Abstract
Objectives. SSc is associated with an increased prevalence of atherosclerosis (ATS). This study assessed the prevalence of subclinical ATS as measured by carotid US and explored serum proteins to identify potential biomarkers of SSc-ATS.
Methods. Forty-six SSc female patients and 46 age- and ethnicity-matched controls underwent carotid US to assess the presence of plaque and carotid intima media thickness (CIMT). Abstracted data included demographics, ATS risk factors and serum measurements [cholesterol, proinflammatory high-density lipoprotein (piHDL), CRP, lipoproteins]. Serum cytokines/proteins analyses included circulating type I IFN activity by quantifying IFN-inducible genes, soluble junctional adhesion molecule A (sJAM-A) and 100 serum proteins by using a microplate-based multiplex platform. Proteins significant at P < 0.05 on bivariate analyses for the presence of plaque were used to develop a composite measure.
Results. Patients with SSc had more plaque (45.6% vs 19.5%, P = 0.01) but similar CIMT compared with controls. Multiplex analysis detected significant associations between serum proteins of inflammation, vasculopathy and fibrosis with ATS in SSc, including IL-2, IL-6, CRP, keratinocyte growth factor, intercellular adhesion molecule 1, endoglin, plasminogen activator inhibitor 1 and insulin-like growth factor binding protein 3 associated with carotid plaque. Myeloid progenitor inhibitory factor 1, serum amyloid A, thrombomodulin, N-terminal pro-brain natriuretic peptide (BNP), and Clara cell secretory protein 16 kD correlated with CIMT. The median composite score for the plaque group was 6 and for the no plaque group it was 2 (P < 0.0001).
Conclusion. Patients with SSc have a higher prevalence of carotid plaque than matched controls, and patients with SSc-plaque vs patients without plaque have elevated serum proteins implicated in both vasculopathy and fibrosis. Further studies are needed to evaluate the role of these proteins in SSc compared with healthy controls.
Keywords: systemic sclerosis, atherosclerosis, serum proteins, endothelial dysfunction, type I interferon, carotid intima media thickness
Introduction
SSc is a connective tissue disease characterized by immune activation, fibrotic processes and widespread vasculopathy. The association between CTDs and atherosclerosis (ATS) has been described in SLE and RA [1, 2]. However, the mechanisms of CTD-ATS remain elusive and may include inflammation burden, dyslipidaemia and disease-specific immune dysregulations [3–7].
The initial event in the pathogenesis of SSc is thought to be vascular injury. Mechanisms include immune-mediated endothelial injury [8, 9] and impaired angiogenesis in response to repeated ischaemia-reperfusion injury [10]. The SSc-related microvascular damage is well characterized and recent data suggest that there is increased carotid intima media thickness (CIMT) in patients with SSc compared with controls [11, 12]. Recent population-based data from Australian and UK registries also suggest increased prevalence of hard cardiac events compared with the general population [13, 14]. With these recent observations, the identification and utility of surrogate markers for SSc-ATS damage and the role of contributing factors to accelerated ATS in SSc still need to be studied.
Therefore we conducted a cross-sectional study to (i) evaluate the prevalence of subclinical atherosclerotic plaque and CIMT in patients with SSc and age- and ethnicity-matched healthy controls, and (ii) explore serum cytokines/proteins associated with carotid plaque and CIMT in patients with SSc.
Methods
Study subjects
Forty-six women who met the 1980 American Rheumatism Association criteria for SSc [15] were recruited. Control subjects were randomly selected from a cohort of 167 controls (initially recruited for the Biomarkers of ATS in SLE Cohort Study, which were women, self-reportedly healthy and without clinical evidence of SLE [13]) and matched to SSc subjects for age and ethnicity. All subjects were at least 18 years of age and provided institution-approved [University of California at Los Angeles (UCLA)] informed consent. Exclusion criteria (obtained directly from the subjects) included history of pre-existing cardiovascular disease (CVD) (myocardial infarction, stroke, peripheral vascular disease), uncontrolled hypertension, uncontrolled diabetes, current pregnancy and current or previous use of statin therapy within 6 months [because statins can reduce proinflammatory high-density lipoprotein (piHDL)] [16].
Clinical data
Sociodemographic information (age, ethnicity), height and weight, along with CVD risk factors including smoking, hypertension, diabetes mellitus and family history of CVD were collected. These data were obtained from the clinic charts and also from a self-administered health history questionnaire, which also assessed the medication. We assessed SSc disease duration (defined as the first non-RP symptom) and scleroderma subtype: limited cutaneous or diffuse cutaneous.
Carotid US
Carotid US was performed using a 5-mHz linear array transducer on a Toshiba 140 US (Toshiba, Tustin, CA, USA). Sonographers measured CIMT and plaque in the right and left common carotid arteries, carotid bulb and the first 1.5 cm of the internal and external carotid arteries. Plaque was defined as a focal projection within the intima media layer ≥50% of the adjacent CIMT. The carotid USs were read by a single blinded reader (N.R.).
Serum analysis
Serum analysis (drawn the morning of the US after overnight fasting) included CRP, ESR, lipid profile [total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL) and triglycerides] and homocysteine. The serum of patients with SSc was further analysed for apolipoprotein A-1 (ApoA-1), apolipoprotein B100 (ApoB100) and lipoprotein.
Proinflammatory high-density lipoprotein
Normal HDL prevents oxidation of LDL and therefore the oxidation of dichlorofluorescein (DCFH), which releases a fluorochrome upon oxidation. To determine the functional properties of our subjects’ HDL, we measured the change in fluorescence intensity resulting from oxidation of DCFH by LDL in the presence or absence of test HDL. Twenty microlitres of LDL solution from normal plasma (final concentration of 50 μg/ml) and 90 μl of test HDL (at a final concentration of 10 μg/ml cholesterol) were incubated in 96-well plates for 1 h. Ten microlitres of DCFH solution (0.2 mg/ml) was then added to each well and incubated for 2 h. Fluorescence was determined with a plate reader (Spectra Max, Gemini XS; Molecular Devices, Sunnyvale, CA, USA). Values of DCFH activated by LDL in the absence of HDL were normalized to 1.0 as the positive control. The piHDL levels were reported as mean (s.d.).
