more modest or zero genetic effects on alpha asymmetry at frontal-central and frontal-lateral electrodes, despite high heritability of alpha power at all frontal sites.
More recently, EEG-indexed functional brain connectivity derived from graph theory has been applied in order to investigate the capacity of the brain for dynamic interaction, rather than activity in a single brain region. Heritability has been reported for measures of synchronization likelihood (40-82%) global (29-63%) and local (25-49%) alpha connectivity (Ref. 129, 130, 131) that shows high phenotypic and genetic stability from adolescence to early adulthood (Ref. 130). This highlights a potential future research direction in ADHD.
A study of alpha asymmetry in multiply affected families (Ref. 132) reported a pattern of increased rightward alpha asymmetry across frontal and central electrode sites, in the offspring of parents with ADHD compared to offspring of parents without ADHD. In the same study, increased rightward alpha asymmetry in parietal regions was associated with a lower familial risk for ADHD. This trait was also found to increase with age only in the offspring of parents who had a childhood diagnosis but were now in remission as compared to the offspring of parents with current (persistent) ADHD. This may suggest a possible adaptive or compensatory mechanism that has a specific familial association with remitting forms of ADHD.
Genetic association studies
Few genetic studies of EEG coherence have been reported. In a sample of 313 undergraduates aged 18-33 participants homozygous for the G-allele of a serotonin IA receptor SNP had significantly greater relative right frontal activity at frontal electrode sites compared to participants with the C-allele (Ref. 133). Previous research associated ADHD with the C/C genotype in 78 ADHD patients and 107 controls (Ref. 134). One study reports significant linkage on chromosome 7 for high theta interhemispheric coherence at centro-parietal scalp locations (Ref. 135). A recent study reports dose-dependent modulation of EEG connectivity by the COMT gene, whereby carriers of the Val/Val genotype exhibited greater connectivity, followed by Val/Met and Met/Met carriers (Ref. 136).
Event-related potentials (ERPs)
ERPs are small voltage fluctuations in the EEG that are evoked from task manipulations and time-locked to the onset of certain cognitive, sensory or affective stimuli (Figures 3 and 4). ERPs are obtained by averaging the ERP response across multiple trials, to average out background EEG signals and extract specific stimulus-locked ERP patterns. ERPs measure covert processing of external stimuli and isolate several performance-related measures that cannot be separated on the basis of performance data alone, by using the millisecond resolution of EEG (Ref. 40).
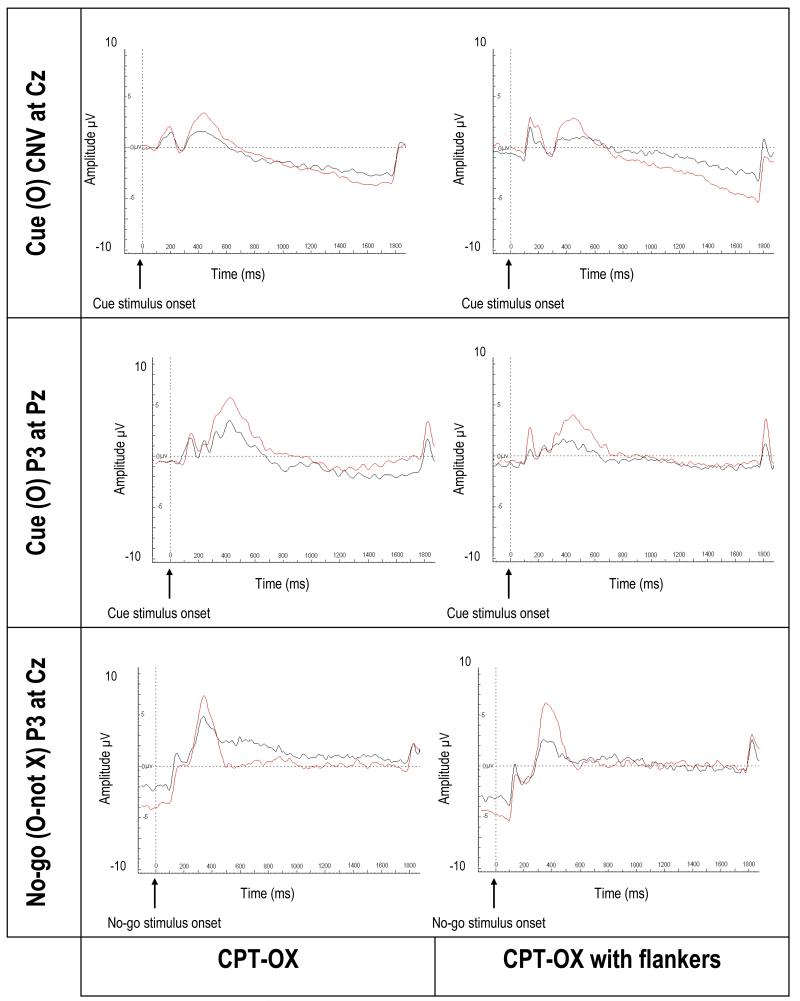
FIGURE 3. Event-related potentials associated with cue and no-go stimuli in a CPT-OX task in ADHD adults and controls (Ref. 152).
In the cued continuous performance test (CPT-OX), participants are instructed to respond to cue-target sequences (i.e. O followed by X). In the Flanker version, letters are flanked by distractor letters on either side. The figure shows CNV and stimulus-locked centro-parietal cue-P3 (in response to the cue stimulus) and no-go-P3 (enhanced positivity at fronto-central locations in response to no-go stimuli) averages for controls (red) and ADHD (black). In ADHD, a reduced CNV indicated abnormal anticipation and preparation, and reduced cue-P3 amplitudes indicated reduced attentional orienting, to cue stimuli. Attenuation of the no-go-P3 indicated the presence of abnormal inhibitory processing in adult ADHD. Figure reproduced from Ref. 144. Abbreviations: ADHD, attention deficit hyperactivity disorder; CNV, contingent negative variation.
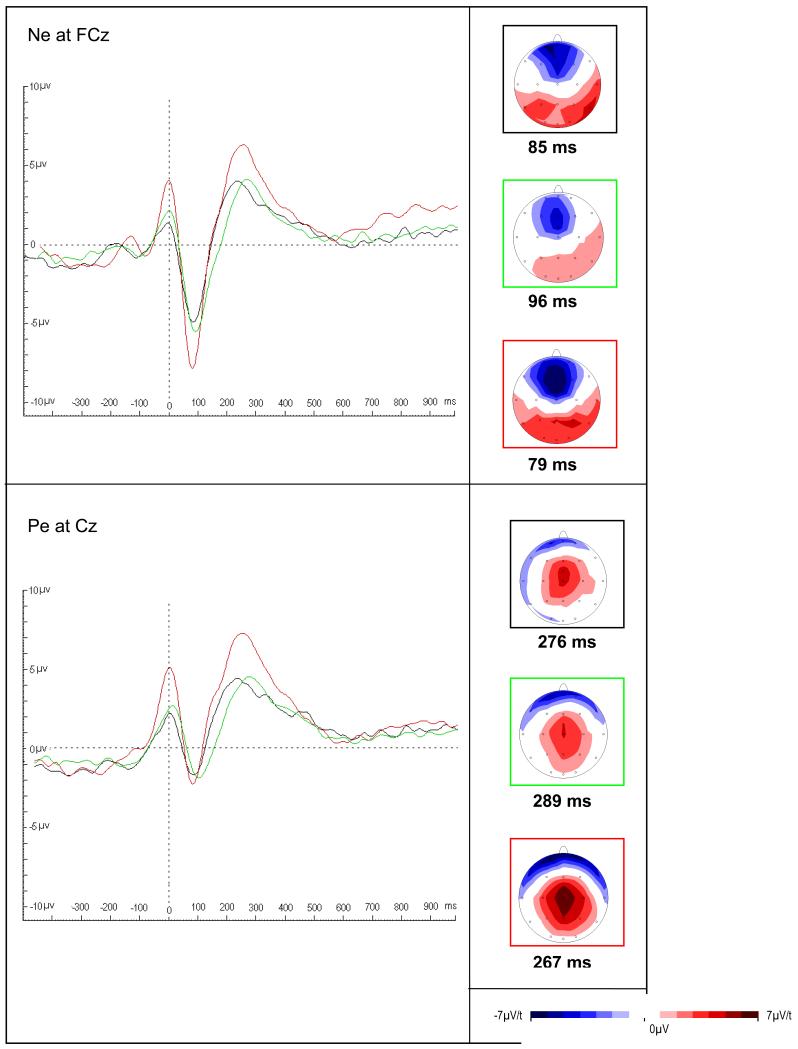
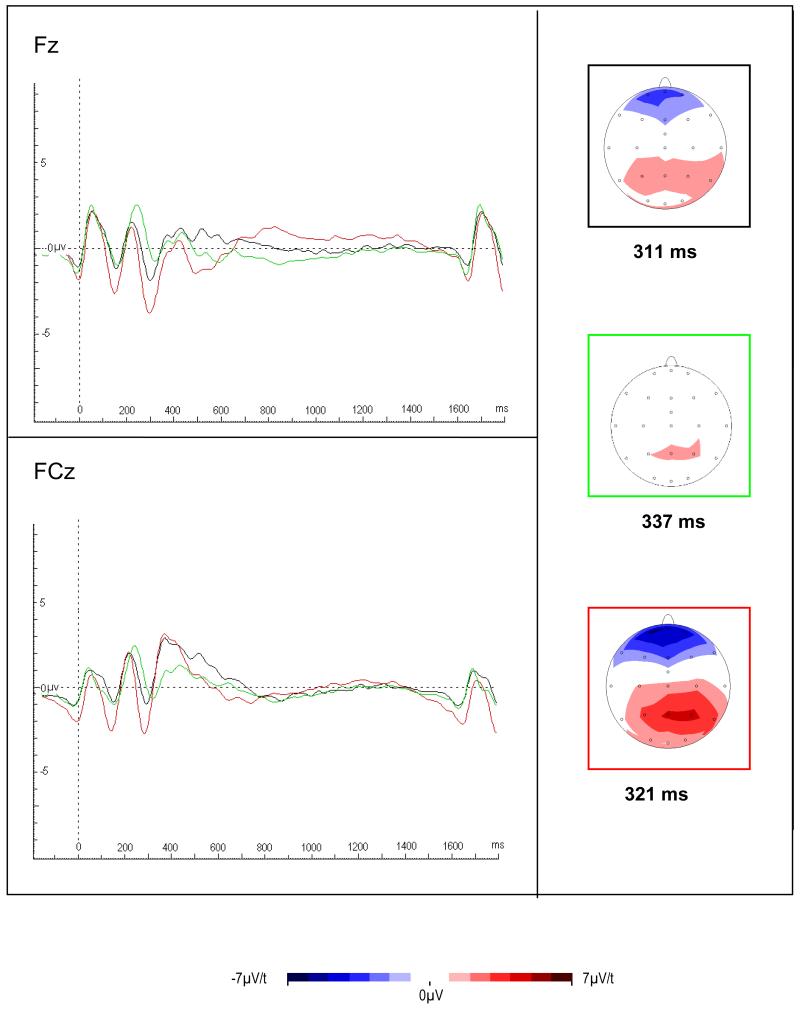
FIGURE 4. Error-related event-related potentials in an arrow flanker task in ADHD adults, fathers of ADHD probands and controls (Ref. 42).
