Abstract
68Ga is a promising emerging radionuclide for positron emission tomography (PET). It is produced using a 68Ge/68Ga-generator, and thus, would enable the cyclotron-independent distribution of PET. However, new 68Ga-labeled radiopharmaceuticals that can replace 18F-labeled agents like [18F]fluorodeoxyglucose (FDG) are needed. Most of the 68Ga-labeled derivatives currently used are peptide agents, but the developments of other agents, such as amino acid derivatives, nitroimidazole derivatives, and glycosylated human serum albumin, are being actively pursued in many laboratories. Thus, appearance of new 68Ga-labeled radiopharmaceuticals with high impact are expected in the near future. Here, we present an overview of 68Ga-labeled agents in terms of their clinical significances and relevances to the management of certain tumors, and pertinent pre-clinical developments.
Keywords: Gallium-68, PET, Peptide, DOTA, NOTA, BAPEN, MSA, Ga-68
Introduction
The introduction of 68Ga positron emission tomography (PET) to clinical practice represents a developmental milestone in the functional and metabolic imaging fields, and was facilitated by the cyclotron-independent availability of 68Ga, enabled by the use of the 68Ge/68Ga radionuclide generator system [1, 2]. 68Ga is an excellent positron emitter. It has the characteristics of low photon emission (1,077 keV, 3.22%) and 89% positron branching [3]. Recent studies have shown that some 68Ga-labeled peptides exhibited distinctly better images than their 111In-labeled analogues [4–6] and than 18F-based radiotracers [7]. Unlike other PET radioisotopes, like 18F or 11C, ionic Ga3+ cannot be bound covalently to targeting vectors but must be conjugated to a target vector using a bifunctional chelating agent (BCA). Nevertheless, the labeling can be done just prior to diagnostic examinations, rapidly with minimum loss of radioactivity. The only stable chemical form of Ga in solution at physiological conditions is Ga3+, and this ion can form stable complexes with chelators that are either free or conjugated with macromolecules or small organic molecules [8].
There are two requirements for using gallium complexes as radiopharmaceuticals: (1) they should be resistant to hydrolysis (the formation of complexes with OH-) and (2) they should be more stable than the Ga(III)–transferrin complex, and thus, the labeled gallium complex must be stabile in the presence of transferrin—a plasma protein. The large formation constant of Ga(III)–transferrin (log K = 20.3) [9] and the high plasma concentration of this protein (0.25 g/100 ml) favor the thermodynamic exchange of Ga(III) complexes with transferrin in vivo, and thus, the majority of radioactive gallium complexes used as radiopharmaceuticals have high thermodynamic and kinetic stabilities.
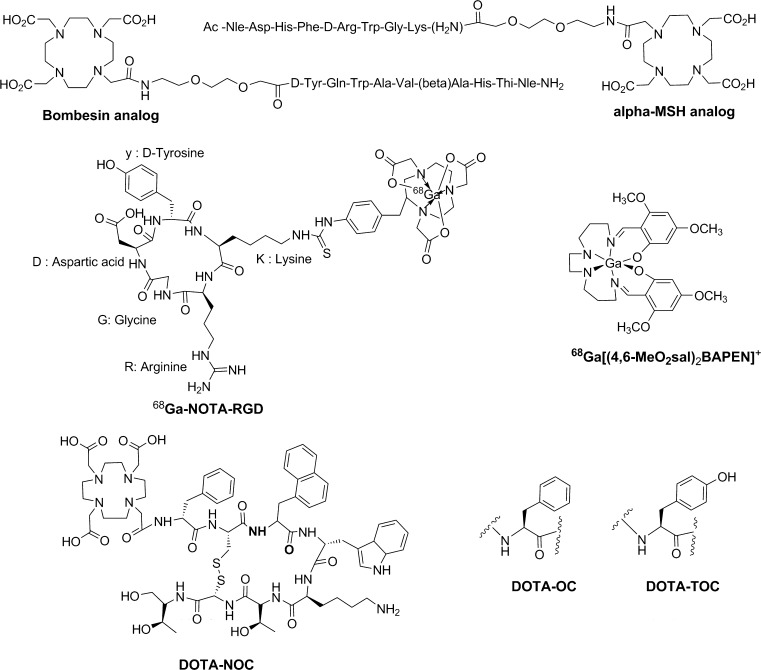
Various 68Ga-labeled radiopharmaceuticals have been developed by conjugating BCAs to peptides, proteins, or small biological molecules via active esters, isothiocyanates, maleimides, hydrazides, or haloamides. 68Ga-1,4,7,10-tetra-azacyclododecane-1,4,7,10-tetraacetic acid–Tyr3-octreotide (DOTA-TOC), 68Ga-DOTA-1-Nal-octreotide (DOTA-NOC), 68Ga-DOTA-bombesin, 68Ga-1,4,7-triazacyclononanetriacetic acid (NOTA)-RGD, 68Ga-DOTA-albumin, and 68Ga-DOTA-human epidermal growth factor (hEGF) are examples of such agents (Fig. 1) [8, 10–15]. Similarly, some agents, such as 68Ga–[(4,6-MeO2sal)2BAPEN]+ and 68Ga-N2S2, are chelates of radioactive gallium and used for myocardial imaging [16–18].
Fig. 1.