Soluble junctional adhesion molecule A
Plasma levels of soluble junctional adhesion molecule A (sJAM-A) were measured using a sandwich ELISA [9]. Reagents used include goat anti-human JAM-A (R&D Systems, Minneapolis, MN, USA) for the capture antibody and mouse anti-JAM-A antibody (Santa Cruz Biotechnology, Dallas, TX, USA) for the primary antibody. A standard curve was produced on each plate using recombinant sJAM-A conjugated to the Fc portion of IgG (R&D Systems). Nanomolar concentrations of IgG/Fc were used as a negative control on each plate to ensure specificity.
Type I IFN activity
Type I IFN activity was quantified as previously described [17]. HeLa cells were cultured in DIFCO/10% FBS/non-essential amino acids/10 mM Hepes at 37°C in 5% CO2, plated at 2 × 105 cells/well in a 24-well plate and exposed to 50% scleroderma or control sera, or recombinant IFN-α (1 kU/well, used as positive control) or recombinant IFN-γ (200 ng/well, used as negative control for type I IFN-inducible genes; Peprotech, Rocky Hill, NJ, USA) for 6 h. TriPure was added and cells were stored at −70°C until RNA extraction. cDNA was made with Superscript II reverse transcriptase (Invitrogen). Real-time PCRs were run on an ABI PRISM 7900HT in duplicate using 2× SYBR GREEN PCR master mix (Applied Biosystems, Foster City, CA, USA) and primers previously described [17], at a concentration of 2.5 μM. The type I IFN-inducible genes quantified by this assay were IFN-induced protein-44 (IFI44), myxovirus resistance-1, IFN-induced protein with tetratricopeptide repeats 1 and double-stranded RNA-activated protein kinase. Samples were normalized to media alone after normalization to the housekeeping gene HPRT-1, and results were reported as fold induction/media (more details are available in Ansell et al. [16]).
Exploratory proteins
We utilized a microplate-based multiplex platform (SearchLight, Aushon Biosystems, Billerica, MA, USA) to quantify 100 proteins in SSc serum samples. The proteins were empirically selected by the authors to reflect processes of vasculopathy, inflammation and fibrosis; some of the proteins are still being explored, as they are involved in more than one pathway (i.e. IL-6). Each well of the microtitre plate was coated with analyte-specific antibodies, followed by washing and detection with a horseradish peroxidase (HRP)-based enzyme assay. Proteins were run in duplicate and the mean is reported (see supplementary Table S1, available at Rheumatology Online).
Statistical analysis
We compared the SSc patients to the controls using paired t-tests, Pearson’s correlation, Wilcoxon matched-pairs signed-rank test and Spearman’s correlation for parametric data.
Wilcoxon rank-sum tests and Spearman’s correlations were used to assess the association of protein values with plaque and average CIMT and P < 0.05 was considered significant.
Development of a composite score
The proteins were examined in bivariate analyses with the presence or absence of plaque using Student’s t-tests or Wilcoxon rank-sum tests. Proteins significant at the α = 5% level were selected for receiver operating characteristic (ROC) analysis with the presence or absence of plaque as the outcome. The optimal cut point was determined for each protein using the criterion of maximum accuracy. Subjects were then classified as positive or negative on each protein. A composite score was determined by summing the number of positives among the selected candidate proteins (score 0–8). The discriminative predicting ability was assessed using ROC analysis.
For IFN analysis, we analysed the IFN signatures for association with plaque and CIMT using three methods: (i) We compared high vs low IFN producers based upon the 95th percentile control cut-offs on at least two IFN-inducible genes. (ii) We performed a principal component analysis (PCA). a separate composite score for each one-, two- and three-component PCA solution was formed by taking the Euclidean distance of the projected points in each case. These three PCA composite scores were then compared between the no-plaque and plaque groups using t-tests or Wilcoxon rank-sum tests. (iii) We also assessed IFN gene expression values by rescaling so that the maximum value for the gene was 1.0. These normalized values were summed across the set of genes to obtain a score for each subject. The composite score was then compared between the no-plaque and plaque groups. Spearman’s correlation coefficient and resulting P-values were computed for the correlation between the above composite scores and (i) average CIMT and (ii) maximum CIMT.
Results
Forty-six females with SSc and 46 matched controls were enrolled (Table 1). Mean (s.d.) disease duration from the first non-RP symptom was 6.5 (5.2) years and 23 patients had limited and 23 had diffuse SSc. The SSc patients and controls had similar BMIs (Table 1).
Table 1.