Response-locked error negativity (Ne, top) and error positivity (Pe, bottom) shown at latencies of maximal amplitude for control participants (red border), parents of ADHD proband (green border) and ADHD participants (black border). Ne but not Pe was attenuated in the ADHD group and fathers compared to controls, which indicates abnormal initial error detection processes that shares familial effects in adult ADHD, suggestive of an informative intermediate phenotype. Figure reproduced from Ref. 48 (© 2009 Elsevier Ltd), with permission from Elsevier. Abbreviations: ADHD, attention deficit hyperactivity disorder.
Cognitive-electrophysiological research in ADHD has largely focused on the analysis of response inhibition and performance monitoring, indexed by ERP components that reflect cognitive performance measures that are impaired in people with ADHD. To date, other aspects of cognitive performance in ADHD, such as choice impulsivity and delay aversion (Ref. 137, 138, 139) have not been explicitly tested in ERP studies and will not be reviewed here.
Inhibitory and attentional processing
A task that is often used to assess different executive processes is the cued Go/No-Go task or the cued-Continuous Performance Task (CPT-OX; also referred to as CPT-AX). The CPT-OX, when used in an ERP paradigm, measures attention and inhibition and additionally attentional orienting to a cue and motor response preparation that do not require an overt response (Figure 3). ERPs associated with these processes are the go-P3 (enhanced positivity in parietal regions in response to the target) and the no-go-N2 (an enhanced negativity at fronto-central locations in response to no-go stimuli and thought to reflect conflict monitoring (Ref. 140)), followed by the no-go-P3 (enhanced positivity at fronto-central locations in response to no-go stimuli that is thought to reflect response inhibition (Ref. 141)). An additional related parameter is no-go-anteriorisation (NGA), a measure of the topographical changes in the P3 from go to no-go trials that are thought to reflect prefrontal response control mechanisms (Ref. 142). In addition, the fronto-central cue-P2, centro-parietal cue-P3 and contingent negative variation (CNV) occur in response to the cue stimulus and are thought to reflect attentional orienting to a cue and motor response preparation respectively (Ref. 143). The go-P3 and no-go-P3 demonstrate high reliability, with intra-class correlations for peak amplitudes of 0.85 and 0.92 respectively, over a period of 30 minutes (Ref. 144). Long-term reliability of topography over an average of 2.7 years found an intra-class correlation of 0.9 (Ref. 145).
Association with ADHD
Numerous studies indicate that children and adults with ADHD exhibit poorer performance (Ref. 146) and altered electrophysiological correlates (Ref. 39, 147, 148) on tasks that require attention and inhibitory control. Impaired target processing to rare targets (oddballs) as indexed by the go-P3 is reduced in children and adults with ADHD (Ref. 148, 149, 150) although this may reflect missed targets or differences in preparatory processing (Ref. 143) or possibly the presence of comorbid conditions (Ref. 149). Individuals with ADHD demonstrate attenuation of the no-go-P3 amplitude suggesting problems of inhibition in children (Ref. 151) and adults with ADHD (Figure 3 (Ref. 152)); and reduced cue-P3 and CNV activity indicating reduced response preparation in children (Ref. 143, 149, 153) and adults with ADHD (Figure 3 (Ref. 152)). In addition individuals with ADHD exhibit a reduced NGA (Ref. 154). Diminished N2 amplitudes are also found in ADHD, although these are mainly related to comorbidities (Ref. 155) or demonstrated in more demanding tasks, such as the Stop-signal task (Ref. 156).
Heritability and genetic overlap with ADHD
Meta-analysis of child and adolescent data confirm average heritability around 60% for P3 amplitude and 51% for P3 latency (Ref. 62), although in paradigms that may elicit functionally distinct components (e.g. (Ref. 157)). More recently comparable heritabilities using a Go/No-Go task were found in an adult twin sample of 60% in the no-go-N2 component and 41% and 58% for go-P3 and no-go-P3 components (Ref. 158). One study reports significant MZ correlations at frontal regions (0.67) but non-significant correlations at centro-parietal regions for visual P3 amplitude (Ref. 159). The extent of genetic influences appears to be stable throughout development, with similar heritabilities reported in children, young adults and middle-aged adults (Ref. 160). Longitudinal studies are however required to test the whether this reflects the same genetic factors throughout development, or whether different sets of genes play a role at different developmental stages. One longitudinal study reported rate of change in P3 amplitude measured at 17, 20 and 23 was genetically influenced (Ref. 161). Slow-cortical potentials such as the CNV demonstrate heritability between 30-43% (Ref. 162). These ERP components therefore appear to index genetically influenced neural processes that are important for cognitive control.
There are only a few studies evaluating the familial association between these variables and ADHD. In one small study, similar P3 amplitude to increased conflict (P3a) was demonstrated when comparing siblings of ADHD probands and typical controls despite a significantly attenuated P3 in the ADHD probands, suggesting that in this study altered P3 showed no familial association with ADHD (Ref. 163). However, other preliminary findings report impaired inhibitory control as indexed by the no-go-P3 in parents of ADHD probands indicating a familial association with adult ADHD (Ref. 164) In addition, attenuated cue-P3 and CNV has been reported in non-affected siblings of ADHD probands compared to controls (Ref. 165) and attenuated cue-P3 in parents of ADHD probands compared to controls (Ref. 164), suggesting impaired attentional orienting and preparatory states might index familial risk for ADHD. A further twin study demonstrated modest phenotypic and genetic overlap between the go-P3 in a visual oddball paradigm and externalising conditions associated with ADHD, including substance abuse disorders, conduct disorder and antisocial behaviour (Ref. 166). This association is likely to be driven by genetic factors alone with an estimated genetic correlation of −.22 (Ref. 167). Further studies particularly those incorporating twin designs in ADHD samples are required to fully determine the familial and genetic associations of these variables with ADHD.
Genetic association studies
Go-P3 in adults has been linked to regions on chromosomes 2, 5, 6 and 17 (Ref. 168) and chromosome 7q (Ref. 169) in genomewide linkage scans, suggesting that genes of moderate to large effect might affect this variable. The P3 has also been associated with specific genes involved in dopamine transmission. The A1 allele of the Taq1A polymorphism in the dopamine D2 receptor gene was associated with a reduction in P3 amplitude to rare targets in visual and auditory oddball tasks (Ref. 170) and a longer parietal go-P3 latency in a visual CPT task (Ref. 171) in individuals “at-risk” for alcoholism, although negative findings were also reported in a sample of 134 young female controls (Ref. 172). Similarly an association between the DRD4-7R allele and reduced P3 amplitude to rare targets in an auditory oddball task has been demonstrated in young boys (Ref. 173) but not reported in young females (Ref. 174), suggesting a gender effect. Healthy individuals with the Val/Val genotype for the COMT gene showed increased go-P3 amplitude and shorter go-P3 latency compared to those bearing the Val/Met homozygote in a visual working memory task (Ref. 175). An enhanced no-go-P3 was reported in Val/Val homozygotes compared to those bearing the Met/Met genotype during a flanker task in a sample of 656 healthy students (Ref. 176), although was not reported in a sample of 187 consisting of individuals with schizophrenia, their relatives and healthy controls (Ref. 177). A reduced NGA has been associated with DAT1-9R in adults with ADHD ((Ref. 178) also see (Ref. 179) for discussion on DAT1-9R as risk allele for adult ADHD) and with putative ADHD risk-alleles on the TPH2 gene in a sample of both controls and ADHD adults (Ref. 180). Finally in a study of event-related oscillations during a Go/No-Go task, DRD4-7R carriers exhibited increased no-go-related theta and reduced go-related beta (Ref. 181). These findings potentially suggest genetic variation of dopamine and serotonin genes might be involved with altered regulation of the P3 response in ADHD, perhaps through prefrontal function.
Performance monitoring
Performance monitoring comprises error detection and conflict monitoring, which are essential prerequisites for adaptively altering behaviour and decision making. Error processing is generally accompanied by a negative component (error negativity; Ne) peaking approximately 40–120 msec after the erroneous response at fronto-central sites (Ref. 182), thought to index mismatch between an intended and actual response (Ref. 182) or response conflict (Ref. 183). The Ne is frequently followed by a more parietal positive deflection (error positivity; Pe) within 200–500 msec after the response (Ref. 184), thought to index conscious processing of errors as it is elicited after errors of which the subject is aware (Ref. 185). Additionally, the no-go-N2 component is implicated in conflict monitoring through resisting the interference caused by distracters in the Flanker task (see below) and may have at least partial overlap with the neural generators of the Ne, with a correlation of 0.6 reported between these components (Ref. 42). The no-go-N2 may therefore represent a general index of conflict monitoring independent of response inhibition (Ref. 42, 140, 141). High split-half and test-retest reliability has been demonstrated across two weeks for both the Ne (intraclass correlations 0.70-0.83 for peak amplitude) and Pe (intraclass correlations 0.71-0.84 for area measures) (Ref. 186).
Association with ADHD
Performance monitoring deficits in response to task demands and post error-slowing have been demonstrated in ADHD (Ref. 39). The Eriksen arrow flanker task (Ref. 184), which requires a high level of conflict monitoring, elicits a diminished N2 amplitude (Ref. 41), reduced early error detection indexed by the Ne (Ref. 41, 187, 188), also shown in the stop-signal task (Ref. 188) and diminished late error detection indexed by the Pe in children with ADHD (Ref. 189, 190); with similar findings elicited in go-no-go task and S1-S2 task (Ref. 190). Altered topography and reduction in N2 and Ne components are found in adults with ADHD (Figures 4 and 5 (Ref. 42)). However, the reported findings are not always consistent and further work is needed to understand the sources of variation across various studies (reviewed in (Ref. 191)) such as sample size, clinical subtypes, comorbidity, task conditions such as duration and provision of feedback and methods of analysis (Ref. 191).
FIGURE 5. Conflict monitoring related event-related potentials in an arrow flanker task in ADHD adults, fathers of ADHD probands and controls (Ref. 42).
The figure shows stimulus-locked N2 averages at Fz (midline frontal) and FCz (midline frontocentral) electrodes to incongruent correct responses of control (red), ADHD participants (black) and parents (green). Scalp maps show topography at the mean latency of the N2 peak for each group, along with t-maps for group comparisons (controls versus ADHD participants and fathers, respectively). An N2 enhancement for incongruent stimuli was highest in the control group with attenuated amplitude in the ADHD group and the fathers, suggesting that reduced conflict monitoring is a genetically influenced intermediate phenotype. Figure reproduced from Ref. 48 (© 2009 Elsevier Ltd), with permission from Elsevier. Abbreviations: ADHD, attention deficit hyperactivity disorder.