Chemical structures of 68Ga-labeled radiopharmaceuticals
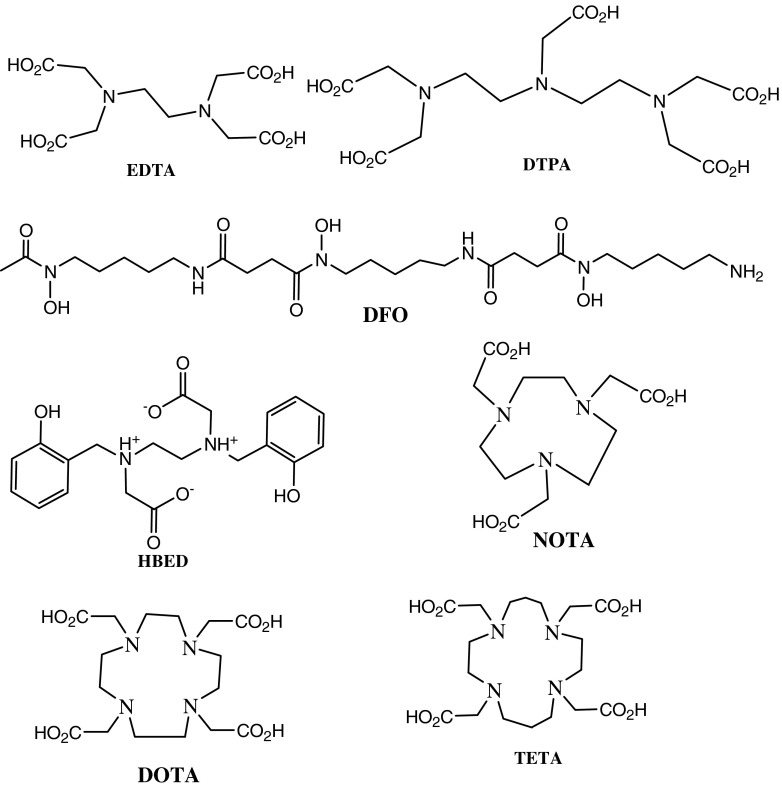
Acyclic BCAs, such as ethylenediaminetetraacetic acid (EDTA), diethylenetriaminepentaacetic acid (DTPA), desferrioxamine (DFO), N,N’-di(2-hydroxybenzyl)ethylenediamine-N,N’-diacetic acid (HBED), and their derivatives, have been used for labeling macromolecules with 111In, 67/68Ga, or 90Y for tumor imaging and therapy [19–22]. However, most of these complexes have low in vivo and in vitro stabilities due to their tendency to undergo acid- or cation-promoted dissociation [23, 24]. These limitations are overcome by using macrocyclic BCAs, such as NOTA, DOTA, or 1,4,8,11-tetraazacyclotetradecanetetraacetic acid (TETA), which can form highly stable complexes with these radiometals (Fig. 2) [23, 25].
Fig. 2.
Some open chain and macrocyclic BCAs used for 68Ga-radiopharmaceutical synthesis
In this review, we present overviews of 68Ga-labeled agents in terms of their present clinical significances and their relevances to the management of certain tumors, and we include details of recent pre-clinical developments.
68Ga-Peptides
During the last decade, the developments of easy and economical production routes for radiolabeled peptides with rapid clearance and tissue penetration and low antigenicity, and the availabilities of simplified purification methods promoted their developments for diagnostic applications. Furthermore, the use of BCAs enabled peptides to be easily labeled with therapeutic radionuclides (90Y, 177Lu).
Most of the early efforts made to label peptides targeted somatostatin and its derivatives. Somatostatin is a regulatory peptide and its action is mediated by membrane-bound receptors (SSTRs) that are present in normal human tissues, such as, in thyroid, brain, the gastrointestinal tract (GIT), pancreas, spleen, and kidneys [26], and they are also highly expressed in many different types of human tumors, notably neuroendocrine tumors (NET) [27], which in clinical practice are usually carcinoid tumors and pheochromocytomas. SSTRs are also expressed, to variable extents, in renal cell carcinoma, small cell lung cancer, breast cancer, prostate cancer, and in malignant lymphoma [4]. There are five SSTR subtypes, but subtype 2 (SSTR2), subtype 5 (SSTR5), and to a lesser extent, subtype 3 (SSTR3) have higher affinities than SSTR1 and 4, and thus, commercially available synthetic somatostatin analogues target these three high-affinity receptors [28]. These analogues are required because somatostatin is rapidly degraded by enzymes in vivo, as reflected by its short biological half-life, and thus, agents with high affinity for SSTR have been developed, which are resistant to enzyme degradation.
Somatostatin analogues, such as, DOTA-TOC show better images than 111In-DTPA-octreotide, the most commonly used somatostatin analogue [29]. The phenylalanine residue at position 3 was replaced by tyrosine in DOTA-TOC, which makes the compound more hydrophilic, increases affinity for SSTR2, and increases uptake by SSTR2-positive tumors [30]. Other peptides have also been linked to DOTA, such as, DOTA-octreotate, which has high affinity for SSTR2 [28], and DOTA-lanreotide, which has high affinity for SSTR5. DOTA-NOC is the newest addition to these compounds, and has high affinity for SSTR2, SSTR3, and SSTR5. Furthermore, these DOTA-peptide products show high radiochemical purity, rapid renal clearance, and high accumulation in tumors, and overall represent remarkable advances over standard peptides [31].
In parallel with the development of the clinical applications of 68Ga-labeled compounds, in vitro and animal testing of various chelators and compounds is on-going. Antunes et al. [4] demonstrated that gallium 67Ga- and 68Ga-DOTA-octapeptides have distinctly better pre-clinical pharmacological performances than 111In-labeled peptides, especially on SSTR2-expressing cells and in animal models. In particular, 68Ga-DFO-octreotide injected into rats bearing SSTR-positive pancreatic tumors demonstrated selective binding to tumor sites with a tumor to background ratio (T/B) of 5 [32]. Subsequently, several DOTA-SST analogues were evaluated in vivo, and 68Ga-DOTA-TOC and 68Ga-DOTA-NOC were found to be the most promising [33–36].