Demographics, CVD risk factors, piHDL and carotid US findings in SSc patients vs controls
|
SSc (n = 46) |
Controls (n = 46) |
||||
|---|---|---|---|---|---|
| n | Mean (s.d.) | n | Mean (s.d.) | P-value | |
| Age, years | 48.6 (13.3) | 48.6 (13.3) | — | ||
| Race, n (%) | — | — | — | ||
| Caucasian | 35 (76.1) | 35 (76.1) | |||
| Black | 4 (8.7) | 4 (8.7) | |||
| Asian | 7 (15.2) | 7 (15.2) | |||
| Ethnicity, n (%)a | |||||
| Hispanic | 5 (10.9) | 5 (10.9) | |||
| Non-Hispanic | 30 (65.2) | 30 (65.2) | |||
| Risk factors, n (%) | — | — | |||
| HTN | 12/41 (29.2) | 9/41 (21.9) | 0.43 | ||
| DM | 1/41 (2.4) | 3/41 (7.3) | 0.15 | ||
| Smoking | |||||
| Current | 1/41 (2.4) | 10/41 (24.4) | <0.001* | ||
| Past | 14/41 (34.1) | 0 (0) | <0.001* | ||
| Family history of CVD | 25/40 (62.5) | 10/40 (25) | <0.001* | ||
| BMI | 35 | 24.8 (4.7) | 35 | 23.9 (4.4) | 0.51 |
| Total cholesterol, mg/dl | 45 | 198.4 (37.5) | 45 | 196.8 (56.7) | 0.60 |
| LDL, mg/dl | 45 | 117.2 (32.6) | 45 | 113.2 (52.5) | 0.22 |
| HDL, mg/dl | 45 | 52.8 (13.2) | 45 | 60.1 (14.3) | <0.001* |
| Triglyceride, mg/dl | 45 | 159.3 (119.6) | 45 | 115.7 (63.3) | 0.01* |
| ESR, mm/h | 32 | 23.8 (18.2) | 32 | 11.3 (11.2) | <0.001* |
| Homocysteine, mg/dl | 22 | 10.2 (3.3) | 22 | 8.6 (2.8) | 0.07 |
| piHDL, FU | 30 | 0.88 (0.92) | 30 | 0.91 (0.54) | 0.20 |
| Plaque, n (%) | 21 (45.6) | — | 9 (19.5) | — | 0.01* |
| Right CIMT, mm | 44 | 0.6 (0.15) | 44 | 0.56 (0.13) | 0.03* |
| Left CIMT, mm | 44 | 0.57 (0.12) | 44 | 0.56 (0.14) | 0.21 |
| Average CIMT, mm | 44 | 0.59 (0.13) | 44 | 0.56 (0.13) | 0.07 |
All patients and controls were women. aEleven patients did not report their ethnicity. FU: fluorescence units; HTN: hypertension; DM: diabetes mellitus; CVD: cardiovascular disease; LDL: low-density lipoprotein; HDL: high-density lipoprotein; piHDL: proinflammatory high-density lipoprotein; CIMT: carotid intima media thickness. *P-values <0.05.
A higher proportion of patients with SSc were reformed smokers, whereas a higher proportion of controls were current smokers (P < 0.001; Table 1). Patients with SSc had a greater family history of CVD compared with controls (P < 0.05; Table 1). Patients with SSc had higher triglycerides and inflammatory markers (ESR) but lower HDL compared with controls (Table 1). Among patients with SSc with and without plaque, there were no differences in inflammatory markers or levels of various lipoproteins (Table 2).
Table 2.
Age, CVD risk factors, cholesterol, CIMT and apolipoproteins in SSc patients with and without plaque
|
Plaque (n = 25) |
No plaque (n = 21) |
||||
|---|---|---|---|---|---|
| n | Mean (s.d.) | n | Mean (s.d.) | P-value | |
| Age, years | 47.2 (13.8) | 50.2 (12.8) | 0.44 | ||
| Risk factors, n (%) | |||||
| HTN | 21 | 6 (28.5) | 20 | 6 (30) | 1 |
| DM | 21 | 1 (4.7) | 20 | 0 (0.0) | 1 |
| Smoking | |||||
| Current | 21 | 0 (0.0) | 20 | 1 (5.0) | 0.48 |
| Past | 21 | 4 (19.0) | 20 | 10 (50.0) | 0.51 |
| Family history of CVD | 21 | 10 (47.6) | 19 | 15 (78.9) | 0.55 |
| Total cholesterol, mg/dl | 21 | 196.8 (34.9) | 25 | 196.6 (42.5) | 0.98 |
| LDL, mg/dl | 21 | 112.7 (32.4) | 25 | 118.4 (34.9) | 0.57 |
| HDL, mg/dl | 21 | 52.4 (11.5) | 25 | 52.9 (14.5) | 0.89 |
| Triglycerides, mg/dl | 21 | 194.2 (158) | 25 | 126.6 (59.4) | 0.14 |
| Average CIMT, mm | 20 | 50.2 (12.8) | 24 | 0.57 (0.13) | 0.35 |
| ESR, mm/h | 21 | 28 (16.5) | 25 | 21.68 (20.38) | 0.13 |
| CRP, mg/dl | 21 | 0.9 (0.7) | 25 | 0.6 (0.46) | 0.09 |
| Homocysteine, mg/dl | 20 | 11.2 (5.7) | 21 | 8.9 (2.32) | 0.13 |
| ApoA-1, ng/ml | 20 | 156.6 (32.1) | 24 | 151.7 (22.1) | 0.62 |
| ApoB100, ng/ml | 20 | 93.9 (22.2) | 24 | 96.7 (22.8) | 0.68 |
| Lipoprotein, ng/ml | 19 | 67.9 (75.6) | 24 | 94.7 (96.6) | 0.99 |
HTN: hypertension; DM: diabetes mellitus; CVD: cardiovascular disease; LDL: low-density lipoprotein; HDL: high-density lipoprotein; CIMT: carotid intima media thickness; ApoA-1: apolipoprotein A-1; ApoB100: apolipoprotein B100.
Carotid US
Patients with SSc had significantly more plaque than the controls (P = 0.01; Table 1). Even though patients with SSc had increased right CIMT, the average CIMT was not different between the groups (Table 1). There was no difference between plaque and CIMT in patients with limited vs diffuse SSc (see supplementary Table S1, available at Rheumatology Online).
Serum proteins analysis
Multiplex analysis
We explored the associations between various proteins and the presence/absence of plaque and CIMT in SSc (see supplementary Table S2, available at Rheumatology Online). Table 3 summarizes proteins significant at P < 0.05 with the presence/absence of plaque or CIMT. We divided the proteins into the following categories: fibrosis, inflammation and vasculopathy. The fibrosis group of proteins was associated with CIMT while the proteins implicated in vasculopathy were mostly associated with plaque. The inflammation proteins were associated with both plaque and CIMT. Among the 100 proteins studied, only 2 were present in both SSc-plaque and SSc-CIMT: N terminal pro-brain natriuretic peptide (NT pro-BNP) and IL-6 (Table 3).
Table 3.