Heritability and genetic overlap with ADHD
Genetic influences on both the Ne and Pe were demonstrated in a small twin sample of young adults (Ref. 158). A larger study (Ref. 192), using the Flanker task in young adolescent males, found heritabilities for the Ne and Pe of 47% and 52% respectively. Familial influences shared between ADHD and the Ne and N2 components were also found in a large study of ADHD probands and their siblings (Ref. 41, 193), with unaffected controls showing significantly greater N2-enhancement and greater Ne and Pe enhancement in response to errors compared to unaffected siblings of ADHD probands, although one small study did not support this finding (Ref. 163). Moreover, fathers of ADHD probands demonstrate significantly attenuated Ne and N2 components compared to typical adults, suggesting that the familial effects are found throughout development (Ref. 42); Figure 4 and 5.
Genetic association studies
The neurobiological role of the anterior cingulate cortex (ACC) has been the focus of considerable attention in relation to performance monitoring. A common interpretation is that ACC activity reflects conflict or outcome monitoring, as studies demonstrate increased ACC activation in tasks that require more cognitive effort (reviewed in (Ref. 194)), and studies indicate that the Ne and the N2 components share sources in the ACC (Ref. 183, 195). In addition, fMRI studies implicate the ACC in ADHD (Ref. 196, 197) and ACC hypoactivation has been associated with DAT1-10R in ADHD (Ref. 198). This is of interest, as the ACC is one of the richest dopaminergic innervated brain regions (Ref. 199), and suggests that the Ne might be generated as part of a dopamine-dependent reinforcement learning process (Ref. 200). In line with this, genetic variants involved in dopamine transmission have been associated with cognitive performance measures and the various ERP variables of performance monitoring tasks. In a sample of 39 healthy individuals, those homozygous for the COMT Met-allele had increased Pe amplitude (Ref. 201). in a sample of 656 students, the DRD4 −521C/T polymorphism (significantly associated with ADHD in meta-analysis (Ref. 19)) was associated with increased Ne following errors and failed inhibitions (Ref. 176) (reviewed in (Ref. 202)). In addition, in a small sample of children with ADHD, ASD and typical controls, significant correlations were found between ADHD symptoms and attenuated Pe; and DAT1-9R carriers were found to display a greater Pe response (Ref. 203). MPH treatment has also be found to normalise the Pe in ADHD strengthening the potential dopaminergic link (Ref. 189). Serotonin genes have also been implicated. In one study of the serotonin transporter gene in 39 healthy individuals, carriers of the promoter S-allele, which is associated with increased extracellular serotonin levels, had a larger Ne than homozygous L carriers (Ref. 204). Such findings warrant further investigation of the association between neural mechanisms of performance monitoring and specific genetic variants in larger samples.
Clinical implications
This review has alluded throughout to developmental outcomes, but these have yet to be systematically studied. Several studies suggest developmental stability for some of the EEG/ERP parameters with comparable electrophysiological findings in children, adolescents and adults with ADHD (e.g. related to performance monitoring; (Ref. 41, 42)). Many of the parameters demonstrate similar heritabilities throughout the lifespan, although this could reflect different genes at different developmental stages. The finding in ADHD that some cognitive-electrophysiological impairments are seen at different ages is important for our understanding of the development course of the disorder. One hypothesis put forward in recent years is that ADHD is associated with enduring subcortical dysfunction, but recovery through development is through improvements in executive (cortical) control (Ref. 205). This and other dual process models that emphasise both bottom-up and top-down dysfunctions (Ref. 206), can be meaningfully studied using EEG, for example by examining the interplay between EEG-indexed arousal and ERP-indexed attentional fluctuations. Future intermediate phenotype studies can systematically study stability and change to aetiological influences throughout development using longitudinal family and twin designs, and by comparing adults with remitted and persistent ADHD, as a way of identifying the brain processes associated with persistence and recovery. Such studies would provide insight into the processes related to the clinical state of ADHD and those that index genetic risk for ADHD independent of clinical status.
EEG in particular has been proposed as a useful tool for the clinical assessment of ADHD (Ref. 207). In order to be a diagnostic tool, however, it must demonstrate both high sensitivity and specificity. Sensitivity has been reported at 90-97% and specificity at 84-94% in ADHD for combined measures of EEG power and coherence (Ref. 208) and combined mean theta-beta power ratio across four tasks at a single electrode (Ref. 209, 210). However, since this has not been found in all studies it remains uncertain whether this is sufficiently robust for use in clinical practice.
Potential problems may be the common association of ADHD with comorbidities, with as many as 65% of children with ADHD having one or more co-occurring condition (Ref. 211). Furthermore there is aetiological, cognitive and neurobiological overlap between ADHD and several other psychiatric disorders (Ref. 212). For example the DMN has also been linked to schizophrenia and autism (Ref. 213), and increased RTV and cognitive performance measures reflecting executive processes are implicated in several other disorders (e.g.(Ref. 214, 215)). This suggests that although these parameters are sensitive to ADHD they are not necessarily specific to ADHD, and may represent general markers of pathophysiology or overlapping neurophysiological processes.
On the other hand it might be possible to find specificity in some cases. ERP paradigms may differentiate children with ADHD with and without conduct and tic disorders (Ref. 149, 153, 216, 217), as well as children with ADHD compared to children with reading disability for inhibitory ERPs (Ref. 218) and children with autism for performance monitoring ERPs (Ref. 219). In addition, EEG power may differentiate ADHD children with high and low autism symptoms (Ref. 220). The effect of comorbidities on the association between ADHD and candidate intermediate phenotypes is a key area for future investigation.
Cognitive-electrophysiological phenotypes may also be sensitive markers of neuropathological or aetiological subtypes and have the potential to delineate processes that can be targeted for the development of specific treatments for subtypes of ADHD. Future studies are required to show whether ERP variables combined with genetic marker data can be used to predict individual patient treatment response and outcomes. Such measures could potentially be utilised in “at-risk” individuals, such as the close relatives of ADHD probands, in order to initiate interventions at an earlier stage.
One successful clinical application using EEG/ERP is through neurofeedback (NF). NF uses operant conditioning to train patients to enhance poorly regulated EEG and ERP patterns. Information on the individual’s brainwave activity is fed into a computer that converts the information into visual or auditory signals in real-time. Improvement is positively rewarded so that individuals learn to control their brainwave patterns. Individualised game-like set ups are especially useful for training children, but may also be highly effective in adults.
Several studies document the efficacy of NF for ADHD (see (Ref. 207) for review of earlier studies). Psychophysiologists have used findings from electrophysiological studies of ADHD to select the most worthwhile treatment approaches. For example, based on the finding of increased theta/beta ratio in ADHD, one study used a task in which a bar on the left side of the screen (representing theta activity) had to be reduced and a bar on the right side (representing beta activity) had to be increased (Ref. 221). The authors reported a decrease in theta activity at post-assessment (one week following the second treatment block of 3-4 weeks) that was associated with improvements in ADHD symptom scores with an effect size of 0.6. Notably, this effect was specific to the NF group compared to a control group that completed attentional skills training that was designed to parallel the NF treatment in terms of training setting, demands upon participants, therapeutic support and expectation and satisfaction with the treatment. Moreover, baseline EEG collected pre-assessment was useful in predicting the overall success of NF. A recent meta-analysis (Ref. 222) concluded that NF treatment for ADHD is “efficacious and specific”, shown through improvements in inattention and impulsivity, and to a lesser extent hyperactivity. Furthermore, these improvements appear to be maintained 2 years after the initial treatment (Ref. 223).
Conclusion
We have shown that EEG and ERP measures related to arousal and attentional processes are potential intermediate phenotypes for ADHD. Each of these domains demonstrates association with ADHD, moderate to high heritability, altered processing similar to ADHD in non-affected first-degree relatives and preliminary reports of association to genetic variants particularly those involving dopamine regulation. Nevertheless, these candidate intermediate phenotypes do not yet meet all criteria; few studies have examined familial and genetic overlap with ADHD, no cognitive-electrophysiological measures has been shown to mediate genetic effects on ADHD and genetic associations reported to date remain unconfirmed.
TABLE 1. Overview of the utility of selected candidate electrophysiological intermediate phenotypes in ADHD.
This table summarises the findings following a systematic review of the literature, described in this paper. Databases Pubmed, Ovid MEDLINE, PsycINFO and EMBASE were searched using combinations of the key words EEG, ERP, electrophysiology, heritability, twin, family, endophenotype, genetic, ADHD within each selected domain. The computer search was supplemented with bibliographic cross-referencing.
| Intermediate phenotype | Association with ADHD | Heritability | Familial/genetic overlap with ADHD | Genetic associations with ADHD risk variants |
|---|---|---|---|---|
|
| ||||
| EEG power | ↑ theta ↓beta ↑theta-beta ratio |
High | High familial correlations for theta and beta in multiply affected families (not unaffected relatives) | SLC6A3 DRD4 |
| Partially inconsistent | Consistent | Limited | Consistent | |
|
| ||||
| EEG power: very low frequency fluctuations | ↓power at rest ↓rest-task attenuation |
Unknown | Unknown | Unknown |
| Limited | ||||
|
| ||||
| EEG: coherence and connectivity | ↑ inter- and intrahemispheric coherence | Moderate | ↑ alpha asymmetry in first-degree relatives indicating familial risk factor | COMT Serotonin 1A receptor |
| Partially inconsistent | Partially inconsistent | Limited | Limited | |
|
| ||||
| ERP: Inhibitory and attentional processing | ↓ go-P3 amplitude ↓ no-go-P3 amplitude ↓ cue-P3 ↓ CNV ↓ no-go-anteriorisation ↓ N2 |
Moderate | ↓ P3 not familial or associated with familial risk for broader externalising conditions ↓ no-go-P3, cue-P3 and CNV demonstrate familial association with ADHD |
SLC6A3 DRD2 DRD4 COMT TPH2 |
| Partially inconsistent | Consistent | Partially inconsistent | Consistent | |
|
| ||||
| ERP: Performance monitoring | ↓ error negativity ↓ error positivity ↓ N2 |
Moderate | ↓ error negativity ↓ error positivity ↓ N2 demonstrated in non-affected first-degree relatives |
SLC6A3 DRD4 COMT 5-HTTLPR |
| Inconsistent | Limited | Limited | Consistent | |
Key: Unknown: Genetically sensitive designs are required to confirm utility.
Inconsistent: Multiple parameters/studies within the domain demonstrate non-replication. Confounding effects of sample and paradigm differences and presence of subtypes and comorbid conditions must be explored.
Partially inconsistent: A small number of parameters/studies within the domain demonstrate non-replication. Confounding effects of sample and paradigm differences and presence of subtypes and comorbid conditions must be explored.
Limited: Promising consistency but further replication is required due to a limited number of studies.
Consistent: To date fulfils this criterion for a potential intermediate phenotype of ADHD but further work in ADHD samples is required.
This review has outlined the potential role of catecholaminergic dysfunctions underlying altered electrophysiological responses in ADHD, in particular highlighting the potential role of dopamine in several domains. Reduced dopaminergic neurotransmission has been linked to underarousal indexed by quantitative EEG, and executive dysfunction indexed by ERPs, as well as indirectly through association with dopaminergic-rich brain regions. However, the mechanisms by involved require further research.
