Abstract
A 32-year-old female who suffered from abdominal pain underwent 18F-fluorodexoyglucose (FDG) positron emission tomography/computed tomography (PET/CT) for the diagnostic workup of pelvic mass lesions. Cystic mass lesions in the bilateral ovaries showed wall thickening and intense hypermetabolism along the rim. In addition, multifocal intense hypermetabolic lymphadenopathies were seen in the left paraaortic lymph node (LN), aortocaval LN, and both common iliac LNs. We interpreted these findings as bilateral ovarian cancer with retroperitoneal metastatic lymphadenopathies rather than endometriosis with reactive lymphadenopathies. However, histopathological examination confirmed the ovarian mass lesions as tubo-ovarian abscesses. We report a case that even if simultaneous hypermetabolic retroperitoneal LNs are seen, intense hypermetabolic lesions in both ovaries can be in consequence of inflammatory change.
Keywords: PET/CT, Ovary, Retroperitoneal lymph node, Tubo-ovarian abscess
Introduction
Recently, 18F-fluorodexoyglucose (FDG) positron emission tomography/computed tomography (PET/CT) has been highlighted for the staging and treatment evaluation of numerous cancers. For instance, localization of the unknown primary site of origin in metastatic head and neck cancer [1], prediction of disease-free survival after induction therapy of esophageal cancer [2], mediastinal lymph node and hilar lymph node staging of non-small cell lung cancer [3], clinical staging and management of breast cancer [4], and following-up of patients with high risk ovarian cancer [5].
However, increased FDG uptake, besides being present in malignant lesions, could also present in benign, inflammatory, or granulomatous lesions [6]. These benign lesions of 18F-FDG uptake may lead to false-positive results for cancer; for example, gallbladder tuberculosis versus gallbladder cancer [7], or osteoradionecosis versus recurrence of nasopharyngeal cancer [8], and so on.
Here, we present a case of pelvic mass with retroperitoneal lymphadenopathies, which showed false-positive findings in FDG-PET/CT.
Case Report
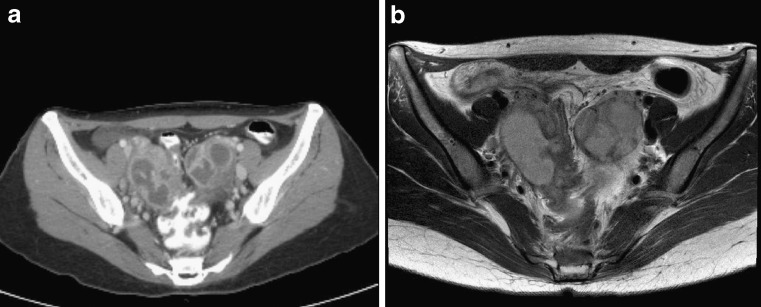
A 32-year-old female who had been suffering from abdominal pain was referred to our medical institute for the diagnostic workup of pelvic mass lesions. Her primary care provider reported ovarian mass lesions in the pelvis on ultrasonography. On admission, blood pressure was 107/61 mmHg and body temperature was 36.0°C. Blood cell count showed mild leukocytosis (12,500 cells/μl, normal range: 4,000-10,000 cells/μl). Further, laboratory studies demonstrated that the serum level of C-reactive protein (CRP) was 0.71 mg/dl (normal range: 0-0.5 mg/d), serum level of carcinoembryonic antigen (CEA), cancer antigen 125 (CA-125), and cancer antigen 19-9 (CA 19-9) were 10.7 ng/ml (normal range: 0-0.5 ng/ml), 525 ng/ml (normal range: 0-37 ng/ml), and 1.0 u/ml (normal range: 0-5 u/ml), respectively. Colonoscopy showed a protruding appearance of the colonic wall by an extrinsic mass. Contrast-enhanced pelvis CT and magnetic resonance (MR) images (Fig. 1a, b) demonstrated lobulating cystic masses in the bilateral adnexae with irregular diffuse wall thickening and infiltration into the surrounding soft tissue. These findings were interpreted as bilateral ovarian cancer with metastatic lymphadenopathy or bilateral endometriosis.
Fig. 1a, b.