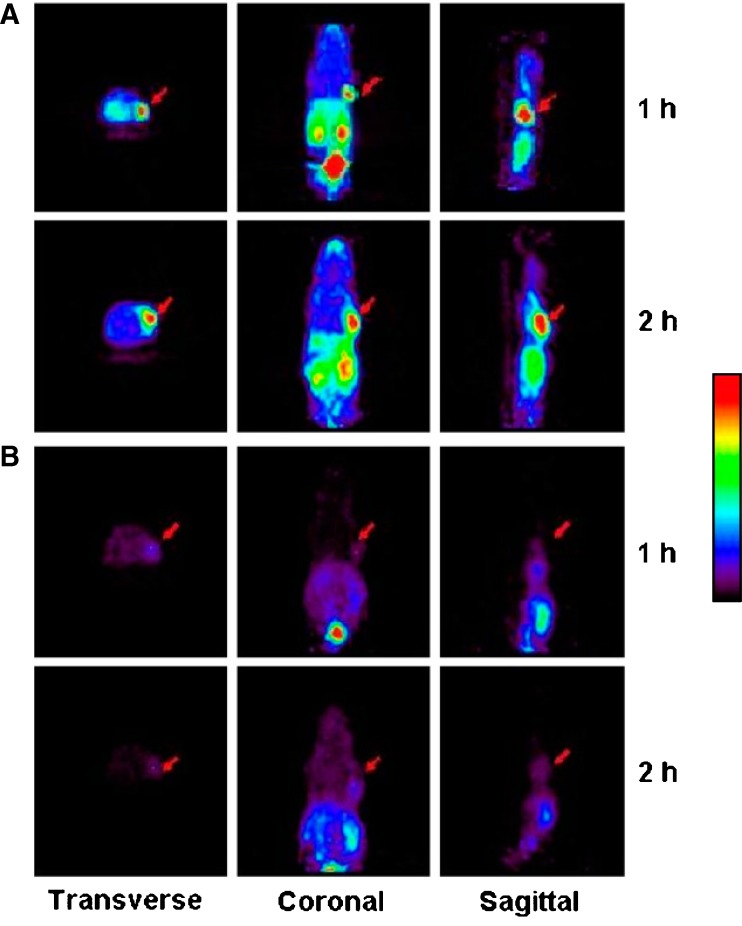
We previously found that the PET imaging agent, 68Ga-NOTA-RGD, can be used to visualize angiogenesis in ischemic tissue, and αvβ3 integrin expression was found to play an important role [13]. Because angiogenesis is known to occur at ischemic lesions during cancer development, we used two animal biodistribution models, a hind limb ischemia and a SNU-C4 (a human colon cancer cell line) xenograft model. Significantly, higher tracer uptakes were observed in ischemic compared with nonischemic muscle tissues. Small-animal PET of mice bearing SNU-C4 xenografts injected with this tracer at 1 and 2 h post-injection with or without cold c(RGDyK) showed specific uptake of tracer by tumors (Fig. 3). Furthermore, in a subsequent biodistribution study, tumor uptake of 68Ga-NOTA-RGD was 5.2 ± 1.0% ID/g, and its tumor-to-blood ratio was 10.4 ± 4.8.
Fig. 3.
Small-animal PET of 68Ga-NOTA-RGD in mice bearing SNU-C4 xenografts at 1 and 2 h after injection without or with cold c(RGDyK) (a and b, respectively). Arrows indicate tumor positions. Reprinted by permission of the Society of Nuclear Medicine from: Jeong JM, et al. (2008) Preparation of a promising angiogenesis PET imaging agent: 68Ga-labeled c(RGDyK)-isothiocyanatobenzyl-1,4,7-triazacyclononane-1,4,7-triacetic acid and feasibility studies in mice. J Nucl Med 49:830–836
68Ga has also been successfully used to label melanocortin peptides, which have many physiologic functions; their receptors are expressed in several cell types, such as, cutaneous melanocytes, keratinocytes, fibroblasts, endothelial cells, antigen-presenting cells, and leukocytes. Melanoma is one of the tumors that can be successfully imaged with radiolabeled MSH, because melanoma overexpresses melanocortin receptor. In particular, 68Ga-DOTA-rhenium-cyclized alphamelanocyte-stimulating hormone (alpha-MSH) analogue [DOTA-ReCCMSH (Arg11)] is a promising agent for the early detection of melanoma in mice [37]. Likewise, 68Ga-DOTA-NAPamide, a short linear alpha-MSH analogue, has been reported to be superior to 111In-DOTA-MSH for the targeting of melanocortin type 1 receptor in murine models of primary and metastatic melanoma [38]. However, receptor density in human melanoma is much lower than in murine tumor models, and thus, more work is needed to improve receptor affinity in man.
Promising pre-clinical studies using the DOTA-analogues of several other peptides, including substance P [39], neurotensin [40], and cholecystokinin (CCK) [41], have also been conducted.
DOTA-conjugated bombesin analogues labeled with 68Ga have been demonstrated to be promising imaging agents and to be useful for the targeted radionuclide treatment of bombesin receptor-positive tumors. These receptors have been reported to be overexpressed in invasive primary prostate carcinoma, associated lymph nodes, breast cancer and gastrointestinal stromal tumor [42, 43].
In terms of the assessment of infection and inflammation, 68Ga-DOTA-VAP-P1, a peptide inhibitor of vascular adhesion protein 1/semicarbazine sensitive amine oxidase (VAP-1/SSAO), is a promising agent for the assessment of inflammatory reactions in healing bones [44]. In addition, studies have suggested that 68Ga PET imaging might be useful in experimental osteomyelitis caused by Staphylococcus aureus [45].
Non-peptide Agents Labeled with 68Ga
Most non-peptide agents labeled with 68Ga are complexes that are not conjugated to specific ligands. However, some non-peptide agents are conjugated with ligands via BCA. For example, three different forms of antisense oligonucleotides targeting activated human K-ras oncogene labeled with 68Ga have been shown to provide a convenient means for in vivo imaging and quantification of oligonucleotide biokinetics in living animals [46].
Various 68Ga-labeled agents have been developed using NOTA as the basic chelating agent, because of the high stability of the chelate formed; for example, the log K value of gallium-NOTA [47, 48] has been reported to be 30.98, which is much higher than that of gallium-DOTA (21.33) [49]. DOTA has eight binding sites available for complexing with metals, but NOTA has only six, and thus, no free binding site remains after complexing NOTA with gallium, which requires all six binding sites. However, it have been shown that amide oxygen or nitrogen can bind with gallium [50], and as a result, various amino acid derivatives conjugated with NOTA have been developed for imaging cancers (Fig. 4) [51]. In addition, another amino acid derivatives conjugated with DO2A and DO3A have also been reported [52].