In order to be successful, intermediate phenotype research must control for confounding variables such as gender, age, treatment effects, specificity of the measure and comorbid psychopathology that may affect the relationship between phenotype and intermediate phenotype (Ref. 212). Furthermore, the evaluation of psychometric properties of reliability (through test-retest paradigms) and construct validity (such as confirmatory factor analysis) is important to ensure consistency and robustness across multi-centre sites. Taking these steps may alleviate the inconsistencies and variable associations between electrophysiological markers and ADHD.
Not only are phenotypic and more so genetic associations with ADHD somewhat variable, if measured in a poorly designed paradigm, ERPs may reflect the superposition of activity in many different overlapping components that themselves reflect different aspects of cognitive processing other than the parameter in question. If this is the case ERPs might not be ideal electrophysiological markers of genetic risk for ADHD due to their potential heterogeneity. One step toward improving the value of ERP findings is using well-validated tasks, such as the CPT-OX and Eriksen Flanker task. In addition, novel spatiotemporal localisation methods of mapping event-related components, such as principal components analysis (PCA; (Ref. 224)), independent components analysis (ICA; (Ref. 225)) and microstate analysis (Ref. 143) are expected to unravel problems of source localisation and reduce heterogeneity of the measures applied.
A general issue for the intermediate phenotype concept is that the genetic and environmental influences on electrophysiological measures may be as complex as those on behavioural phenotypes; and it remains uncertain whether they reflect simpler phenotypes that better target aetiological influences (Ref. 226). Causal tests of mediation will also be necessary to identify electrophysiological markers that mediate aetiological effects on ADHD (Ref. 227). For example shared genetic effects between ADHD and electrophysiological markers may reflect pleiotropy (or epiphenomena) rather than reflecting causal processes on ADHD (Ref. 228). Furthermore, familial effects identified in most family study designs cannot distinguish between genetic or environmental effects, although like ADHD, there is limited evidence of familial environmental effects on most of the cognitive-electrophysiological measures. The use of multivariate twin model-fitting alongside longitudinal designs and molecular genetic studies would however enable better dissection of the genetic and environmental factors involved and causal hypotheses to be tested (Ref. 229).
In conclusion, EEG/ERP is inexpensive and generates reliable and heritable data making it possible to study brain-behaviour relations with the large sample sizes needed for both quantitative and molecular genetics research; and addresses parameters that are of particular importance in understanding the nature of the cognitive performance deficits in ADHD. Multiple biological, cognitive and behavioural measures can be incorporated into multivariate approaches to provide a detailed dissection of the aetiological influences involved.
Acknowledgements and funding
Charlotte Tye is supported by a UK Medical Research Council (MRC) studentship. Gráinne McLoughlin’s work is supported by The Waterloo Foundation and the Steel Charitable Trust. Jonna Kuntsi is funded by an Action Medical Research Project Grant for her EEG work on ADHD. Philip Asherson is funded by NIHR for electrophysiological studies of adults with ADHD and the Biomedical Research Centre (BRC) for electrophysiological studies of ADHD. We would like to thank the peer reviewers for their helpful comments on an earlier manuscript.
References
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th edition Washington, DC: 2000. [Google Scholar]
- 2.Polanczyk G, Jensen P. Epidemiologic Considerations in Attention Deficit Hyperactivity Disorder: A Review and Update. Child and Adolescent Psychiatric Clinics of North America. 2008;17(2):245–260. doi: 10.1016/j.chc.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Faraone SV, et al. Diagnosing Adult Attention Deficit Hyperactivity Disorder: Are Late Onset and Subthreshold Diagnoses Valid? Am J Psychiatry. 2006;163(10):1720–1729. doi: 10.1176/ajp.2006.163.10.1720. [DOI] [PubMed] [Google Scholar]
- 4.Faraone SV, Biederman J, Monuteaux MC. Attention-deficit disorder and conduct disorder in girls: evidence for a familial subtype. Biological Psychiatry. 2000;48(1):21–29. doi: 10.1016/s0006-3223(00)00230-4. [DOI] [PubMed] [Google Scholar]
- 5.Faraone SV, et al. Molecular Genetics of Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2005;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Goodman R, Stevenson J. A twin study of hyperactivity--II. The aetiological role of genes, family relationships and perinatal adversity. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1989;30(5):691–709. doi: 10.1111/j.1469-7610.1989.tb00782.x. [DOI] [PubMed] [Google Scholar]
- 7.Biederman J, et al. Convergence of the Child Behavior Checklist with Structured Interview-based Psychiatric Diagnoses of ADHD Children with and without Comorbidity. Journal of Child Psychology and Psychiatry. 1993;34(7):1241–1251. doi: 10.1111/j.1469-7610.1993.tb01785.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, et al. DSM-IV combined type ADHD shows familial association with sibling trait scores: a sampling strategy for QTL linkage. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics. 2008;147B(8):1450–60. doi: 10.1002/ajmg.b.30672. [DOI] [PubMed] [Google Scholar]
- 9.Levy F, et al. Genetic Analysis of a Large-Scale Twin Study. 1997. Attention-Deficit Hyperactivity Disorder: A Category or a Continuum? pp. 737–744. [DOI] [PubMed] [Google Scholar]
- 10.McLoughlin G, et al. Genetic support for the dual nature of attention deficit hyperactivity disorder: substantial genetic overlap between the inattentive and hyperactive-impulsive components. J Abnorm Child Psychol. 2007;35(6):999–1008. doi: 10.1007/s10802-007-9149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plomin R, Owen M, McGuffin P. The genetic basis of complex human behaviors. Science. 1994;264(5166):1733–1739. doi: 10.1126/science.8209254. [DOI] [PubMed] [Google Scholar]
- 12.Swanson J, et al. Etiologic Subtypes of Attention-Deficit/Hyperactivity Disorder: Brain Imaging, Molecular Genetic and Environmental Factors and the Dopamine Hypothesis. Neuropsychology Review. 2007;17(1):39–59. doi: 10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
- 13.Li D, et al. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD) Hum. Mol. Genet. 2006;15(14):2276–2284. doi: 10.1093/hmg/ddl152. [DOI] [PubMed] [Google Scholar]
- 14.Brookes KJ, et al. Association of ADHD with genetic variants in the 5prime-region of the dopamine transporter gene: Evidence for allelic heterogeneity. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics. 2008;147B(8):1519–1523. doi: 10.1002/ajmg.b.30782. [DOI] [PubMed] [Google Scholar]
- 15.Asherson P, et al. Confirmation That a Specific Haplotype of the Dopamine Transporter Gene Is Associated With Combined-Type ADHD. Am J Psychiatry. 2007;164(4):674–677. doi: 10.1176/ajp.2007.164.4.674. [DOI] [PubMed] [Google Scholar]
- 16.Maher BS, et al. Dopamine system genes and attention deficit hyperactivity disorder: a meta-analysis. Psychiatric Genetics. 2002;12(4):207–215. doi: 10.1097/00041444-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Purper-Ouakil D, et al. Meta-analysis of family-based association studies between the dopamine transporter gene and attention deficit hyperactivity disorder. Psychiatric Genetics. 2005;15(1):53–59. doi: 10.1097/00041444-200503000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Yang B, et al. A meta-analysis of association studies between the 10-repeat allele of a VNTR polymorphism in the 3prime-UTR of dopamine transporter gene and attention deficit hyperactivity disorder. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics. 2007;144B(4):541–550. doi: 10.1002/ajmg.b.30453. [DOI] [PubMed] [Google Scholar]
- 19.Gizer I, Ficks C, Waldman I. Candidate gene studies of ADHD: a meta-analytic review. Human Genetics. 2009;126(1):51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- 20.Lucki I. The spectrum of behaviors influenced by serotonin. Biological Psychiatry. 1998;44(3):151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 21.Spivak B, et al. Circulatory levels of catecholamines, serotonin and lipids in attention deficit hyperactivity diiorder. Acta Psychiatrica Scandinavica. 1999;99(4):300–304. doi: 10.1111/j.1600-0447.1999.tb07229.x. [DOI] [PubMed] [Google Scholar]
- 22.Kuntsi J, et al. The IMAGE project: Methodological issues for the molecular genetic analysis of ADHD. Behavioral and Brain Functions. 2006;2:27. doi: 10.1186/1744-9081-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neale BM, et al. Genome-wide association scan of attention deficit hyperactivity disorder. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics. 2008;147B(8):1337–1344. doi: 10.1002/ajmg.b.30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neale BM, et al. Meta-Analysis of Genome-Wide Association Studies of Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(9):884–897. doi: 10.1016/j.jaac.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brookes K, et al. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry. 2006;11(10):934–953. doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]
- 26.Lasky-Su J, et al. Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics. 2008;147B(8):1345–54. doi: 10.1002/ajmg.b.30867. [DOI] [PubMed] [Google Scholar]
- 27.Lesch KP, et al. Molecular genetics of adult ADHD: converging evidence from genome-wide association and extended pedigree linkage studies. J Neural Transm. 2008;115(11):1573–85. doi: 10.1007/s00702-008-0119-3. [DOI] [PubMed] [Google Scholar]
- 28.Franke B, Neale BM, Faraone SV. Genome-wide association studies in ADHD. Hum Genet. 2009;126(1):13–50. doi: 10.1007/s00439-009-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou K, et al. Meta-analysis of genome-wide linkage scans of attention deficit hyperactivity disorder. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics. 2008;147B(8):1392–8. doi: 10.1002/ajmg.b.30878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elia J, et al. Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol Psychiatry. 2010;15:637–646. doi: 10.1038/mp.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams NM, et al. Rare chromosomal deletions and duplications in attention-deficit hyperactivity disorder: a genome-wide analysis. Lancet. 2010;376:1401–1408. doi: 10.1016/S0140-6736(10)61109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottesman, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 33.Green AE, et al. Using genetic data in cognitive neuroscience: from growing pains to genuine insights. Nat Rev Neurosci. 2008;9(9):710–720. doi: 10.1038/nrn2461. [DOI] [PubMed] [Google Scholar]