A 32-year-old woman who complained of abdominal pain had CT and MR imaging of the pelvis to diagnose the disease. Transaxial CT (a) and T2-weighted transaxial MRI (b) images reveal bilateral lobulating cystic masses in the bilateral adnexae, suggesting ovarian cancer or endometriosis
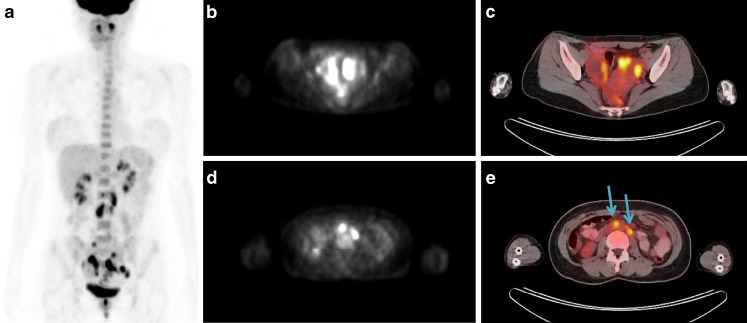
For the differentiation of the lesions, an FDG-PET/CT scan was performed (Fig. 2a-e) after 8 h of fasting. Sixty minutes after intravenous administration of 370 MBq of FDG, a PET/CT scan was performed using a PET/CT scanner (Biograph LSO; Siemens Medical Systems). Cystic mass lesions in the bilateral ovaries were found with hypermetabolism along the rim and wall thickening area with maximum standardized uptake value (maxSUV) of 8.4 (Fig. 2b, c). Additionally, multifocal lymphadenopathies were detected with hypermetabolism in left paraaortic LN (maxSUV: 4.1, right arrow), aortocaval LN (maxSUV: 5.7, left arrow), and both common iliac LNs (maxSUV: right 3.8 and left 2.6) (Fig. 2a, d, e). These findings were interpreted as bilateral ovarian cancer with retroperitoneal metastatic lymphadenopathies rather than endometriosis with reactive lymphadenopathies.
Fig. 2.
a-e Anterior maximum intensity projection (MIP) image of FDG-PET/CT (a) shows intense FDG uptake in bilateral ovaries and retroperitoneal lymph nodes. PET (b), and fusion PET/CT (c) images show high FDG uptake along the wall thickening area with maxSUV 8.4. Further, PET (d) and fusion PET/CT (e) images of the abdomen demonstrate focal intense FDG uptake in retroperiteum. Lymphadenopathies in the left paraaortic lymph nodes (maxSUV 4.1, right arrow), aortocaval lymph nodes (maxSUV 5.7, left arrow) are shown with intense FDG uptake
In suspicion of bilateral ovarian tumors, explorative laparotomy with right ovarian wedge resection was performed. The bilateral adnexae, posterior wall of the uterus and sigmoid colon were adhered, and the bilateral salpinges were swollen by inflammation, with pus in them. Pus drainage was performed with adhesiolysis. The result of a frozen biopsy of the ovarian wall demonstrated an abscess with no evidence of cancerous lesion. The histopathological examination reported the ovarian lesion as a benign cystic lesion with hemorrhage and no epithelial lining, suggestive of hemorrhagic corpus luteum and cystic follicle. Washing cytology of the peritoneum was also negative for malignant cells. Considering the histopathological results, the patient was diagnosed as having a tubo-ovarian abscess, and treated with intravenous antibiotics. After the operation, her vital signs were stable without acute complication and post operation laboratory serum tests were normalized; CRP was changed from 0.71 mg/dl to 0.32 mg/dl and white blood cells (WBC) from 12,500/μl to 5,790/μl. The patient was discharged 9 days after the operation.
Discussion
FDG-PET/CT is a useful tool for the diagnosis and management of cancers. However, the accumulation of FDG in PET/CT scans only indicates enhanced glucose metabolism, irrespective of the nature of the cells. Therefore, healthy tissue with physiologic FDG uptake or benign disease such as inflammation or posttraumatic lesions could be mistaken for a cancerous lesion [9]. Accurate interpretation of FDG-PET/CT scans requires differentiating inflammatory or physiologic lesions from malignant tumors with thorough knowledge. Ho [10] insisted that, except for tuberculosis, many inflammatory lesions have a maxSUV < 2.5, and the pattern of involvement usually gives clues to differentiate an infective inflammatory cause from real malignancy.
Pelvic mass malignant lesions should be differentiated from benign inflammatory lesions such as endometriosis [11], hemorrhagic corpus luteum [12], which also show high FDG uptake. In ovarian cancer, FDG-PET/CT has a fair sensitivity to detect recurrence in the setting of a rising CA-125 and negative conventional imaging studies [13]. However, in detecting primary ovarian cancer, increased FDG uptake in both ovaries may indicate either malignant or benign functional uptake in premenopausal patients [14]. The result of a previous study insisted that, during the late follicular to early luteal phase, physiologic hypermetabolic lesions of the ovary in premenoposal women should also be checked [15].
Drieskens et al. [16] demonstrated that 18F-FDG-PET uptake for the retroperitoneal lymph nodes were correct in all cases of ovarian cancer patients. However, in our case, the ovarian masses with intense FDG uptake (maxSUV 8.4) were not confirmed as malignant lesions even with simultaneous high FDG uptake of retroperitoneal lymph nodes (maxSUV 5.7). This may be because of increased FDG uptake in inflammatory cells. Therefore, further studies including histopathological confirmation, imaging (such as CT or MRI) and laboratory findings might be needed in patients with high FDG uptake in ovarian lesions with retroperitoneal lymph nodes. Especially in young premenopausal patients, inflammatory lesion or physiologic FDG uptake should be considered once more.