Fig. 4.
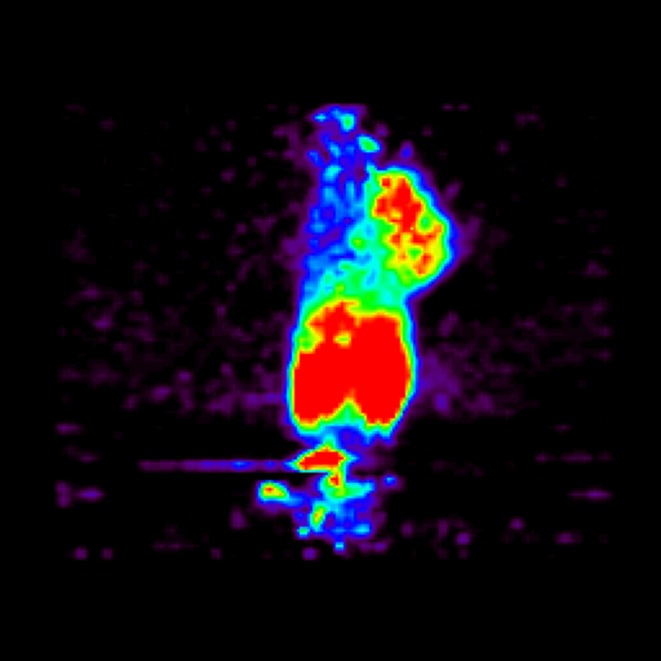
Micro-PET image of 68Ga-NOTA-aminoalanine in mouse colon cancer (CT-26) showing clear uptake by the cancer lesion located at the right shoulder
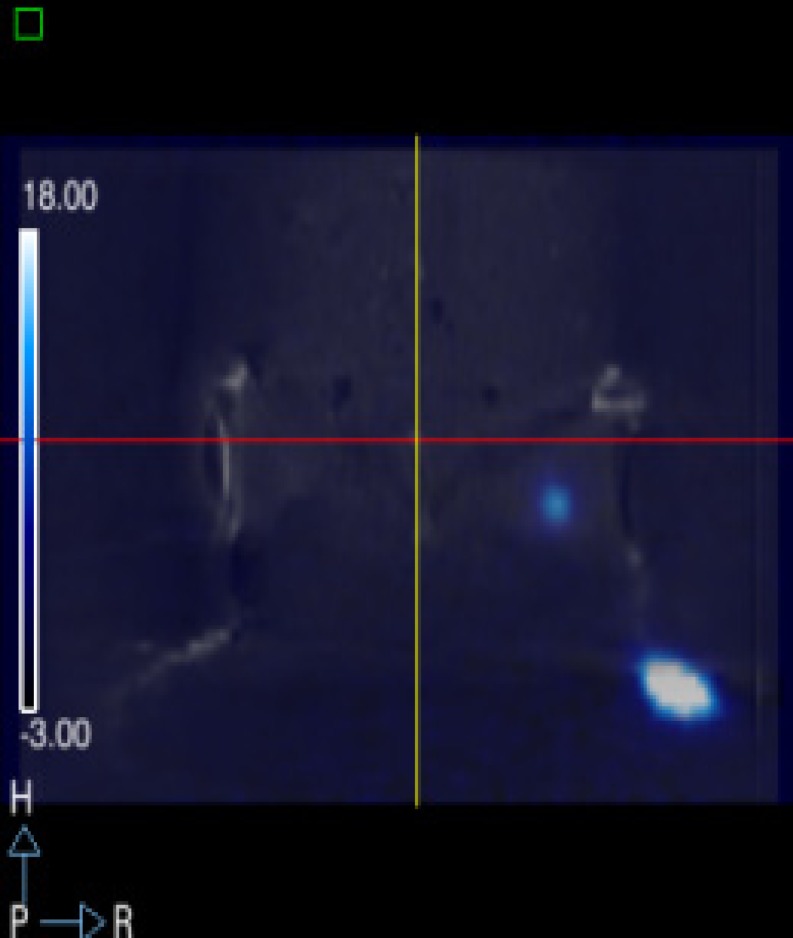
An in vitro cellular uptake and biodistribution study in breast cancer-bearing rats, using 68Ga metronidazole and ethylenedicysteine (EC) as a chelator, demonstrated the feasibility of this tracer for the assessment of tumor hypoxia [53]. Conjugates of nitroimidazole and NOTA derivatives have also been developed [54]. The molecular imaging of the functional transport activity of multidrug resistance (MDR1) P-glycoprotein (Pgp) using 67Ga/68Ga-(3-ethoxy-ENBDMPI) may also enable non-invasive monitoring of the blood-brain barrier, chemotherapeutic regiments, and MDR1 gene treatment protocols in vivo [55]. In addition, 68Ga-mannosylated human serum albumin (MSA) has been reported to be a promising agent for sentinel node detection (Fig. 5) [56]. MSA targets the mannose receptor of macrophages present in lymph nodes after subcutaneous injection. Furthermore, this heat labile agent can be labeled straightforwardly at room temperature, because NOTA is used as the BCA.
Fig. 5.
A micro-PET-CT image of 68Ga-MSA after injection into a mouse footpad. Radioactivity was present at the inguinal lymph node
We have formulated 68Ga-BAPEN [68Ga-Tris(4,6-dimethoxysalicylaldimine)-N,N′-bis(3-aminopropyl)-N,N′-ethylenediamine] as a kit for myocardial PET imaging and biodistribution studies [57]. This kit allows 68Ga-labeled agents to be easily prepared.