- 34.Munafò MR, Brown SM, Hariri AR. Serotonin Transporter (5-HTTLPR) Genotype and Amygdala Activation: A Meta-Analysis. Biological Psychiatry. 2008;63(9):852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuntsi J, et al. Separation of cognitive impairments in attention deficit hyperactivity disorder into two familial factors. Arch Gen Psychiatry. 2010;67:1159–1166. doi: 10.1001/archgenpsychiatry.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuntsi J, et al. Reaction time, inhibition, working memory and performance: genetic influences and their interpretation. Psychological Medicine. 2006;36(11):1613–1624. doi: 10.1017/S0033291706008580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doyle A, et al. Are endophenotypes based on measures of executive functions useful for molecular genetic studies of ADHD? J Child Psychol Psychiatry. 2005;46:774–803. doi: 10.1111/j.1469-7610.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- 38.Castellanos F, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3(8):617–28. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 39.Kuntsi J, McLoughlin G, Asherson P. Attention deficit hyperactivity disorder. NeuroMolecular Medicine. 2006;8(4):461–484. doi: 10.1385/NMM:8:4:461. [DOI] [PubMed] [Google Scholar]
- 40.McLoughlin G, et al. Electrophysiological parameters in psychiatric research: ADHD. Psychiatry. 2005;4(12):14–18. [Google Scholar]
- 41.Albrecht B, et al. Action Monitoring in Boys With Attention-Deficit/Hyperactivity Disorder, Their Nonaffected Siblings, and Normal Control Subjects: Evidence for an Endophenotype. Biological Psychiatry. 2008;64(7):615, 625. doi: 10.1016/j.biopsych.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLoughlin G, et al. Performance monitoring is altered in adult ADHD: A familial event-related potential investigation. Neuropsychologia. 2009;47(14):3134–3142. doi: 10.1016/j.neuropsychologia.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams LM, et al. The test-retest reliability of a standardized neurocognitive and neurophysiological test battery: “Neuromarker”. International Journal of Neuroscience. 2005;115(12):1605–1630. doi: 10.1080/00207450590958475. [DOI] [PubMed] [Google Scholar]
- 44.Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clinical Neurophysiology. 2003;114(2):171–183. doi: 10.1016/s1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- 45.Bresnahan SM, Anderson JW, Barry RJ. Age-related changes in quantitative EEG in attention-deficit/hyperactivity disorder. Biological Psychiatry. 1999;46(12):1690–1697. doi: 10.1016/s0006-3223(99)00042-6. [DOI] [PubMed] [Google Scholar]
- 46.Chabot RJ, Serfontein G. Quantitative electroencephalographic profiles of children with attention deficit disorder. Biological Psychiatry. 1996;40(10):951–963. doi: 10.1016/0006-3223(95)00576-5. [DOI] [PubMed] [Google Scholar]
- 47.Clarke AR, et al. EEG-defined subtypes of children with attention-deficit/hyperactivity disorder. Clinical Neurophysiology. 2001;112(11):2098–2105. doi: 10.1016/s1388-2457(01)00668-x. [DOI] [PubMed] [Google Scholar]
- 48.Janzen T, et al. Differences in baseline EEG measures for ADD and Normally Achieving preadolescent males. Biofeedback and self-regulation. 1995;20(1):65–82. doi: 10.1007/BF01712767. [DOI] [PubMed] [Google Scholar]
- 49.Snyder SM, Hall JR. A Meta-analysis of Quantitative EEG Power Associated With Attention-Deficit Hyperactivity Disorder. Journal of Clinical Neurophysiology. 2006;23(5):440–455. doi: 10.1097/01.wnp.0000221363.12503.78. [DOI] [PubMed] [Google Scholar]
- 50.Clarke AR, et al. Excess beta activity in children with attention-deficit/hyperactivity disorder: an atypical electrophysiological group. Psychiatry Research. 2001;103(2-3):205–218. doi: 10.1016/s0165-1781(01)00277-3. [DOI] [PubMed] [Google Scholar]
- 51.Koehler S, et al. Increased EEG power density in alpha and theta bands in adult ADHD patients. Journal of Neural Transmission. 2009;116(1):97–104. doi: 10.1007/s00702-008-0157-x. [DOI] [PubMed] [Google Scholar]
- 52.Makeig S, Jung T-P. Tonic, phasic, and transient EEG correlates of auditory awareness in drowsiness. Cognitive Brain Research. 1996;4(1):15–25. doi: 10.1016/0926-6410(95)00042-9. [DOI] [PubMed] [Google Scholar]
- 53.Yordanova J, et al. Abnormal early stages of task stimulus processing in children with attention-deficit hyperactivity disorder - evidence from event-related gamma oscillations. Clinical Neurophysiology. 2001;112(6):1096–1108. doi: 10.1016/s1388-2457(01)00524-7. [DOI] [PubMed] [Google Scholar]
- 54.Benninger C, Matthis P, Scheffner D. EEG development of healthy boys and girls. Results of a longitudinal study. Electroencephalography and Clinical Neurophysiology. 1984;57(1):1–12. doi: 10.1016/0013-4694(84)90002-6. [DOI] [PubMed] [Google Scholar]
- 55.Hudspeth WJ, Pribram KH. Psychophysiological indices of cerebral maturation. International Journal of Psychophysiology. 1992;12(1):19–29. doi: 10.1016/0167-8760(92)90039-e. [DOI] [PubMed] [Google Scholar]
- 56.Mann CA, et al. Quantitative analysis of EEG in boys with attention-deficit-hyperactivity disorder: Controlled study with clinical implications. Pediatric Neurology. 8(1):30, 36. doi: 10.1016/0887-8994(92)90049-5. [DOI] [PubMed] [Google Scholar]
- 57.McGuire KA, et al. Genetic influences on the spontaneous EEG: An examination of 15-year-old and 17-year-old twins. Developmental Neuropsychology. 1998;14(1):7–18. [Google Scholar]
- 58.Christian JC, et al. Genetic analysis of the resting electroencephalographic power spectrum in human twins. Psychophysiology. 1996;33(5):584–591. doi: 10.1111/j.1469-8986.1996.tb02435.x. [DOI] [PubMed] [Google Scholar]
- 59.Lykken DT, Tellegen A, Iacono WG. EEG spectra in twins: Evidence for a neglected mechanism of genetic determination. Physiological Psychology. 1982;10(1):60–65. [Google Scholar]
- 60.Beijsterveldt C. E. M. v., Geus E. J. C. d., Boomsma DI. Heritability of human brain functioning as assessed by electroencephalography. American Journal of Human Genetics. 1996;58(3):562–573. [PMC free article] [PubMed] [Google Scholar]
- 61.Van Baal GCM, De Geus EJC, Boomsma DI. Genetic architecture of EEG power spectra in early life. Electroencephalography and Clinical Neurophysiology. 1996;98(6):502–514. doi: 10.1016/0013-4694(96)95601-1. [DOI] [PubMed] [Google Scholar]
- 62.van Beijsterveldt CEM, van Baal GCM. Twin and family studies of the human electroencephalogram: a review and a meta-analysis. Biological Psychology. 2002;61(1-2):111–138. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- 63.Zietsch BP, et al. Common and specific genetic influences on EEG power bands delta, theta, alpha, and beta. Biological Psychology. 2007;75(2):154–164. doi: 10.1016/j.biopsycho.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 64.Tang Y, et al. Heritability of Bipolar EEG Spectra in a Large Sib-pair Population. Behavior Genetics. 2007;37(2):302–313. doi: 10.1007/s10519-006-9133-0. [DOI] [PubMed] [Google Scholar]
- 65.Loo SK, et al. Familial clustering and DRD4 effects on electroencephalogram measures in multiplex families with attention deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 49(4):368–377. [PMC free article] [PubMed] [Google Scholar]
- 66.Loo SK, Smalley SL. Preliminary report of familial clustering of EEG measures in ADHD. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics. 2008;147B(1):107–109. doi: 10.1002/ajmg.b.30575. [DOI] [PubMed] [Google Scholar]
- 67.Basar E, Güntekin B. A review of brain oscillations in cognitive disorders and the role of neurotransmitters. Brain Research. 2008;1235:172–193. doi: 10.1016/j.brainres.2008.06.103. [DOI] [PubMed] [Google Scholar]
- 68.Loo SKPD, et al. Functional Effects of the DAT1 Polymorphism on EEG Measures in ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42(8):986–993. doi: 10.1097/01.CHI.0000046890.27264.88. [DOI] [PubMed] [Google Scholar]
- 69.Clarke AR, et al. Effects of stimulant medications on the EEG of children with Attention-Deficit/Hyperactivity Disorder Predominantly Inattentive type. International Journal of Psychophysiology. 2003;47(2):129–137. doi: 10.1016/s0167-8760(02)00119-8. [DOI] [PubMed] [Google Scholar]
- 70.Loo SK, et al. EEG Correlates of Methylphenidate Response in ADHD: Association With Cognitive and Behavioral Measures. Journal of Clinical Neurophysiology. 2004;21(6):457–464. doi: 10.1097/01.wnp.0000150890.14421.9a. [DOI] [PubMed] [Google Scholar]
- 71.Debener S, et al. Top-down attentional processing enhances auditory evoked gamma band activity. NeuroReport. 2003;14(5):683–686. doi: 10.1097/00001756-200304150-00005. [DOI] [PubMed] [Google Scholar]
- 72.Herrmann CS, Mecklinger A. Gamma activity in human EEG is related to highspeed memory comparisons during object selective attention. Visual Cognition. 2001;8(3):593–608. [Google Scholar]
- 73.Demiralp T, et al. DRD4 and DAT1 Polymorphisms Modulate Human Gamma Band Responses. Cereb. Cortex. 2007;17(5) doi: 10.1093/cercor/bhl011. [DOI] [PubMed] [Google Scholar]
- 74.Klein C, et al. Intra-Subject Variability in Attention-Deficit Hyperactivity Disorder. Biological Psychiatry. 2006;60(10):1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 75.Nigg JT, et al. Evaluating the Endophenotype Model of ADHD Neuropsychological Deficit: Results for Parents and Siblings of Children With ADHD Combined and Inattentive Subtypes. Journal of Abnormal Psychology. 2004;113(4):614–625. doi: 10.1037/0021-843X.113.4.614. [DOI] [PubMed] [Google Scholar]
- 76.Andreou P, et al. Reaction time performance in ADHD: improvement under fast-incentive condition and familial effects. Psychological Medicine. 2007;37(12):1703–1715. doi: 10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Uebel H, et al. Performance variability, impulsivity errors and the impact of incentives as gender-independent endophenotypes for ADHD. Journal of Child Psychology and Psychiatry. 51(2):210–218. doi: 10.1111/j.1469-7610.2009.02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bidwell LC, et al. Testing for Neuropsychological Endophenotypes in Siblings Discordant for Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2007;62(9):991–998. doi: 10.1016/j.biopsych.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuntsi J, Stevenson J. Psychological Mechanisms in Hyperactivity: II The Role of Genetic Factors. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42(02):211–219. [PubMed] [Google Scholar]
- 80.Kuntsi J, Oosterlaan J, Stevenson J. Psychological Mechanisms in Hyperactivity: I Response Inhibition Deficit, Working Memory Impairment, Delay Aversion, or Something Else? The Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42(02):199–210. [PubMed] [Google Scholar]
- 81.Wood A, et al. The relationship between ADHD and key cognitive phenotypes is not mediated by shared familial effects with IQ. Psychol Med. 2010;4:1–11. doi: 10.1017/S003329171000108X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wood AC, et al. Separation of genetic influences on attention deficit hyperactivity disorder symptoms and reaction time performance from those on IQ. Psychological Medicine. 2010;40:1027–1037. doi: 10.1017/S003329170999119X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bellgrove MA, et al. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: Sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia. 2005;43(13):1847–1857. doi: 10.1016/j.neuropsychologia.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 84.O’Connell RG, et al. Reduced electrodermal response to errors predicts poor sustained attention performance in attention deficit hyperactivity disorder. NeuroReport. 2004;15(16):2535–2538. doi: 10.1097/00001756-200411150-00021. [DOI] [PubMed] [Google Scholar]