Associations of serum proteins (P < 0.05) with the presence/absence of plaque and CIMT in SSc
|
CIMT (n = 44) |
|||||
|---|---|---|---|---|---|
| Biomarker (pg/ml) | No plaque, mean (s.d.) (n = 25) | Plaque, mean (s.d.) (n = 21) | P-value | Correlation coefficients | P-value |
| Fibrosis | |||||
| MPIF-1 | 901.2 (143.7) | 265.8 (223.3) | 0.17 | 0.31 | 0.04* |
| CC16 | 14 492.7 (6631.9) | 14 845.4 (7555.1) | 0.93 | 0.37 | 0.01* |
| Inflammation | |||||
| IL-2 | 3.0 (2.6) | 5.0 (3.8) | 0.02* | −0.17 | 0.25 |
| CRP | 1 627 772.0 (2 437 838.6) | 3 856 899.5 (4 734 651.2) | 0.00* | 0.14 | 0.35 |
| IL-6 | 11.0 (13.5) | 25.2 (48.5) | 0.04* | 0.28 | 0.06* |
| A-SAA | 18 790 613.4 (30 238 222.3) | 23 431 668.8 (30 070 001.2) | 0.43 | 0.31 | 0.04* |
| Vasculopathy | |||||
| KGF | 0.8 (2.1) | 1.6 (2.1) | 0.01* | 0.19 | 0.22 |
| ICAM-1 | 403 423.3 (131 159.9) | 539 501.8 (242 768.3) | 0.04* | 0.16 | 0.29 |
| PAI-1 | 9607.8 (6527.1) | 6680.8 (5898.0) | 0.04* | −0.10 | 0.29 |
| Endoglin | 22 444.1 (9253.0) | 8860.8 (0.0394) | 0.03* | 0.07 | 0.51 |
| IGFBP-3 | 404 848.8 (116 838.8) | 322 117.3 (93 709.0) | 0.00* | 0.13 | 0.66 |
| TM | 4826.3 (1865.1) (n = 24) | 4412.1 (1262.7) | 0.00 | 0.32 | 0.03* |
CIMT: carotid intima media thickness; MPIF-1: myeloid progenitor inhibitory factor 1; CC16: Clara cell secretory protein 16 kDa; A-SAA: serum amyloid A; KGF: keratinocyte growth factor; ICAM-1: intercellular adhesion molecule 1; PAI-1: plasminogen activator inhibitor 1; IGFBP-3: insulin-like growth factor binding protein 3; TM: thrombomodulin. *P-values <0.05.
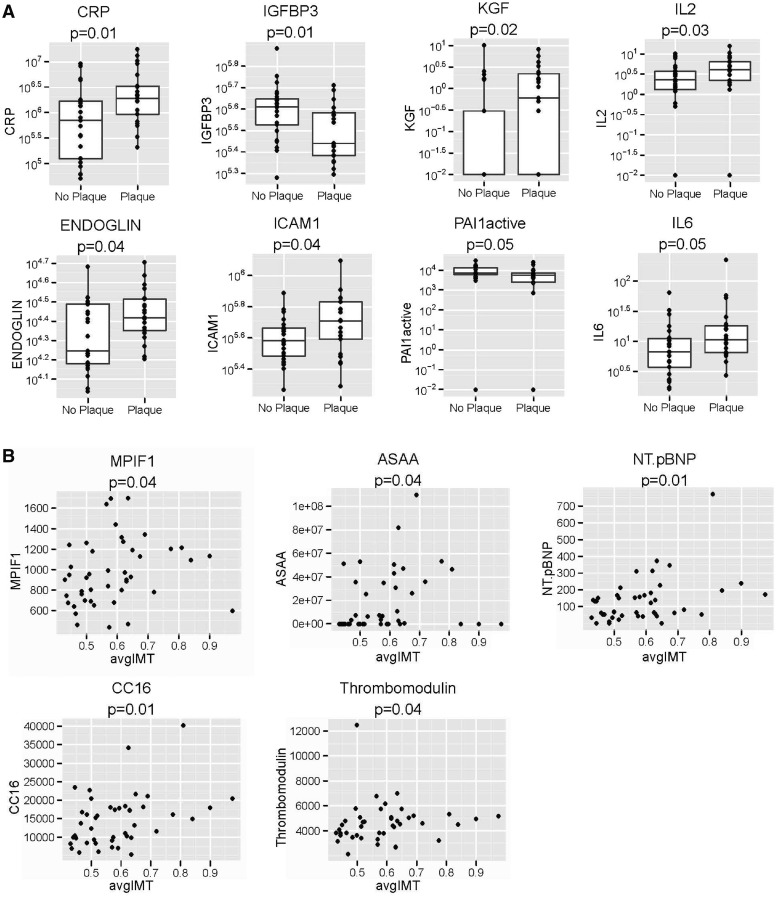
We found that eight proteins were associated with the presence of plaque (P < 0.05): CRP, insulin-like growth factor binding protein 3 (IGFBP-3), keratinocyte growth factor (KGF), IL-2, endoglin, intercellular adhesion molecule 1 (ICAM-1), plasminogen activator inhibitor 1 active (PAI-1) and IL-6 (Fig. 1A). Interestingly, five different proteins were associated with increased CIMT (P < 0.05): myeloid progenitor inhibitory factor 1 (MPIF-1), serum amyloid A (A-SAA), NT pro-BNP, Clara cell secretory protein 16 kD (CC16) and thrombomodulin (Fig. 1B). IGFBP-3 and PAI-1 were lower in patients with SSc-plaque, while the rest of the proteins were significantly higher (Fig. 1A). There were no differences in the piHDL, type I IFN and sJAM-A in SSc subjects vs controls (Table 2 and supplementary Tables S1and S2, available at Rheumatology Online).
Fig. 1.