Clinical Applications of 68Ga Peptides
Hofmann et al. [58] presented the first impressive 68Ga-DOTA-TOC images of neuroendocrine tumors, compared with 111In-octeriotide scintigraphy, in eight patients with carcinoid tumors. 68Ga-DOTA-TOC identified all lesions, whereas 111In-octreotide identified only 85%. Furthermore, quantitative analysis of these lesions showed higher tumor to non-tumor contrast ratios and low kidney accumulation for 68Ga-DOTA-TOC PET imaging [4]. The pharmacokinetics of 68Ga-DOTA-TOC and 18F-fluorodeoxyglucose (FDG) in metastatic NET patients demonstrated uptakes by 57 of 63 lesions and by 43 of 63 lesions, respectively [59]. Another comparison between these two radiopharmaceuticals in a small group of patients (n = 4) with metastatic NET showed that 68Ga-DOTA-TOC was better at depicting smaller lesions with low tracer uptake, especially when tumors bore somatostatin receptors at low densities [60].
A comparative study between 68Ga-DOTA-TOC PET and 99mTc-HYNIC-octreotide was performed by Gabriel et al. [6] in 88 patients with as neuroendocrine tumor. 68Ga-DOTA-TOC PET showed a sensitivity of 97%, a specificity of 92%, and an overall accuracy of 96%, which were significantly higher than those of single photon emission computed tomography (SPECT) using 99mTc-labeled tracer (Fig. 3).
A viability study of 68Ga-DOTA-TATE for pheochromocytoma PET imaging was conducted in patients who had previously undergone surgical resection of malignant pheochromocytomas. 68Ga-DOTA-TATE was positive in all five patients studied, whereas only three patients were positive by 123I-metaiodobenzylguanidine (MIBG) scan [61, 62]. Due to the difficulties of diagnosing meningioma by computed tomography (CT) and magnetic resonance imaging (MRI), other methods of characterizing these intracranial lesions are being sought. Imaging meningiomas with 68Ga-DOTA-TOC can provide useful clinical indications, because SSTR2 is highly expressed in most meningiomas. The first use of 68Ga-DOTA-TOC in meningiomas in three patients was reported by Milker-Zabel and co-workers; the same group evaluated its kinetic parameters in meningioma [63].
Conclusion
The use of a 68Ge/68Ga-generator that can consistently supply 68Ga, which has a half-life of 271 days, provides a convenient way of producing 68Ga for more than a year. Furthermore, the cost of the generator is comparable with those of other radionuclides used for PET. In addition, diagnostic approaches based on 68Ga-labeled agents have the additional advantage of facilitating treatment. For example, when a diagnostic scan is positive, these agents can be labeled with therapeutic radionuclides, such as, yttrium-90, lutetium-177, or rhenium-188. Ongoing experimental work suggests the feasibility of 68Ga labeling with different biomolecules for the imaging of different tumors, myocardium, and infection. The commercial availability of 68Ge/68Ga-generator will undoubtedly encourage further preclinical research and clinical studies, and may open the door to new possibilities for PET.
References
- 1.Breeman WA, Verbruggen AM. The 68Ge/68Ga generator has high potential, but when can we use 68Ga-labelled tracers in clinical routine? Eur J Nucl Med Mol Imaging. 2007;34:978–981. doi: 10.1007/s00259-007-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrhardt GJ, Welch MJ. A new germanium-63/gallium-68 generator. J Nucl Med. 1978;19:925–929. [PubMed] [Google Scholar]
- 3.Zhernosekov KP, Filosofov DV, Baum RP, Aschoff P, Bihl H, Razbash AA, et al. Processing of generator-produced 68Ga for medical application. J Nucl Med. 2007;48:1741–1748. doi: 10.2967/jnumed.107.040378. [DOI] [PubMed] [Google Scholar]
- 4.Antunes P, Ginj M, Zhang H, Waser B, Baum RP, Reubi JC, et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging. 2007;34:982–993. doi: 10.1007/s00259-006-0317-x. [DOI] [PubMed] [Google Scholar]
- 5.Buchmann I, Henze M, Engelbrecht S, Eisenhut M, Runz A, Schafer M, et al. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2007;34:1617–1626. doi: 10.1007/s00259-007-0450-1. [DOI] [PubMed] [Google Scholar]
- 6.Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–518. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 7.Ambrosini V, Tomassetti P, Castellucci P, Campana D, Montini G, Rubello D, et al. Comparison between 68Ga-DOTA-NOC and 18F-DOPA PET for the detection of gastro-entero-pancreatic and lung neuro-endocrine tumours. Eur J Nucl Med Mol Imaging. 2008;35:1431–1438. doi: 10.1007/s00259-008-0769-2. [DOI] [PubMed] [Google Scholar]
- 8.Parker D. Tumour targeting with radiolabelled macrocycle–antibody conjugates. Chem Soc Rev. 1990;19:271–291. doi: 10.1039/cs9901900271. [DOI] [Google Scholar]
- 9.Harris WR, Pecoraro VL. Thermodynamic binding constants for gallium transferrin. Biochemistry. 1983;22:292–299. doi: 10.1021/bi00271a010. [DOI] [PubMed] [Google Scholar]