To sum up, even if simultaneous hypermetabolic retroperitoneal lymph nodes are seen, intense hypermetabolism in both ovaries can be caused by inflammatory change. So we should be careful in assessing the pelvic hypermetabolic lesion to avoid false-positive PET/CT diagnosis if an inflammatory process could not be excluded completely.
References
- 1.AA OS, Fischbein NJ, Caputo GR, Kaplan MJ, Price DC, Singer MI, et al. Metastatic head and neck cancer: role and usefulness of FDG PET in locating occult primary tumors. Radiology. 1999;210:177–181. doi: 10.1148/radiology.210.1.r99ja48177. [DOI] [PubMed] [Google Scholar]
- 2.Downey RJ, Akhurst T, Ilson D, Ginsberg R, Bains MS, Gonen M, et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol. 2003;21:428–432. doi: 10.1200/JCO.2003.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Konishi J, Yamazaki K, Tsukamoto E, Tamaki N, Onodera Y, Otake T, et al. Mediastinal lymph node staging by FDG-PET in patients with non-small cell lung cancer: analysis of false-positive FDG-PET findings. Respiration. 2003;70:500–506. doi: 10.1159/000074207. [DOI] [PubMed] [Google Scholar]
- 4.Yap CS, Seltzer MA, Schiepers C, Gambhir SS, Rao J, Phelps ME, et al. Impact of whole-body 18F-FDG PET on staging and managing patients with breast cancer: the referring physician’s perspective. J Nucl Med. 2001;42:1334–1337. [PubMed] [Google Scholar]
- 5.Nakamoto Y, Saga T, Ishimori T, Mamede M, Togashi K, Higuchi T, et al. Clinical value of positron emission tomography with FDG for recurrent ovarian cancer. AJR Am J Roentgenol. 2001;176:1449–1454. doi: 10.2214/ajr.176.6.1761449. [DOI] [PubMed] [Google Scholar]
- 6.Kostakoglu L, Hardoff R, Mirtcheva R, Goldsmith SJ. PET-CT fusion imaging in differentiating physiologic from pathologic FDG uptake. Radiographics. 2004;24:1411–1431. doi: 10.1148/rg.245035725. [DOI] [PubMed] [Google Scholar]
- 7.Ramia JM, Muffak K, Fernandez A, Villar J, Garrote D, Ferron JA. Gallbladder tuberculosis: false-positive PET diagnosis of gallbladder cancer. World J Gastroenterol. 2006;12:6559–6560. doi: 10.3748/wjg.v12.i40.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu SH, Chang JT, Ng SH, Chan SC, Yen TC. False positive fluorine-18 fluorodeoxy-D-glucose positron emission tomography finding caused by osteoradionecrosis in a nasopharyngeal carcinoma patient. Br J Radiol. 2004;77:257–260. doi: 10.1259/bjr/69516821. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbaum SJ, Lind T, Antoch G, Bockisch A. False-positive FDG PET uptake—the role of PET/CT. Eur Radiol. 2006;16:1054–1065. doi: 10.1007/s00330-005-0088-y. [DOI] [PubMed] [Google Scholar]
- 10.Ho CL. Clinical PET imaging—an Asian perspective. Ann Acad Med Singapore. 2004;33:155–165. [PubMed] [Google Scholar]
- 11.Jeffry L, Kerrou K, Camatte S, Metzger U, Lelievre L, Talbot JN, et al. Endometriosis with FDG uptake on PET. Eur J Obstet Gynecol Reprod Biol. 2004;117:236–239. doi: 10.1016/j.ejogrb.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 12.Ames J, Blodgett T, Meltzer C. 18F-FDG uptake in an ovary containing a hemorrhagic corpus luteal cyst: false-positive PET/CT in a patient with cervical carcinoma. AJR Am J Roentgenol. 2005;185:1057–1059. doi: 10.2214/AJR.04.1282. [DOI] [PubMed] [Google Scholar]
- 13.Havrilesky LJ, Kulasingam SL, Matchar DB, Myers ER. FDG-PET for management of cervical and ovarian cancer. Gynecol Oncol. 2005;97:183–191. doi: 10.1016/j.ygyno.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Lerman H, Metser U, Grisaru D, Fishman A, Lievshitz G, Even-Sapir E. Normal and abnormal 18F-FDG endometrial and ovarian uptake in pre- and postmenopausal patients: assessment by PET/CT. J Nucl Med. 2004;45:266–271. [PubMed] [Google Scholar]
- 15.Nishizawa S, Inubushi M, Okada H. Physiological 18F-FDG uptake in the ovaries and uterus of healthy female volunteers. Eur J Nucl Med Mol Imaging. 2005;32:549–556. doi: 10.1007/s00259-004-1703-x. [DOI] [PubMed] [Google Scholar]
- 16.Drieskens O, Stroobants S, Gysen M, Vandenbosch G, Mortelmans L, Vergote I. Positron emission tomography with FDG in the detection of peritoneal and retroperitoneal metastases of ovarian cancer. Gynecol Obstet Invest. 2003;55:130–134. doi: 10.1159/000071525. [DOI] [PubMed] [Google Scholar]