- 85.Sergeant JA. Modeling Attention-Deficit/Hyperactivity Disorder: A Critical Appraisal of the Cognitive-Energetic Model. Biological Psychiatry. 2005;57(11):1248–1255. doi: 10.1016/j.biopsych.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 86.Johnson K, et al. Dissociation in performance of children with ADHD and high-functioning autism on a task of sustained attention. Neuropsychologia. 2007;45:2234–45. doi: 10.1016/j.neuropsychologia.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson KA, et al. Response variability in Attention Deficit Hyperactivity Disorder: Evidence for neuropsychological heterogeneity. Neuropsychologia. 2007;45(4):630–638. doi: 10.1016/j.neuropsychologia.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 88.O’Connell RG, et al. Two Types of Action Error: Electrophysiological Evidence for Separable Inhibitory and Sustained Attention Neural Mechanisms Producing Error on Go/No-go Tasks. Journal of Cognitive Neuroscience. 2009;21(1):93–104. doi: 10.1162/jocn.2009.21008. [DOI] [PubMed] [Google Scholar]
- 89.Raichle ME, et al. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rösler F, Heil M, Röder B. Slow negative brain potentials as reflections of specific modular resources of cognition. Biological Psychology. 1997;45(1-3):109–141. doi: 10.1016/s0301-0511(96)05225-8. [DOI] [PubMed] [Google Scholar]
- 91.Vanhatalo S, et al. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(14):5053–5057. doi: 10.1073/pnas.0305375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.He BJ, Raichle ME. The fMRI signal, slow cortical potential and consciousness. Trends in Cognitive Sciences. 2009;13(7):302–309. doi: 10.1016/j.tics.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Castellanos FX, et al. Varieties of Attention-Deficit/Hyperactivity Disorder-Related Intra-Individual Variability. Biological Psychiatry. 2005;57(11):1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Di Martino A, et al. Decomposing Intra-Subject Variability in Children with Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2008;64(7) doi: 10.1016/j.biopsych.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sonuga-Barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neuroscience and Biobehavioral Reviews. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 96.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Giambra LM. A Laboratory Method for Investigating Influences on Switching Attention to Task-Unrelated Imagery and Thought. Consciousness and Cognition. 1995;4(1):1–21. doi: 10.1006/ccog.1995.1001. [DOI] [PubMed] [Google Scholar]
- 98.Castellanos FX, et al. Cingulate-Precuneus Interactions: A New Locus of Dysfunction in Adult Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2008;63(3):332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fassbender C, et al. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Research. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tian L, et al. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neuroscience Letters. 2006;400(1-2):39–43. doi: 10.1016/j.neulet.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 101.Uddin LQ, et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. Journal of Neuroscience Methods. 2008;169(1):249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 102.Eichele T, et al. Prediction of human errors by maladaptive changes in event-related brain networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(16):6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kelly AMC, et al. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. European Journal of Neuroscience. 2004;19(11):3105–3112. doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- 104.Suskauer SJ, et al. fMRI of Intrasubject Variability in ADHD: Anomalous Premotor Activity With Prefrontal Compensation. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(10):1141–1150. doi: 10.1097/CHI.0b013e3181825b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weissman DH, et al. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 106.Khader P, et al. On the relationship between slow cortical potentials and BOLD signal changes in humans. International Journal of Psychophysiology. 2008;67(3):252–261. doi: 10.1016/j.ijpsycho.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 107.Helps S, et al. Very low frequency EEG oscillations and the resting brain in young adults: a preliminary study of localisation, stability and association with symptoms of inattention. Journal of Neural Transmission. 2008;115(2):279–285. doi: 10.1007/s00702-007-0825-2. [DOI] [PubMed] [Google Scholar]
- 108.Helps SK, et al. The Attenuation of Very Low Frequency Brain Oscillations in Transitions from a Rest State to Active Attention. Journal of Psychophysiology. 2010;23(4):191–198. [Google Scholar]
- 109.Helps SK, et al. Altered spontaneous low frequency brain activity in Attention Deficit/Hyperactivity Disorder. Brain Research. 2010;1322:134–143. doi: 10.1016/j.brainres.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 110.Glahn DC, et al. Genetic control over the resting brain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(3):1223–1228. doi: 10.1073/pnas.0909969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meyer-Lindenberg A. Neural connectivity as an intermediate phenotype: Brain networks under genetic control. Wiley Subscription Services, Inc., A Wiley Company; 2009. pp. 1938–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu B, et al. Prefrontal-Related Functional Connectivities within the Default Network Are Modulated by COMT val158met in Healthy Young Adults. J. Neurosci. 2010;30(1):64–69. doi: 10.1523/JNEUROSCI.3941-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.French CC, Beaumont JG. A critical review of EEG coherence studies of hemisphere function. International Journal of Psychophysiology. 1984;1(3):241–254. doi: 10.1016/0167-8760(84)90044-8. [DOI] [PubMed] [Google Scholar]
- 114.Hagemann D, et al. Does Resting Electroencephalograph Asymmetry Reflect a Trait? An Application of Latent State-Trait Theory. Journal of Personality and Social Psychology. 2002;82(4):619–641. [PubMed] [Google Scholar]
- 115.Barry RJ, et al. EEG coherence in attention-deficit/hyperactivity disorder: a comparative study of two DSM-IV types. Clinical Neurophysiology. 2002;113(4):579–585. doi: 10.1016/s1388-2457(02)00036-6. [DOI] [PubMed] [Google Scholar]
- 116.Chabot RJ, et al. Behavioral and Electrophysiologic Predictors of Treatment Response to Stimulants in Children with Attention Disorders. J Child Neurol. 1999;14(6):343–351. doi: 10.1177/088307389901400601. [DOI] [PubMed] [Google Scholar]
- 117.Murias M, et al. Resting State Cortical Connectivity Reflected in EEG Coherence in Individuals With Autism. Biological Psychiatry. 2007;62(3):270–273. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tcheslavski GV, Beex AA. Phase synchrony and coherence analyses of EEG as tools to discriminate between children with and without attention deficit disorder. Biomedical Signal Processing and Control. 2006;1(2):151–161. [Google Scholar]
- 119.Thatcher RW, Krause PJ, Hrybyk M. Cortico-cortical associations and EEG coherence: A two-compartmental model. Electroencephalography and Clinical Neurophysiology. 1986;64(2):123–143. doi: 10.1016/0013-4694(86)90107-0. [DOI] [PubMed] [Google Scholar]
- 120.Montagu JD. The hyperkinetic child: a behavioural, electrodermal and EEG investigation. Developmental Medicine and Child Neurology. 1975;17(3):299–305. [PubMed] [Google Scholar]
- 121.Baving L, Laucht M, Schmidt MH. Atypical Frontal Brain Activation in ADHD: Preschool and Elementary School Boys and Girls. 1999. pp. 1363–1371. [DOI] [PubMed] [Google Scholar]
- 122.Hale TS, et al. Atypical alpha asymmetry in adults with ADHD. Neuropsychologia. 2009;47(10):2082–2088. doi: 10.1016/j.neuropsychologia.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stassen HH, et al. Genetic determination of the human EEG. Survey of recent results on twins reared together and apart. Human Genetics. 1988;80(2):165–176. doi: 10.1007/BF00702862. [DOI] [PubMed] [Google Scholar]
- 124.Van Beijsterveldt CEM, Boomsma DI. Genetics of the human electroencephalogram (EEG) and event-related brain potentials (ERPs): A review. Human Genetics. 1994;94(4):319–330. doi: 10.1007/BF00201587. [DOI] [PubMed] [Google Scholar]
- 125.Van Beijsterveldt CEM, et al. Genetic and environmental influences on EEG coherence. Behavior Genetics. 1998;28(6):443–453. doi: 10.1023/a:1021637328512. [DOI] [PubMed] [Google Scholar]
- 126.van Baal GCM, de Geus EJC, Boomsma DI. Genetic Influences on EEG Coherence in 5-Year-Old Twins. Behavior Genetics. 1998;28(1):9–19. doi: 10.1023/a:1021400613723. [DOI] [PubMed] [Google Scholar]
- 127.Chorlian DB, et al. Heritability of EEG coherence in a large sib-pair population. Biological Psychology. 2007;75(3):260–266. doi: 10.1016/j.biopsycho.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Anokhin AP, Heath AC, Myers E. Genetic and environmental influences on frontal EEG asymmetry: A twin study. Biological Psychology. 2006;71(3):289–295. doi: 10.1016/j.biopsycho.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Posthuma D, et al. Genetic components of functional connectivity in the brain: The heritability of synchronization likelihood. Wiley; 2005. pp. 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Smit D, et al. Endophenotypes in a Dynamically Connected Brain. Behavior Genetics. 2010;40(2):167–177. doi: 10.1007/s10519-009-9330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smit DJA, et al. Heritability of “small-world” networks in the brain: A graph theoretical analysis of resting-state EEG functional connectivity. Wiley; 2008. pp. 1368–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hale TS, et al. ADHD familial loading and abnormal EEG alpha asymmetry in children with ADHD. Journal of Psychiatric Research. 2010;44(9):605–615. doi: 10.1016/j.jpsychires.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bismark AW, et al. Polymorphisms of the HTR1a allele are linked to frontal brain electrical asymmetry. Biological Psychology. 2010;83(2):153–158. doi: 10.1016/j.biopsycho.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shim S-H, et al. A case-control association study of serotonin 1A receptor gene and tryptophan hydroxylase 2 gene in attention deficit hyperactivity disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34(6):974–979. doi: 10.1016/j.pnpbp.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 135.Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. TheScientificWorldJournal. 2007;7(SUPPL. 2):131–141. doi: 10.1100/tsw.2007.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee T-W, et al. The effects of catechol-O-methyl-transferase polymorphism Val158Met on functional connectivity in healthy young females: a resting EEG study. Brain Research. 1377:21–31. doi: 10.1016/j.brainres.2010.12.073. [DOI] [PubMed] [Google Scholar]
- 137.Sonuga-Barke EJS. Psychological heterogeneity in AD/HD--a dual pathway model of behaviour and cognition. Behavioural Brain Research. 2002;130(1-2):29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- 138.Marco R, et al. Delay and Reward Choice in ADHD: An Experimental Test of the Role of Delay Aversion. Neuropsychology. 2009;23(3):367–380. doi: 10.1037/a0014914. [DOI] [PubMed] [Google Scholar]
- 139.Paloyelis Y, Asherson P, Kuntsi J. Are ADHD Symptoms Associated With Delay Aversion or Choice Impulsivity? A General Population Study. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(8):837–846. doi: 10.1097/CHI.0b013e3181ab8c97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nieuwenhuis S, et al. Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cognitive, Affective, & Behavioral Neuroscience. 2003;3(1):17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- 141.Donkers FCL, van Boxtel GJM. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain and Cognition. 2004;56(2):165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 142.Fallgatter AJ, Brandeis D, Strik WK. A robust assessment of the NoGo-anteriorisation of P300 microstates in a cued Continuous Performance Test. Brain Topography. 1997;9(4):295–302. doi: 10.1007/BF01464484. [DOI] [PubMed] [Google Scholar]