- 10.Chong HS, Ma X, Le T, Kwamena B, Milenic DE, Brady ED, et al. Rational design and generation of a bimodal bifunctional ligand for antibody-targeted radiation cancer therapy. J Med Chem. 2008;51:118–125. doi: 10.1021/jm070401q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong HS, Song HA, Ma X, Milenic DE, Brady ED, Lim S, et al. Novel bimodal bifunctional ligands for radioimmunotherapy and targeted MRI. Bioconjug Chem. 2008;19:1439–1447. doi: 10.1021/bc800050x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffend J, Mier W, Schuhmacher J, Schmidt K, Dimitrakopoulou-Strauss A, Strauss LG, et al. Gallium-68-DOTA-albumin as a PET blood-pool marker: experimental evaluation in vivo. Nucl Med Biol. 2005;32:287–292. doi: 10.1016/j.nucmedbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Jeong JM, Hong MK, Chang YS, Lee YS, Kim YJ, Cheon GJ, et al. Preparation of a promising angiogenesis PET imaging agent: 68Ga-labeled c(RGDyK)-isothiocyanatobenzyl-1, 4, 7-triazacyclononane-1, 4, 7-triacetic acid and feasibility studies in mice. J Nucl Med. 2008;49:830–836. doi: 10.2967/jnumed.107.047423. [DOI] [PubMed] [Google Scholar]
- 14.Sabatino G, Chinol M, Paganelli G, Papi S, Chelli M, Leone G, et al. A new biotin derivative-DOTA conjugate as a candidate for pretargeted diagnosis and therapy of tumors. J Med Chem. 2003;46:3170–3173. doi: 10.1021/jm030789z. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka K, Masuyama T, Hasegawa K, Tahara T, Mizuma H, Wada Y, et al. A submicrogram-scale protocol for biomolecule-based pet imaging by rapid 6p-azaelectrocyclization: visualization of sialic acid dependent circulatory residence of glycoproteins. Angew Chem Int Ed. 2008;47:102–105. doi: 10.1002/anie.200702989. [DOI] [PubMed] [Google Scholar]
- 16.Tsang BW, Mathias CJ, Fanwick PE, Green MA. Structure-distribution relationships for metal-labeled myocardial imaging agents: comparison of a series of cationic gallium (III) complexes with hexadentate bis(salicylaldimine) ligands. J Med Chem. 1994;37:4400–4406. doi: 10.1021/jm00051a018. [DOI] [PubMed] [Google Scholar]
- 17.Tsang BW, Mathias CJ, Green MA. A gallium-68 radiopharmaceutical that is retained in myocardium: 68Ga[(4, 6-MeO2sal)2BAPEN]+ J Nucl Med. 1993;34:1127–1131. [PubMed] [Google Scholar]
- 18.Kung HF, Liu BL, Mankoff D, Kung MP, Billings JJ, Francesconi L, et al. A new myocardial imaging agent: synthesis, characterization, and biodistribution of gallium-68-BAT-TECH. J Nucl Med. 1990;31:1635–1640. [PubMed] [Google Scholar]
- 19.Fichna J, Janecka A. Synthesis of target-specific radiolabeled peptides for diagnostic imaging. Bioconjug Chem. 2003;14:3–17. doi: 10.1021/bc025542f. [DOI] [PubMed] [Google Scholar]
- 20.Janoki G, Harwig J, Chanachai W, Wolf W. 67Ga desferrioxamine–HSA: synthesis of chelon protein conjugates using carbodiimide as a coupling agent. Int J Appl Radiat Isot. 1983;34:871–877. doi: 10.1016/0020-708X(83)90145-X. [DOI] [PubMed] [Google Scholar]
- 21.Koizumi M, Endo K, Kunimatsu M, Sakahara H, Nakashima T, Kawamura Y, et al. Preparation of 67Ga-labeled antibodies using deferoxamine as a bifunctional chelate. An improved method. J Immunol Methods. 1987;104:93–102. doi: 10.1016/0022-1759(87)90492-3. [DOI] [PubMed] [Google Scholar]
- 22.Mathias CJ, Sun YZ, Welch MJ, Connett JM, Philpott GW, Martell AE. N,N'-bis(2-hydroxybenzyl)-1-(4-bromoacetamidobenzyl)-1,2-ethylenediamine-N,N'-diacetic acid: a new bifunctional chelate for radiolabeling antibodies. Bioconjug Chem. 1990;1:204–211. doi: 10.1021/bc00003a005. [DOI] [PubMed] [Google Scholar]
- 23.Broan C, Cox J, Craig A, Kataky R, Parker D, Harrison A, et al. Structure and solution stability of indium and gallium complexes of 1,4,7-triazacyclononanetriacetate and of yttrium complexes of 1,4,7,10-tetraazacyclododecanetetraacetate and related ligands: kinetically stable complexes for use in imaging and radioimmunotherapy. X-ray molecular structure of the indium and gallium complexes of 1,4,7-triazacyclononane-1,4,7-triacetic acid. J Chem Soc Perkin Trans. 1991;2:87–99. [Google Scholar]
- 24.Liu S, Edwards DS. Synthesis and characterization of two 111In-labeled DTPA-peptide conjugates. Bioconjug Chem. 2001;12:630–634. doi: 10.1021/bc010013h. [DOI] [PubMed] [Google Scholar]
- 25.Chong HS, Garmestani K, Ma D, Milenic DE, Overstreet T, Brechbiel MW. Synthesis and biological evaluation of novel macrocyclic ligands with pendent donor groups as potential yttrium chelators for radioimmunotherapy with improved complex formation kinetics. J Med Chem. 2002;45:3458–3464. doi: 10.1021/jm0200759. [DOI] [PubMed] [Google Scholar]
- 26.Reubi JC, Schaer JC, Markwalder R, Waser B, Horisberger U, Laissue J. Distribution of somatostatin receptors in normal and neoplastic human tissues: recent advances and potential relevance. Yale J Biol Med. 1997;70:471–479. [PMC free article] [PubMed] [Google Scholar]
- 27.Reubi JC. Regulatory peptide receptors as molecular targets for cancer diagnosis and therapy. Q J Nucl Med. 1997;41:63–70. [PubMed] [Google Scholar]
- 28.Schonbrunn A. Somatostatin receptors present knowledge and future directions. Ann Oncol. 1999;10:S17–S21. doi: 10.1023/A:1027392228418. [DOI] [PubMed] [Google Scholar]
- 29.Reubi JC, Schar JC, Waser B, Wenger S, Heppeler A, Schmitt JS, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27:273–282. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 30.Kwekkeboom DJ, Kooij PP, Bakker WH, Macke HR, Krenning EP. Comparison of 111In-DOTA-Tyr3-octreotide and 111In-DTPA-octreotide in the same patients: biodistribution, kinetics, organ and tumor uptake. J Nucl Med. 1999;40:762–767. [PubMed] [Google Scholar]