(A) Proteins significantly associated with the presence/absence of carotid plaque; (B) proteins significantly associated with increased CIMT
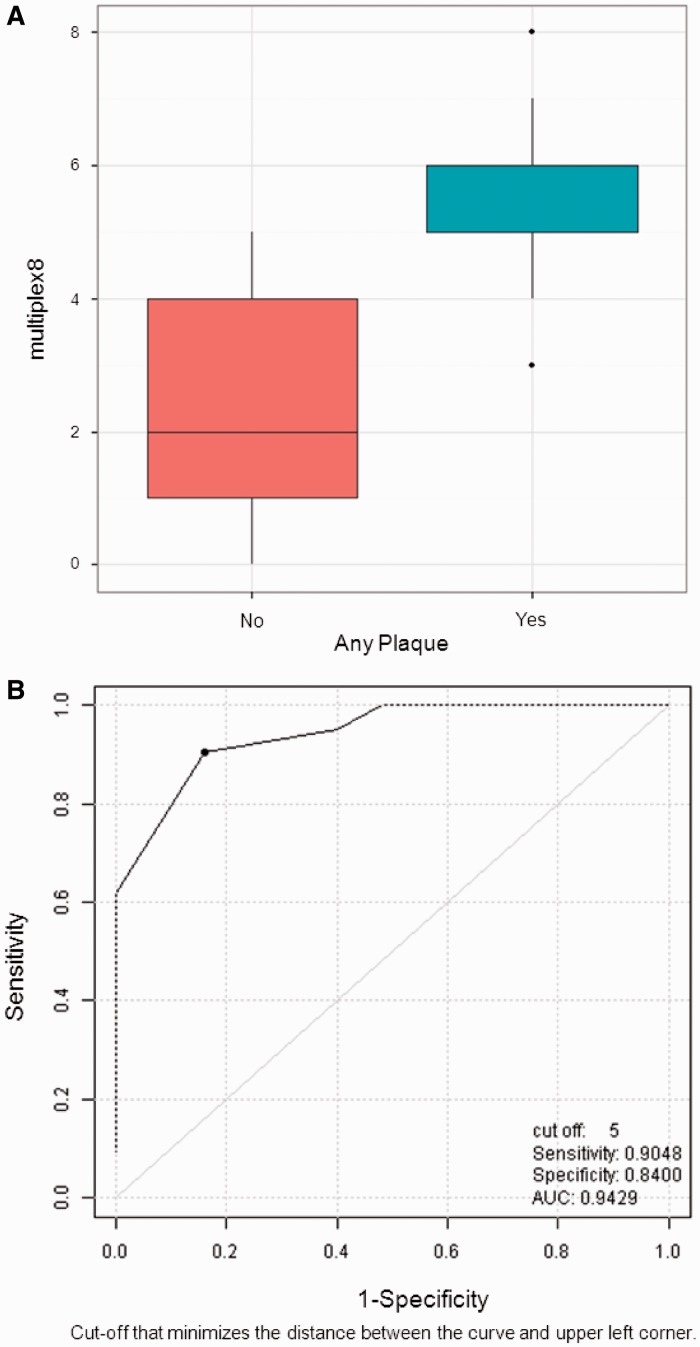
Association of plaque with multiplex proteins
We modelled the predictive value of the eight proteins associated with plaque at P < 0.05 (Table 3) by deriving a composite score. The median (25th–75th percentile) composite score for the plaque group was 6 (5–6) and for the no plaque group 2 (1–4), P < 0.0001 (Fig. 2A). The ROC analysis showed that the optimal cut point of the composite score for predicting plaque is five (Fig. 2B). Specifically, the presence of more than five of the eight proteins is associated with a sensitivity/specificity of 0.90/0.84 for having SSc-plaque.
Fig. 2.
(A) Composite score to model the predictive value of the eight proteins associated with plaque; (B) ROC analysis showing the ability of the five of eight proteins to predict the presence of plaque
Discussion
The association with ATS is well described in other CTDs, but remains controversial in SSc. The mortality due to macrovascular disease is not sufficiently understood [18], although a recent meta-analysis suggests increased CIMT and reduced flow-mediated dilatation (both associated with increased risk of CVD) in SSc [12]. In addition, a recent population-based cohort study using the Health Improvement Network from the UK found that in patients with SSc, the risk of incident MI and stroke were increased 2-fold, while the risk for peripheral vascular disease was increased 4-fold compared with patients without SSc [14]. A similar cross-sectional cohort study involving patients from the Australian Scleroderma Cohort Study showed that the odds ratio of coronary heart disease was 3.2 when adjusted for cardiovascular risk factors [13]. These recent observations have kindled interest in assessing the surrogate markers that are associated or play a role in the pathogenesis of SSc-ATS.
Our study demonstrates that subclinical ATS, as measured by carotid plaque, is significantly higher in patients with SSc compared with controls. We also found that certain novel proteins are independently associated with carotid plaque and CIMT and we have developed a composite score that discriminates patients with and without plaque.
SSc-ATS has been described in coronary arteries [19, 20] and the cerebrovascular vasculature [20], but the most robust data are derived from carotid US [12]. We elected to utilize carotid US for our study because it is a non-invasive measurement [21] and because it is a surrogate marker of ATS in the general population [22] and rheumatic diseases [11].
Carotid artery plaque burden is a strong predictor of atherosclerotic-related mortality, as evidenced by longitudinal and population studies [23]. Coronary angiography and the coronary calcium score in patients with SSc [24, 25] are not well studied. However, there has been discussion about the contribution of CIMT as an independent risk factor for CVD: analysis from the Rotterdam study showed the additional predictive value of CIMT for CVD risk is small [26]. A recent report addressing the impact of untreated hypertension in a cohort of treatment-naive patients showed that CIMT is increased starting at an early level of blood pressure (BP) elevation and that it further correlates with higher levels of BP [27]. Based on these reports, the CIMT measurement may reflect an intrinsic vascular reaction (such as media thickness) in response to hypertension rather than incipient plaque.
In our bivariate analysis, we found that the four proteins grouped under fibrosis were associated with increased CIMT in SSc, while the vasculopathy markers had greater associations with plaque (Table 3). The function and studies supporting the role of each protein in ATS are summarized in supplementary Table S3, available at Rheumatology Online.
Our analyses showed associations of serum cytokines that have been previously described in human ATS and RA, such as IL-6, TNF-α and CRP [28, 29]. We also discovered new associations of serum proteins that have not been described in CTD-ATS and may play a unique mechanistic role in SSc. MPIF-1 is a specific inhibitor of myeloid progenitor cells and is the most potent activator of monocytes [30], with a monomeric structure that makes MPIF-1 a more attractive therapeutic target [31]. MPIF-1 plays a role in the angiogenesis process and is involved in human ATS via up-regulation of MMP-2 [31, 32]. Vascular endothelial cells, when exposed to MPIF-1 in vitro, showed markedly enhanced migration ability [31]. CC16 is found in airway secretions and serum and has been described as a highly sensitive biomarker of altered lung epithelial permeability [33] and a potential marker for active SSc-related pulmonary fibrosis [34]. We also found significant associations with SSc-plaque and KGF, an epithelial growth factor that controls epithelial cell differentiation and enhances T cell immune reconstitution in murine models of allogeneic umbilical cord blood transplant [35]. It has been linked to angiogenesis and fibroblast biology [36], which makes it an interesting target in T cell–mediated SSc manifestations. The role of these novel findings need to be explored. It is yet unknown whether these proteins are associated with SSc-vasculopathy or play a role in SSc-ATS.