- 143.van Leeuwen TH, et al. The continuous performance test revisited with neuroelectric mapping: impaired orienting in children with attention deficits. Behavioural Brain Research. 1998;94(1):97–110. doi: 10.1016/s0166-4328(97)00173-3. [DOI] [PubMed] [Google Scholar]
- 144.Fallgatter AJ, et al. Test-retest reliability of electrophysiological parameters related to cognitive motor control. Clinical Neurophysiology. 2001;112(1):198–204. doi: 10.1016/s1388-2457(00)00505-8. [DOI] [PubMed] [Google Scholar]
- 145.Fallgatter AJ, et al. Long-term Reliability of Electrophysiologic Response Control Parameters. Journal of Clinical Neurophysiology. 2002;19(1):61–66. doi: 10.1097/00004691-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 146.Willcutt E, et al. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–46. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 147.Valko L, et al. Differences in Neurophysiological Markers of Inhibitory and Temporal Processing Deficits in Children and Adults with ADHD. Journal of Psychophysiology. 2010;23(4):235–246. [Google Scholar]
- 148.Szuromi B, et al. P300 deficits in adults with attention deficit hyperactivity disorder: a meta-analysis. Psychological Medicine. 2010;20:1–10. doi: 10.1017/S0033291710001996. [DOI] [PubMed] [Google Scholar]
- 149.Banaschewski T, et al. Questioning inhibitory control as the specific deficit of ADHD – evidence from brain electrical activity. Journal of Neural Transmission. 2004;111(7):841–864. doi: 10.1007/s00702-003-0040-8. [DOI] [PubMed] [Google Scholar]
- 150.Verbaten M, et al. Methylphenidate influences on both early and late ERP waves of ADHD children in a continuous performance test. Journal of Abnormal Child Psychology. 1994;22(5):561–578. doi: 10.1007/BF02168938. [DOI] [PubMed] [Google Scholar]
- 151.Brandeis D, et al. Multicenter P300 Brain Mapping of Impaired Attention to Cues in Hyperkinetic Children. 2002. pp. 990–998. [DOI] [PubMed] [Google Scholar]
- 152.McLoughlin G, et al. Electrophysiological evidence for abnormal preparatory states and inhibitory processing in adult ADHD. Behavioral and Brain Functions. 2010;6(1):66. doi: 10.1186/1744-9081-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Banaschewski T, et al. Association of ADHD and conduct disorder – brain electrical evidence for the existence of a distinct subtype. Journal of Child Psychology and Psychiatry. 2003;44(3):356–376. doi: 10.1111/1469-7610.00127. [DOI] [PubMed] [Google Scholar]
- 154.Fallgatter AJ, et al. Altered response control and anterior cingulate function in attention-deficit/hyperactivity disorder boys. Clinical Neurophysiology. 2004;115(4):973–981. doi: 10.1016/j.clinph.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 155.Wiersema R, et al. Event rate and event-related potentials in ADHD. Journal of Child Psychology and Psychiatry. 2006;47(6):560–567. doi: 10.1111/j.1469-7610.2005.01592.x. [DOI] [PubMed] [Google Scholar]
- 156.Albrecht B, et al. Response inhibition deficits in externalizing child psychiatric disorders: An ERP-study with the Stop-task. Behavioral and Brain Functions. 2005;1(1):22. doi: 10.1186/1744-9081-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dien J, Spencer KM, Donchin E. Parsing the late positive complex: Mental chronometry and the ERP components that inhabit the neighborhood of the P300. Psychophysiology. 2004;41(5):665–678. doi: 10.1111/j.1469-8986.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 158.Anokhin AP, Heath AC, Myers E. Genetics, prefrontal cortex, and cognitive control: a twin study of event-related brain potentials in a response inhibition task. Neuroscience Letters. 2004;368(3):314–318. doi: 10.1016/j.neulet.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 159.Bestelmeyer PEG, et al. The P300 as a possible endophenotype for schizophrenia and bipolar disorder: Evidence from twin and patient studies. Psychiatry Research. 2009;169(3):212–219. doi: 10.1016/j.psychres.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 160.Smit D, et al. Genetic Contribution to the P3 in Young and Middle-Aged Adults. Twin Research and Human Genetics. 2007;10(2):335–347. doi: 10.1375/twin.10.2.335. [DOI] [PubMed] [Google Scholar]
- 161.Carlson SR, Iacono WG. Heritability of P300 amplitude development from adolescence to adulthood. Blackwell Publishing Inc; 2006. pp. 470–480. [DOI] [PubMed] [Google Scholar]
- 162.Smit J, et al. Phenotypic and genetic correlations between evoked EEG/ERP measures during the response anticipation period of a delayed response task. Blackwell Publishing Inc; 2009. pp. 344–356. [DOI] [PubMed] [Google Scholar]
- 163.Wild-Wall N, et al. Neural activity associated with executive functions in adolescents with attention-deficit/hyperactivity disorder (ADHD) International Journal of Psychophysiology. 2009;74(1):19–27. doi: 10.1016/j.ijpsycho.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 164.McLoughlin G, et al. 47th Annual Meeting of the Society for Psychophysiological Research. Blackwell Publishing; Savannah, GA: 2007. ERP endophenotypes of adult ADHD; pp. S47–S47. [Google Scholar]
- 165.Brandeis D, et al. Brain mapping of attention and inhibition in ADHD sib pairs using CPT variants; 17th European Network on Hyperkinetic Disorders (EUNETHYDIS) Meeting; Brugge, Belgium. 2006. [Google Scholar]
- 166.Gilmore C, Malone S, Iacono W. Brain Electrophysiological Endophenotypes for Externalizing Psychopathology: A Multivariate Approach. Behavior Genetics. 2010;40(2):186–200. doi: 10.1007/s10519-010-9343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hicks BM, et al. Genes mediate the association between P3 amplitude and externalizing disorders. Blackwell Publishing Inc; 2007. pp. 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Begleiter H, et al. Quantitative trait loci analysis of human event-related brain potentials: P3 voltage. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. 1998;108(3):244–250. doi: 10.1016/s0168-5597(98)00002-1. [DOI] [PubMed] [Google Scholar]
- 169.Wright MJ, et al. QTLs Identified for P3 Amplitude in a Non-Clinical Sample: Importance of Neurodevelopmental and Neurotransmitter Genes. Biological Psychiatry. 2008;63(9):864–873. doi: 10.1016/j.biopsych.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 170.Hill SY, et al. Genetic Association between Reduced P300 Amplitude and the DRD2 Dopamine Receptor A1 Allele in Children at High Risk for Alcoholism. Biological Psychiatry. 1998;43(1):40–51. doi: 10.1016/s0006-3223(97)00203-5. [DOI] [PubMed] [Google Scholar]
- 171.Noble EP, et al. Prolonged P300 latency in children with the D[sub 2] dopamine receptor A1 allele. American Journal of Human Genetics. 1994;54(4):658–668. [PMC free article] [PubMed] [Google Scholar]
- 172.Lin C-H, et al. Association analysis for dopamine D2 receptor Taq 1 polymorphism with P300 event-related potential for normal young females. Psychiatric Genetics. 2001;11(3):165–168. doi: 10.1097/00041444-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 173.Vogel CIG, et al. Association of DRD4 exon III polymorphism with auditory P300 amplitude in 8-year-old children. Journal of Neural Transmission. 2006;113(12):1935–1941. doi: 10.1007/s00702-006-0497-3. [DOI] [PubMed] [Google Scholar]
- 174.Tsai S-J, et al. Association study of a functional catechol-O-methyltransferase-gene polymorphism and cognitive function in healthy females. Neuroscience Letters. 2003;338(2):123–126. doi: 10.1016/s0304-3940(02)01396-4. [DOI] [PubMed] [Google Scholar]
- 175.Yue C, et al. Comparison of visual evoked-related potentials in healthy young adults of different catechol-O-methyltransferase genotypes in a continuous 3-back task. NeuroReport. 2009;20(5):521–524. doi: 10.1097/wnr.0b013e328317f3b1. doi: 10.1097/WNR.0b013e328317f3b1. [DOI] [PubMed] [Google Scholar]
- 176.Kramer UM, et al. The Impact of Catechol-O-Methyltransferase and Dopamine D4 Receptor Genotypes on Neurophysiological Markers of Performance Monitoring. J. Neurosci. 2007;27(51):14190–14198. doi: 10.1523/JNEUROSCI.4229-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bramon E, et al. Is there an association between the COMT gene and P300 endophenotypes? European Psychiatry. 2006;21(1):70–73. doi: 10.1016/j.eurpsy.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 178.Dresler T, et al. Dopamine Transporter (SLC6A3) Genotype Impacts Neurophysiological Correlates of Cognitive Response Control in an Adult Sample of Patients with ADHD. Neuropsychopharmacology. 35(11):2193–2202. doi: 10.1038/npp.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Franke B, et al. Multicenter Analysis of the SLC6A3/DAT1 VNTR Haplotype in Persistent ADHD Suggests Differential Involvement of the Gene in Childhood and Persistent ADHD. Neuropsychopharmacology. 2009;35(3):656–664. doi: 10.1038/npp.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Baehne CG, et al. Tph2 gene variants modulate response control processes in adult ADHD patients and healthy individuals. Mol Psychiatry. 2008;14(11):1032–1039. doi: 10.1038/mp.2008.39. [DOI] [PubMed] [Google Scholar]
- 181.Kramer U, et al. ADHD candidate gene (DRD4 exon III) affects inhibitory control in a healthy sample. BMC Neuroscience. 2009;10(1):150. doi: 10.1186/1471-2202-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gehring WJ, et al. A Neural System for Error Detection and Compensation. Psychological Science. 1993;4(6):385–390. [Google Scholar]
- 183.Carter CS, et al. Anterior Cingulate Cortex, Error Detection, and the Online Monitoring of Performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 184.Falkenstein M, et al. ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology. 2000;51(2-3):87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- 185.Nieuwenhuis S, et al. Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology. 2001;38(05):752–760. [PubMed] [Google Scholar]
- 186.Olvet DM, Hajcak G. Reliability of error-related brain activity. Brain Research. 2009;1284:89–99. doi: 10.1016/j.brainres.2009.05.079. [DOI] [PubMed] [Google Scholar]
- 187.van Meel CS, et al. Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): The role of error processing. Psychiatry Research. 2007;151(3):211–220. doi: 10.1016/j.psychres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 188.Liotti M, et al. Abnormal Brain Activity Related to Performance Monitoring and Error Detection in Children with ADHD. Cortex. 2005;41(3):377–388. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- 189.Jonkman LM, et al. Methylphenidate improves deficient error evaluation in children with ADHD: An event-related brain potential study. Biological Psychology. 2007;76(3):217–229. doi: 10.1016/j.biopsycho.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 190.Wiersema JR, van der Meere JJ, Roeyers H. ERP correlates of impaired error monitoring in children with ADHD. Journal of Neural Transmission. 2005;112(10):1417–1430. doi: 10.1007/s00702-005-0276-6. [DOI] [PubMed] [Google Scholar]