- 31.Rufini V, Calcagni ML, Baum RP. Imaging of neuroendocrine tumors. Semin Nucl Med. 2006;36:228–247. doi: 10.1053/j.semnuclmed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Smith-Jones PM, Stolz B, Bruns C, Albert R, Reist HW, Fridrich R, et al. Gallium-67/gallium-68-[DFO]-octreotide—a potential radiopharmaceutical for PET imaging of somatostatin receptor-positive tumors: synthesis and radiolabeling in vitro and preliminary in vivo studies. J Nucl Med. 1994;35:317–325. [PubMed] [Google Scholar]
- 33.Breeman WA, de Jong M, Kwekkeboom DJ, Valkema R, Bakker WH, Kooij PP, et al. Somatostatin receptor-mediated imaging and therapy: basic science, current knowledge, limitations and future perspectives. Eur J Nucl Med. 2001;28:1421–1429. doi: 10.1007/s002590100502. [DOI] [PubMed] [Google Scholar]
- 34.Kwekkeboom DJ, Mueller-Brand J, Paganelli G, Anthony LB, Pauwels S, Kvols LK, et al. Overview of results of peptide receptor radionuclide therapy with 3 radiolabeled somatostatin analogs. J Nucl Med. 2005;46(Suppl 1):62S–66S. [PubMed] [Google Scholar]
- 35.Wild D, Macke HR, Waser B, Reubi JC, Ginj M, Rasch H, et al. 68Ga-DOTANOC: a first compound for PET imaging with high affinity for somatostatin receptor subtypes 2 and 5. Eur J Nucl Med Mol Imaging. 2005;32:724. doi: 10.1007/s00259-004-1697-4. [DOI] [PubMed] [Google Scholar]
- 36.Wild D, Schmitt JS, Ginj M, Macke HR, Bernard BF, Krenning E, et al. DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometals. Eur J Nucl Med Mol Imaging. 2003;30:1338–1347. doi: 10.1007/s00259-003-1255-5. [DOI] [PubMed] [Google Scholar]
- 37.Wei L, Miao Y, Gallazzi F, Quinn TP, Welch MJ, Vavere AL, et al. Gallium-68-labeled DOTA-rhenium-cyclized alpha-melanocyte-stimulating hormone analog for imaging of malignant melanoma. Nucl Med Biol. 2007;34:945–953. doi: 10.1016/j.nucmedbio.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Froidevaux S, Calame-Christe M, Schuhmacher J, Tanner H, Saffrich R, Henze M, et al. A gallium-labeled DOTA-alpha-melanocyte- stimulating hormone analog for PET imaging of melanoma metastases. J Nucl Med. 2004;45:116–123. [PubMed] [Google Scholar]
- 39.van Hagen PM, Breeman WA, Reubi JC, Postema PT, van den Anker-Lugtenburg PJ, Kwekkeboom DJ, et al. Visualization of the thymus by substance P receptor scintigraphy in man. Eur J Nucl Med. 1996;23:1508–1513. doi: 10.1007/BF01254476. [DOI] [PubMed] [Google Scholar]
- 40.de Visser M, Janssen PJ, Srinivasan A, Reubi JC, Waser B, Erion JL, et al. Stabilised 111In-labelled DTPA- and DOTA-conjugated neurotensin analogues for imaging and therapy of exocrine pancreatic cancer. Eur J Nucl Med Mol Imaging. 2003;30:1134–1139. doi: 10.1007/s00259-003-1189-y. [DOI] [PubMed] [Google Scholar]
- 41.Behr TM, Behe MP. Cholecystokinin-B/Gastrin receptor-targeting peptides for staging and therapy of medullary thyroid cancer and other cholecystokinin-B receptor-expressing malignancies. Semin Nucl Med. 2002;32:97–109. doi: 10.1053/snuc.2002.31028. [DOI] [PubMed] [Google Scholar]
- 42.Reubi JC, Wenger S, Schmuckli-Maurer J, Schaer JC, Gugger M. Bombesin receptor subtypes in human cancers: detection with the universal radioligand 125I-[D-TYR(6), beta-ALA(11), PHE(13), NLE(14)] bombesin(6-14) Clin Cancer Res. 2002;8:1139–1146. [PubMed] [Google Scholar]
- 43.Zhang H, Schuhmacher J, Waser B, Wild D, Eisenhut M, Reubi JC, et al. DOTA-PESIN, a DOTA-conjugated bombesin derivative designed for the imaging and targeted radionuclide treatment of bombesin receptor-positive tumours. Eur J Nucl Med Mol Imaging. 2007;34:1198–1208. doi: 10.1007/s00259-006-0347-4. [DOI] [PubMed] [Google Scholar]
- 44.Lankinen P, Makinen TJ, Poyhonen TA, Virsu P, Salomaki S, Hakanen AJ, et al. 68Ga-DOTAVAP-P1 PET imaging capable of demonstrating the phase of inflammation in healing bones and the progress of infection in osteomyelitic bones. Eur J Nucl Med Mol Imaging. 2008;35:352–364. doi: 10.1007/s00259-007-0637-5. [DOI] [PubMed] [Google Scholar]
- 45.Makinen TJ, Lankinen P, Poyhonen T, Jalava J, Aro HT, Roivainen A. Comparison of 18F-FDG and 68Ga PET imaging in the assessment of experimental osteomyelitis due to Staphylococcus aureus. Eur J Nucl Med Mol Imaging. 2005;32:1259–1268. doi: 10.1007/s00259-005-1841-9. [DOI] [PubMed] [Google Scholar]
- 46.Roivainen A, Tolvanen T, Salomaki S, Lendvai G, Velikyan I, Numminen P, et al. 68Ga-labeled oligonucleotides for in vivo imaging with PET. J Nucl Med. 2004;45:347–355. [PubMed] [Google Scholar]
- 47.Clarke ET, Martell AE. Stabilities of the Fe(Iii), Ga(Iii) and in(Iii) Chelates of N,N',N''-triazacyclononanetriacetic acid. Inorg Chim Acta. 1991;181:273–280. doi: 10.1016/S0020-1693(00)86821-8. [DOI] [Google Scholar]
- 48.Clarke ET, Martell AE. Potentiometric and spectrophotometric determination of the stabilities of in(Iii), Ga(Iii) and Fe(Iii) complexes of N,N',N''-tris(3,5-dimethyl-2-hydroxybenzyl)-1,4,7-triazacyclononane. Inorg Chim Acta. 1991;186:103–111. doi: 10.1016/S0020-1693(00)87938-4. [DOI] [Google Scholar]
- 49.Clarke ET, Martell AE. Stabilities of trivalent metal-ion complexes of the tetraacetate derivatives of 12-membered, 13-membered and 14-membered tetraazamacrocycles. Inorg Chim Acta. 1991;190:37–46. doi: 10.1016/S0020-1693(00)80229-7. [DOI] [Google Scholar]
- 50.Shetty D, Jeong JM, Hoigebazar L, Lee YS, Lee DS, Chung JK, et al. (2010) Formation and characterization of gallium(III) complexes with monoamide derivatives of 1,4,7-triazacyclononane-1,4,7-triacetic acid: a pH dependant structural study. Eur J Inorg Chem 2010:In press.