It is unclear why we found that patients with SSc-plaque had lower levels of PAI-1 active and IGFBP-3 (Fig. 1A). PAI-1 is the main inhibitor of the plasminogen activator and has been found to be elevated in ATS, RA and SLE [37]. A possible explanation could be the fact that aside from a circadian variation, PAI-1 active spontaneously converts to latent forms [37]. IGFBP-3 has been found to be elevated in both ATS [38] and SSc [39], but no data about SSc-ATS exists.
We explored a composite index that can differentiate patients with plaque vs no plaque. The advantage of a composite index is that it can increase accuracy and reduce the variability associated with individual markers [40]. The eight-protein index is the first step in exploring the association with carotid plaque and has been successful in recent work by Farina et al. [40], where the four-gene biomarker was found to have a higher association with skin score than individual genes. Future studies need to assess if the eight-protein index has a higher association or is predictive of SSc-ATS as assessed by the presence of plaque and hard events.
Abnormally functioning piHDL has been associated with ATS in the general population, in subjects with SLE or RA [41, 42] and in a small SSc study [24]. piHDL may increase the atherosclerotic risk by potentiating LDL oxidation [43]. In our study there was no difference in piHDL relative to plaque or CIMT (P = 0.09), which might be explained by the heterogeneity of SSc and by the small sample size.
Recent evidence indicates that type I IFNs could play important roles in the development of vasculopathy and premature ATS in SLE [13, 17, 44]. IFN-α promotes an imbalance of endothelial damage and repair by interfering with proper vasculogenesis [17], promoting foam cell formation [13] and platelet activation [10] in patients with SLE. Type I IFNs may be associated to the development of ATS in humans and murine models in the absence of overt autoimmune disease [14, 45] and has been reported in other CTDs to correlate well with disease activity [46]. In our relatively small study, type I IFN serum activity did not correlate with CIMT or plaque in SSc. It is still possible that type I IFNs may contribute to aberrant vascular repair in SSc.
sJAM-A is a novel vascular biomarker that has been shown to be increased in SSc compared with controls [47]. sJAM-A plays an important role in the inflammatory thrombosis leading to atherogenesis [48] and correlates with the severity of coronary artery disease [49]. In this study there was no association between CIMT or plaque, which questions the role of sJAM-A in SSc-ATS.
Strengths and limitations
Our study is the first to look at correlations between subclinical SSc-ATS and serum proteins. We were able to identify a distinct set of proteins that could predict the presence of atherosclerotic plaque in patients with SSc.
Our study is not without limitations. First, it is limited by the lack of a control group for the serum proteins study (we only compared the piHDL and type I IFN in both cases and controls). In addition, some of the observed differences in the protein levels might be secondary to confounders such as age, ethnicity or treatment regimens that were not accounted for in the present study. Another major limitation of our study is our sample size. Although we matched for age and race, we found some differences in the CVD risk factors (i.e. smoking, history of diabetes). It is well known that SSc is phenotypically heterogeneous, and our small sample size did not allow for subclassification of the disease. Although we used carotid US to measure subclinical ATS, our study did not include measures of endothelial function such as flow-mediated dilatation or capture hard events such as myocardial infarction or cerebrovascular events. Although the role of steroid exposure and aspirin use are well defined in the pathophysiology of ATS, our study did not collect data on a cohort of patients large enough to permit further analysis. We recorded the presence/absence of diabetes mellitus but did not include glycosylated haemoglobin or microalbuminuria. Lastly, our study has the limitations of a cross-sectional design. The relationship between progression of atherosclerotic plaque and various serum proteins found to have significant associations still needs to be studied prospectively.
Conclusions
In conclusion, our study suggests that patients with SSc have a higher prevalence of carotid plaque than matched controls, and patients with SSc-ATS (as defined by presence of carotid plaque) have elevated ATS serum proteins that are implicated in both vasculopathy and fibrosis. Further prospective studies are needed to validate our preliminary data and evaluate the pathophysiological role of ATS-associated cytokines in SSc-related vasculopathy.
Rheumatology key messages.
Scleroderma patients have increased carotid plaque compared with age- and race-matched controls.
Certain serum proteins implicated in vasculopathy/fibrosis are higher in SSc-plaque.
Supplementary Material
Acknowledgements
K.M.A. and this project were supported by the ACR Research and Education Foundation (REF) Physician Scientist Development Award. D.K. is supported by the National Institutes of Health. D.E.F. is partially supported by the National Institutes of Health. The sJAM-A assay was done with funds from Jonathan and Lisa Rye and the Marvin and Betty Danto Scleroderma Research Endowments to the University of Michigan. The type I IFN assay was done in M.J.K.’s laboratory, which is supported by the National Institutes of Health. Authors’ contributions: E.S. analysed the data and wrote the first draft of the manuscript; K.M.A. designed the protocol, carried out the project, collected the data, organized it and drafted the manuscript; M.A.M., M.J.K., W.Z. and A.D. carried out the biomarker measurements and significantly contributed to the writing of the manuscript; R.R.S. and N.R. performed the radiology portion of the study and contributed to the design and the manuscript; D.E.F. and P.J.C. reviewed the design and the manuscript; P.M. did all the statistical analysis and D.K. conceived the study and coordinated the final version of the manuscript. All authors read and approved the final manuscript.
Funding statement: The study was partly funded by the National Institutes of Health/NIAMS (grants K23 AR053858 and K24 AR063120) to D.K.