- 191.Shiels K, Hawk LW., Jr Self-regulation in ADHD: The role of error processing. Clinical Psychology Review. 2010;30(8):951–961. doi: 10.1016/j.cpr.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Anokhin AP, Golosheykin S, Heath AC. Heritability of frontal brain function related to action monitoring. Psychophysiology. 2008;45(4):524–534. doi: 10.1111/j.1469-8986.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- 193.Albrecht B, et al. Action monitoring in children with or without a family history of ADHD - Effects of gender on an endophenotype parameter. Neuropsychologia. 2010;48(4):1171–1177. doi: 10.1016/j.neuropsychologia.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 195.Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3(5):516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- 196.Bush G, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting stroop. Biological Psychiatry. 1999;45(12):1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 197.Paloyelis Y, et al. Functional MRI in ADHD: a systematic literature review. Expert Review of Neurotherapeutics. 2007;7:1337–1356. doi: 10.1586/14737175.7.10.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Brown AB, et al. Effect of dopamine transporter gene (SLC6A3) variation on dorsal anterior cingulate function in attention-deficit/hyperactivity disorder. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics. 2010;153B(2):365–375. doi: 10.1002/ajmg.b.31022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Progress in Neurobiology. 2004;74(1):1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 200.Holroyd CB, Coles MGH. The Neural Basis of Human Error Processing: Reinforcement Learning, Dopamine, and the Error-Related Negativity. Psychological Review. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- 201.Frank MJ. Cross-task individual differences in error processing: Neural, electrophysiological, and genetic components. Cognitive, Affective, & Behavioural Neuroscience. 2007;7:297–308. doi: 10.3758/cabn.7.4.297. [DOI] [PubMed] [Google Scholar]
- 202.Ullsperger M. Genetic association studies of performance monitoring and learning from feedback: The role of dopamine and serotonin. Neuroscience & Biobehavioral Reviews. 2010;34(5):649–659. doi: 10.1016/j.neubiorev.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 203.Althaus M, et al. Variants of the SLC6A3 (DAT1) polymorphism affect performance monitoring-related cortical evoked potentials that are associated with ADHD. Biological Psychology. 2010;85(1):19–32. doi: 10.1016/j.biopsycho.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 204.Fallgatter AJ, et al. Allelic Variation of Serotonin Transporter Function Modulates the Brain Electrical Response for Error Processing. Neuropsychopharmacology. 2004;29(8):1506–1511. doi: 10.1038/sj.npp.1300409. [DOI] [PubMed] [Google Scholar]
- 205.Halperin JM, et al. Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. Journal of Child Psychology and Psychiatry. 2008;49(9):958–966. doi: 10.1111/j.1469-7610.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Johnson KA, Wiersema JR, Kuntsi J. What would Karl Popper say? Are current psychological theories of ADHD falsifiable? Behav Brain Funct. 2009;5:15. doi: 10.1186/1744-9081-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Loo SK, Barkley RA. Clinical Utility of EEG in Attention Deficit Hyperactivity Disorder. Applied Neuropsychology. 2005;12(2):64–76. doi: 10.1207/s15324826an1202_2. [DOI] [PubMed] [Google Scholar]
- 208.Chabot RJ, et al. Sensitivity and specificity of QEEG in children with attention deficit or specific developmental learning disorders. Clinical EEG electroencephalography. 1996;27(1):26–34. doi: 10.1177/155005949602700105. [DOI] [PubMed] [Google Scholar]
- 209.Monastra VJ, Lubar JF, Linden M. The development of a quantitative electroencephalographic scanning process for attention deficit-hyperactivity disorder: reliability and validity studies. Neuropsychology. 2001;15(1):136–144. doi: 10.1037//0894-4105.15.1.136. [DOI] [PubMed] [Google Scholar]
- 210.Monastra VJ, et al. Assessing Attention Deficit Hyperactivity Disorder via Quantitative Electroencephalography: An Initial Validation Study. Neuropsychology. 1999;13(3):424–433. doi: 10.1037/0894-4105.13.3.424. [DOI] [PubMed] [Google Scholar]
- 211.Dalsgaard S, et al. Conduct problems, gender and adult psychiatric outcome of children with attention-deficit hyperactivity disorder. The British Journal of Psychiatry. 2002;181(5):416–421. doi: 10.1192/bjp.181.5.416. [DOI] [PubMed] [Google Scholar]
- 212.Rommelse N, et al. Comorbid Problems in ADHD: Degree of Association, Shared Endophenotypes, and Formation of Distinct Subtypes. Implications for a Future DSM. Journal of Abnormal Child Psychology. 2009;37(6):793–804. doi: 10.1007/s10802-009-9312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Broyd SJ, et al. Default-mode brain dysfunction in mental disorders: A systematic review. Neuroscience & Biobehavioral Reviews. 2009;33(3):279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 214.Geurts HM, et al. Intra-individual variability in ADHD, autism spectrum disorders and Tourette’s syndrome. Neuropsychologia. 2008;46(13):3030–3041. doi: 10.1016/j.neuropsychologia.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 215.Sergeant JA, Geurts H, Oosterlaan J. How specific is a deficit of executive functioning for Attention-Deficit/Hyperactivity Disorder? Behavioural Brain Research. 2002;130(1-2):3–28. doi: 10.1016/s0166-4328(01)00430-2. [DOI] [PubMed] [Google Scholar]
- 216.Rothenberger A, et al. Comorbidity in ADHD-children: effects of coexisting conduct disorder or tic disorder on event-related brain potentials in an auditory selective-attention task. European Archives of Psychiatry and Clinical Neuroscience. 2000;250(2):101–110. doi: 10.1007/s004060070042. [DOI] [PubMed] [Google Scholar]
- 217.Yordanova J, et al. Frontocortical activity in children with comorbidity of tic disorder and attention-deficit hyperactivity disorder. Biological Psychiatry. 1997;41(5):585–594. doi: 10.1016/s0006-3223(96)00096-0. [DOI] [PubMed] [Google Scholar]
- 218.Liotti M, et al. Evidence for specificity of ERP abnormalities during response inhibition in ADHD children: A comparison with reading disorder children without ADHD. Brain and Cognition. 2010;72(2):228–237. doi: 10.1016/j.bandc.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Groen Y, et al. Error and feedback processing in children with ADHD and children with autistic spectrum disorder: An EEG event-related potential study. Clinical Neurophysiology. 2008;119(11):2476–2493. doi: 10.1016/j.clinph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 220.Clarke AR, et al. Children with attention-deficit/hyperactivity disorder and autistic features: EEG evidence for comorbid disorders. Psychiatry Research. 2011;185(1-2):225–231. doi: 10.1016/j.psychres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 221.Gevensleben H, et al. Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. Journal of Child Psychology and Psychiatry. 2009;50(7):780–789. doi: 10.1111/j.1469-7610.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 222.Arns M, et al. Efficacy of neurofeedback treatment in ADHD: the effects on inattention, impulsivity and hyperactivity: a meta-analysis. Clinical EEG and Neuroscience. 2009;40(3):180–189. doi: 10.1177/155005940904000311. [DOI] [PubMed] [Google Scholar]
- 223.Gani C, et al. Neurofeedback treatment for attention-deficit/hyperactivity disorder in children: A comparison with methylphenidate. Applied Psychophysiology and Biofeedback. 2003;28:1–12. doi: 10.1023/a:1022353731579. [DOI] [PubMed] [Google Scholar]
- 224.Jurgen K, Craig ET. Optimizing PCA methodology for ERP component identification and measurement: theoretical rationale and empirical evaluation. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2003;114(12):2307–2325. doi: 10.1016/s1388-2457(03)00241-4. [DOI] [PubMed] [Google Scholar]
- 225.Makeig S, et al. Independent component analysis of electroencephalographic data. Advances in Neural Information Processing Systems. 1996;8:145–151. [Google Scholar]
- 226.Flint J, Munafo M. The endophenotype concept in psychiatric genetics. Psychological Medicine. 2007;37(2):163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Walters JTR, Owen MJ. Endophenotypes in psychiatric genetics. Mol Psychiatry. 12(10):886–890. doi: 10.1038/sj.mp.4002068. [DOI] [PubMed] [Google Scholar]
- 228.Kovas Y, Plomin R. Generalist genes: implications for the cognitive sciences. Trends Cogn Sci. 2006;10(5):198–203. doi: 10.1016/j.tics.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 229.Kendler KS, Neale MC. Endophenotype: a conceptual analysis. Mol Psychiatry. 15(8):789–797. doi: 10.1038/mp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.de Geus EJC. Introducing genetic psychophysiology. Biological Psychology. 2002;61(1-2):1–10. doi: 10.1016/s0301-0511(02)00049-2. [DOI] [PubMed] [Google Scholar]
- 231.Kovas Y, Plomin R. Generalist genes: implications for the cognitive sciences. Trends in Cognitive Sciences. 2006;10(5):198–203. doi: 10.1016/j.tics.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 232.Ronald A, et al. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. Journal of Child Psychology and Psychiatry. 2008;49(5):535–542. doi: 10.1111/j.1469-7610.2007.01857.x. [DOI] [PubMed] [Google Scholar]
- 233.Willcutt EG, et al. Understanding comorbidity: A twin study of reading disability and attention-deficit/hyperactivity disorder. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics. 2007;144B(6):709–714. doi: 10.1002/ajmg.b.30310. [DOI] [PubMed] [Google Scholar]
- 234.Malmivuo J, Plonsey R. Bioelectromagnetism. Oxford University Press; New York: 1995. [Google Scholar]
Further reading, resources and contacts
Selected papers:
- Rommelse NJ. Endophenotypes in the genetic research of ADHD over the last decade: have they lived up to their expectations? Expert Rev. Neurother. 2008;8(10):1425–1429. doi: 10.1586/14737175.8.10.1425. [This paper provides an overview and critical discussion of the endophenotype approach in ADHD in various domains.] [DOI] [PubMed] [Google Scholar]
- De Geus EJC. From genotype to EEG endophenotype: a route for post-genomic understanding of complex psychiatric disease? Genome Medicine. 2010;2(9):63. doi: 10.1186/gm184. [A broad outline of the utility of electrophysiological intermediate phenotypes in psychiatric genetics to date.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AC, Neale MC. Twin studies and their implications for molecular genetic studies: endophenotypes integrate quantitative and molecular genetics in ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(9):874–83. doi: 10.1016/j.jaac.2010.06.006. [This paper provides an overview of and a rationale for the use of quantitative genetic methods in ADHD endophenotype research.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman ID. Statistical approaches to complex phenotypes: evaluating neuropsychological endophenotypes for attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:1347–1356. doi: 10.1016/j.biopsych.2005.03.002. [This paper outlines criteria and associated statistical tests for ADHD neuropsychological endophenotypes, providing a useful and necessary framework for future electrophysiological endophenotype research.] [DOI] [PubMed] [Google Scholar]
Websites
- Provides an outline of the interdisciplinary research being undertaken at the MRC Social. Genetic and Developmental Psychiatry (SGDP) Centre, Institute of Psychiatry, KCL.; http://www.iop.kcl.ac.uk/departments/?locator=10. [Google Scholar]
- The UK Adult ADHD Network (UKAAN) provides support, education, research and training for mental health professionals working with adults with ADHD. http://ukaan.org/