- 51.Shetty D, Ju CH, Kim YJ, Lee JY, Lee YS, Lee DS, et al. (2010) Synthesis and evaluation of macrocyclic amino acid derivatives for tumor imaging by gallium-68 positron emission tomography. Bioorg Med Chem 18. doi:10.1016/j.bmc.2010.09.022 [DOI] [PubMed]
- 52.Shetty D, Ju CH, Lee YS, Jeong SY, Choi JY, Yang BY, et al. (2010) Synthesis of novel 68Ga labeled amino acid derivatives for positron emission tomography of cancer cells. Nucl Med Biol 37. doi:10.1016/j.nucmedbio.2010.06.003 [DOI] [PubMed]
- 53.Ito M, Yang DJ, Mawlawi O, Mendez R, Oh CS, Azhdarinia A, et al. PET and planar imaging of tumor hypoxia with labeled metronidazole. Acad Radiol. 2006;13:598–609. doi: 10.1016/j.acra.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Hoigebazar L, Jeong JM, Choi SY, Choi JY, Shetty D, Lee YS, et al. Synthesis and characterization of nitroimidazole derivatives for 68Ga-labeling and testing in tumor xenografted mice. J Med Chem. 2010;53:6378–6385. doi: 10.1021/jm100545a. [DOI] [PubMed] [Google Scholar]
- 55.Sharma V, Prior JL, Belinsky MG, Kruh GD, Piwnica-Worms D. Characterization of a 67Ga/68Ga radiopharmaceutical for SPECT and PET of MDR1 P-glycoprotein transport activity in vivo: validation in multidrug-resistant tumors and at the blood-brain barrier. J Nucl Med. 2005;46:354–364. [PubMed] [Google Scholar]
- 56.Choi JY, Yoo BC, Kim K, Kim Y, Yang BY, Lee YS, et al. (2010) Development of 68Ga-labeled mannosylated human serum albumin (MSA) as a lymph node imaging agent for positron emission tomography. Nucl Med Biol 37. doi:10.1016/j.nucmedbio.2010.09.010 [DOI] [PubMed]
- 57.Yang BY, Jeong JM, Kim YJ, Choi JY, Lee YS, Lee DS, et al. Formulation of 68Ga BAPEN kit for myocardial positron emission tomography imaging and biodistribution study. Nucl Med Biol. 2009;37:149–155. doi: 10.1016/j.nucmedbio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 58.Hofmann M, Maecke H, Borner R, Weckesser E, Schoffski P, Oei L, et al. Biokinetics and imaging with the somatostatin receptor PET radioligand 68Ga-DOTATOC: preliminary data. Eur J Nucl Med. 2001;28:1751–1757. doi: 10.1007/s002590100639. [DOI] [PubMed] [Google Scholar]
- 59.Koukouraki S, Strauss LG, Georgoulias V, Schuhmacher J, Haberkorn U, Karkavitsas N, et al. Evaluation of the pharmacokinetics of 68Ga-DOTATOC in patients with metastatic neuroendocrine tumours scheduled for 90Y-DOTATOC therapy. Eur J Nucl Med Mol Imaging. 2006;33:460–466. doi: 10.1007/s00259-005-0006-1. [DOI] [PubMed] [Google Scholar]
- 60.Kowalski J, Henze M, Schuhmacher J, Macke HR, Hofmann M, Haberkorn U. Evaluation of positron emission tomography imaging using [68Ga]-DOTA-D Phe(1)-Tyr(3)-Octreotide in comparison to [111In]-DTPAOC SPECT. First results in patients with neuroendocrine tumors. Mol Imaging Biol. 2003;5:42–48. doi: 10.1016/S1536-1632(03)00038-6. [DOI] [PubMed] [Google Scholar]
- 61.Win Z, Al-Nahhas A, Towey D, Todd JF, Rubello D, Lewington V, et al. 68Ga-DOTATATE PET in neuroectodermal tumours: first experience. Nucl Med Commun. 2007;28:359–363. doi: 10.1097/MNM.0b013e32808ea0b0. [DOI] [PubMed] [Google Scholar]
- 62.Win Z, Rahman L, Murrell J, Todd J, Al-Nahhas A. The possible role of 68Ga-DOTATATE PET in malignant abdominal paraganglioma. Eur J Nucl Med Mol Imaging. 2006;33:506. doi: 10.1007/s00259-005-0035-9. [DOI] [PubMed] [Google Scholar]
- 63.Henze M, Dimitrakopoulou-Strauss A, Milker-Zabel S, Schuhmacher J, Strauss LG, Doll J, et al. Characterization of 68Ga-DOTA-D-Phe1-Tyr3-octreotide kinetics in patients with meningiomas. J Nucl Med. 2005;46:763–769. [PubMed] [Google Scholar]