Disclosure statement: E.S. was a member of the United Therapeutics Speaker’s Bureau until April 2013 and received grants/research support from United Therapeutics, Actelion, Medimmune, Celgene, InterMune, BMS, Pfizer, and MediQuest. D.K. is a consultant or serves on speaker bureaus for Actelion, Bayer, Gilead, Roche, Merck, InterMune, DIGNA, and United Therapeutics. D.F. has received grants or research support from AbbVie, Actelion, Amgen, BMS, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech and UCB; is a consultant for and has received honoraria from AbbVie, Actelion, Amgen, BMS, Janssen, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech and UCB and has served on speaker’s bureaus (CME only) for AbbVie, Actelion and UCB. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Asanuma Y, Oeser A, Shintani A, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407–15. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 2.Rho Y, Chung C, Oeser A, et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009;61:1580–85. doi: 10.1002/art.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soltész P, Kerekes G, Dér H, et al. Comparative assessment of vascular function in autoimmune rheumatic diseases: considerations of prevention and treatment. Autoimmun Rev. 2011;10:416–25. doi: 10.1016/j.autrev.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill S, Giles I, Lambrianides A, et al. Antibodies to apolipoprotein A-I, high-density lipoprotein, and C-reactive protein are associated with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2010;62:845–54. doi: 10.1002/art.27286. [DOI] [PubMed] [Google Scholar]
- 5.McMahon M, Grossman J, Skaggs B, et al. Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheum. 2009;60:2428–37. doi: 10.1002/art.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borba E, Bonfá E. Dyslipoproteinemias in systemic lupus erythematosus: influence of disease, activity, and anticardiolipin antibodies. Lupus. 1997;6:533–9. doi: 10.1177/096120339700600610. [DOI] [PubMed] [Google Scholar]
- 7.Hansson G. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 8.Ihn H, Sato S, Fujimoto M, et al. Characterization of autoantibodies to endothelial cells in systemic sclerosis (SSc): association with pulmonary fibrosis. Clin Exp Immunol. 2000;119:203–9. doi: 10.1046/j.1365-2249.2000.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou Y, Rabquer B, Gerber M, et al. Junctional adhesion molecule-A is abnormally expressed in diffuse cutaneous systemic sclerosis skin and mediates myeloid cell adhesion. Ann Rheum Dis. 2010;69:249–54. doi: 10.1136/ard.2008.102624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manetti M, Guiducci S, Ibba-Manneschi L, et al. Mechanisms in the loss of capillaries in systemic sclerosis: angiogenesis versus vasculogenesis. J Cell Mol Med. 2010;14:1241–54. doi: 10.1111/j.1582-4934.2010.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tyrrell P, Beyene J, Feldman B, et al. Rheumatic disease and carotid intima-media thickness: a systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2010;30:1014–26. doi: 10.1161/ATVBAHA.109.198424. [DOI] [PubMed] [Google Scholar]
- 12.Au K, Singh M, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum. 2011;63:2078–90. doi: 10.1002/art.30380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ngian G, Sahhar J, Proudman S, et al. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis. 2012;71:1980–3. doi: 10.1136/annrheumdis-2011-201176. [DOI] [PubMed] [Google Scholar]
- 14.Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis. 2013;72:1188–93. doi: 10.1136/annrheumdis-2012-202007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preliminary criteria for the classification of systemic sclerosis (scleroderma) Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 16.Ansell B, Navab M, Hama S, et al. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 2003;108:2751–6. doi: 10.1161/01.CIR.0000103624.14436.4B. [DOI] [PubMed] [Google Scholar]
- 17.Denny M, Thacker S, Mehta H, et al. Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood. 2007;110:2907–15. doi: 10.1182/blood-2007-05-089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nussinovitch U, Shoenfeld Y. Atherosclerosis and macrovascular involvement in systemic sclerosis: myth or reality. Autoimmun Rev. 2011;10:259–66. doi: 10.1016/j.autrev.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 19.D’Angelo W, Fries J, Masi A, et al. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med. 1969;46:428–40. doi: 10.1016/0002-9343(69)90044-8. [DOI] [PubMed] [Google Scholar]
- 20.Youssef P, Brama T, Englert H, et al. Limited scleroderma is associated with increased prevalence of macrovascular disease. J Rheumatol. 1995;22:469–72. [PubMed] [Google Scholar]
- 21.Salonen R, Haapanen A, Salonen J. Measurement of intima-media thickness of common carotid arteries with high-resolution B-mode ultrasonography: inter- and intra-observer variability. Ultrasound Med Biol. 1991;17:225–30. doi: 10.1016/0301-5629(91)90043-v. [DOI] [PubMed] [Google Scholar]
- 22.Zureik M, Ducimetière P, Touboul P, et al. Common carotid intima-media thickness predicts occurrence of carotid atherosclerotic plaques: longitudinal results from the Aging Vascular Study (EVA) study. Arterioscler Thromb Vasc Biol. 2000;20:1622–9. doi: 10.1161/01.atv.20.6.1622. [DOI] [PubMed] [Google Scholar]
- 23.Störk S, van den Beld A, von Schacky C, et al. Carotid artery plaque burden, stiffness, and mortality risk in elderly men: a prospective, population-based cohort study. Circulation. 2004;110:344–8. doi: 10.1161/01.CIR.0000134966.10793.C9. [DOI] [PubMed] [Google Scholar]
- 24.Khurma V, Meyer C, Park G, et al. A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum. 2008;59:591–7. doi: 10.1002/art.23540. [DOI] [PubMed] [Google Scholar]
- 25.Tarek e-G, Yasser A, Gheita T. Coronary angiographic findings in asymptomatic systemic sclerosis. Clin Rheumatol. 2006;25:487–90. doi: 10.1007/s10067-005-0073-5. [DOI] [PubMed] [Google Scholar]
- 26.del Sol A, Moons K, Hollander M, et al. Is carotid intima-media thickness useful in cardiovascular disease risk assessment? The Rotterdam Study. Stroke. 2001;32:1532–8. doi: 10.1161/01.str.32.7.1532. [DOI] [PubMed] [Google Scholar]
- 27.Pasha S, Wiria A, Wammes L, et al. Blood pressure class and carotid artery intima-media thickness in a population at the secondary epidemiological transition. J Hypertens. 2011;29:2194–200. doi: 10.1097/HJH.0b013e32834bbba8. [DOI] [PubMed] [Google Scholar]
- 28.Protogerou A, Zampeli E, Fragiadaki K, et al. A pilot study of endothelial dysfunction and aortic stiffness after interleukin-6 receptor inhibition in rheumatoid arthritis. Atherosclerosis. 2011;219:734–6. doi: 10.1016/j.atherosclerosis.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Kerekes G, Soltész P, Szucs G, et al. Effects of adalimumab treatment on vascular disease associated with early rheumatoid arthritis. Isr Med Assoc J. 2011;13:147–52. [PubMed] [Google Scholar]
- 30.Rajarathnam K, Li Y, Rohrer T, et al. Solution structure and dynamics of myeloid progenitor inhibitory factor-1 (MPIF-1), a novel monomeric CC chemokine. J Biol Chem. 2001;276:4909–16. doi: 10.1074/jbc.M005085200. [DOI] [PubMed] [Google Scholar]
- 31.Son K, Hwang J, Kwon B, et al. Human CC chemokine CCL23 enhances expression of matrix metalloproteinase-2 and invasion of vascular endothelial cells. Biochem Biophys Res Commun. 2006;340:498–504. doi: 10.1016/j.bbrc.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 32.Kim C, Kang J, Cho H, et al. Potential involvement of CCL23 in atherosclerotic lesion formation/progression by the enhancement of chemotaxis, adhesion molecule expression, and MMP-2 release from monocytes. Inflamm Res. 2011;60:889–95. doi: 10.1007/s00011-011-0350-5. [DOI] [PubMed] [Google Scholar]
- 33.Hermans C, Knoops B, Wiedig M, et al. Clara cell protein as a marker of Clara cell damage and bronchoalveolar blood barrier permeability. Eur Resp J. 1999;13:1014–21. doi: 10.1034/j.1399-3003.1999.13e14.x. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa M, Fujimoto M, Hamaguchi Y, et al. Use of serum clara cell 16-kDa (CC16) levels as a potential indicator of active pulmonary fibrosis in systemic sclerosis. J Rheumatol. 2011;38:877–84. doi: 10.3899/jrheum.100591. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Chen G, Qiao S, et al. Keratinocyte growth factor enhanced immune reconstitution in murine allogeneic umbilical cord blood cell transplant. Leuk Lymphoma. 2011;52:1556–66. doi: 10.3109/10428194.2011.573037. [DOI] [PubMed] [Google Scholar]
- 36.Peng C, He Q, Luo C. Lack of keratinocyte growth factor retards angiogenesis in cutaneous wounds. J Int Med Res. 2011;39:416–23. doi: 10.1177/147323001103900209. [DOI] [PubMed] [Google Scholar]
- 37.Lijnen H. Pleiotropic functions of plasminogen activator inhibitor-1. J Thromb Haemost. 2005;3:35–45. doi: 10.1111/j.1538-7836.2004.00827.x. [DOI] [PubMed] [Google Scholar]
- 38.Martin R, Gunnell D, Whitley, et al. Associations of insulin-like growth factor (IGF)-I, IGF-II, IGF binding protein (IGFBP)-2 and IGFBP-3 with ultrasound measures of atherosclerosis and plaque stability in an older adult population. J Clin Endocrinol Metab. 2008;93:1331–8. doi: 10.1210/jc.2007-2295. [DOI] [PubMed] [Google Scholar]
- 39.Hamaguchi Y, Fujimoto M, Matsushita T, et al. Elevated serum insulin-like growth factor (IGF-1) and IGF binding protein-3 levels in patients with systemic sclerosis: possible role in development of fibrosis. J Rheumatol. 2008;35:2363–71. doi: 10.3899/jrheum.080340. [DOI] [PubMed] [Google Scholar]
- 40.Farina G, Lafyatis D, Lemaire R, et al. A four-gene biomarker predicts skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 2010;62:580–8. doi: 10.1002/art.27220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMahon M, Grossman J, FitzGerald J, et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54:2541–9. doi: 10.1002/art.21976. [DOI] [PubMed] [Google Scholar]
- 42.Charles-Schoeman C, Khanna D, Furst DE, et al. Effects of high-dose atorvastatin on antiinflammatory properties of high density lipoprotein in patients with rheumatoid arthritis: a pilot study. J Rheumatol. 2007;34:1459–64. [PubMed] [Google Scholar]
- 43.Navab M, Hama S, Hough G, et al. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res. 2001;42:1308–17. [PubMed] [Google Scholar]
- 44.Szucs G, Tímár O, Szekanecz Z, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis—relevance for prevention of vascular complications. Rheumatology. 2007;46:759–62. doi: 10.1093/rheumatology/kel426. [DOI] [PubMed] [Google Scholar]
- 45.LeRoy E, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 46.Higgs B, Liu Z, White B, et al. Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann Rheum Dis. 2011;70:2029–36. doi: 10.1136/ard.2011.150326. [DOI] [PubMed] [Google Scholar]
- 47.Hou Y, Rabquer B, Gerber M, et al. Junctional adhesion molecule-A is abnormally expressed in diffuse cutaneous systemic sclerosis skin and mediates myeloid cell adhesion. Ann Rheum Dis. 2010;69:249–54. doi: 10.1136/ard.2008.102624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostermann G, Fraemohs L, Baltus T, et al. Involvement of JAM-A in mononuclear cell recruitment on inflamed or atherosclerotic endothelium: inhibition by soluble JAM-A. Arterioscler Thromb Vasc Biol. 2005;25:729–35. doi: 10.1161/01.ATV.0000157154.14474.3b. [DOI] [PubMed] [Google Scholar]
- 49.Cavusoglu E, Kornecki E, Sobocka M, et al. Association of plasma levels of F11 receptor/junctional adhesion molecule-A (F11R/JAM-A) with human atherosclerosis. J Am Coll Cardiol. 2007;50:1768–76. doi: 10.1016/j.jacc.2007.05.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.