Abstract
Purpose
This study was performed to assess the usefulness of 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) or PET/computed tomography (CT) for distinguishing thymic epithelial tumors according to World Health Organization (WHO) classifications.
Methods
We analyzed a total of 45 patients (range, 29–75 years of age; mean, 55 years) with pathologically confirmed thymic epithelial tumors who underwent pretreatment 18F-FDG PET or PET/CT between November 2003 and October 2009. The size, visual grading of uptake value, peak standardized uptake value (SUVpeak), uptake pattern, and contour of each tumor, and associated findings on PET or PET/CT, were analyzed relative to the three simplified WHO subgroups: less-invasive thymomas (types A and AB), more-invasive thymomas (types B1, B2, and B3) and thymic carcinomas. We statistically assessed the relationship of 18F-FDG PET or PET/CT findings with these simplified subgroups.
Results
Of the 45 patients, ten had less-invasive thymomas, 23 had more-invasive thymomas, and 12 had thymic carcinomas. The SUVpeak of the less- and more-invasive thymomas were significantly lower than those of thymic carcinomas (p < 0.000), but there was no difference in SUVpeak between less- and more-invasive thymomas. The visual grading scale (p < 0.000), uptake pattern (p = 0.001), and contour (p < 0.000) of the tumors differed significantly among the three simplified subgroups.
Conclusion
The image findings of 18F-FDG PET or PET/CT differed significantly by histologic subgroups. Pre-treatment evaluation with 18F-FDG PET or PET/CT might be helpful in differentiating subgroups of thymic epithelial tumors.
Keywords: 18F-fluorodeoxyglucose, PET, Thymus, Thymoma, Thymic carcinoma
Introduction
According to the World Health Organization (WHO) classification system, thymic epithelial tumors can be classified into six types: thymomas A, AB, B1, B2, B3, and thymic carcinomas. Prognostic factors for thymic epithelial tumors include Masaoka stage, WHO histology, complete resection status, and size [1]. Surgery is the standard of care for patients with early-stage disease, whereas chemotherapy, with or without radiation therapy, has shown good results in patients with unresectable disease [1]. Recently, less invasive surgical procedures have been developed, including video-assisted thoracoscopic surgery (VATS) and robotic-assisted thoracoscopic surgery (RATS), both of which have been found effective as alternatives to conventional median sternotomy [2].
Information on the histologic type and invasiveness of each thymic epithelial tumor is needed to determine an appropriate therapeutic plan. However, conventional imaging modalities may be unable to differentiate among the various WHO histologic subtypes [3]. Recently, several studies were reported regarding the clinical role of positron emission tomography (PET) or PET/computed tomography (CT) in the evaluation of thymic epithelial tumors. Sung et al. [4] reported that 18F-fluorodeoxyglucose (18F-FDG) PET/CT was useful for differentiating among the subgroups of thymic epithelial tumors and for staging the extent of disease. Shibata et al. [5] reported that maximum standardized uptake value (SUVmax) in 18F-FDG PET and 11C-acetate PET could be used to predict the histologic type of thymoma. However, still the studies about the usefulness of 18F-FDG PET for the pretreatment evaluation of thymic epithelial tumor are limited. Therefore, we compared 18F-FDG PET findings among the patients with various histopathologically classified thymic epithelial tumors to determine whether 18F-FDG PET or PET/CT could be used at the pretreatment stage to distinguish between less-invasive thymomas, more-invasive thymomas, and thymic carcinomas.
Materials and methods
Subjects
Our patient cohort consisted of 52 consecutive patients with pathologically confirmed thymic epithelial tumors who underwent pretreatment 18F-FDG PET or PET/CT scans between November 2003 and October 2009 at our institution. Of these, we excluded six patients for whom we had no information on WHO histologic classification or whose biopsy specimens were obtained from metastatic lesions; and one patient because of errors in the measurement of his standard uptake value (SUV), which could not be adjusted because of the retrospective design of the study. Thus, we analyzed 45 thymic epithelial tumors in 45 patients (21 men, 24 women; mean age, 55 years; range, 29–75 years).
PET scanning
Imaging and data acquisition were performed using a PET or combined PET/CT system. All patients fasted for at least 6 h before injection of 18F-FDG, and mean blood glucose concentration before the scan was 100 mg/dl (range 67–137 mg/dl). Whole-body images were acquired from the skull bone to the upper thigh, starting approximately 60 min after intravenous injection of 518 ± 74 MBq 18F-FDG.
Six patients underwent whole-body PET scans with an ECAT Exact HR-Plus scanner (Siemens-CTI, Knoxville, Tenn.), consisting of a transmission scan of 4 min using a Ge-68 rotating pin source and an emission scan of 6 min per table position. PET images were reconstructed using an ordered-subsets expectation maximization algorithm for 16 subsets and two iterations (a voxel size of final image 0.264 cm3). The remaining 39 patients underwent whole-body PET/CT imaging with PET/CT systems (Biograph Sensation 16 or True Point 40; Siemens; Knoxville, Tenn.; or DSTe 8; GE, Milwaukee, Wis.), which could acquire coregistered CT and PET images from the same patient during a single imaging session. CT data were acquired first, followed by PET data, in the three-dimensional (3D) mode. The acquisition time for PET was 2.0–3.0 min per table position. CT data were used for attenuation correction of PET images, which were reconstructed using an ordered-subsets expectation maximization algorithm for 14–20 subsets and two to three iterations for all three PET/CT scanners. The image reconstruction matrix was 168 × 168 with the Biograph True Point 40 (a voxel size of final image 0.163 cm3), and 128 × 128 with the other scanners (a voxel size of final image 0.265 cm3 for Biograph Sensation 16 and 0.128 cm3 for DSTe 8), with a transverse field of view of 50 cm. The PET component of each scanner had an in-plane spatial resolution of 4.4–5.8 mm.
Histopathological examination
Twenty-six (58%) of the 45 patients underwent surgical resection and the remaining 19 underwent needle biopsy before chemotherapy or radiation therapy. We also recorded the results of preoperative needle biopsy for 13 (50%) of the 26 patients who underwent surgical resection; for these patients, we utilized the pathologic results obtained after surgical resection. However, nine patients were primarily diagnosed by needle biopsy and received chemotherapy before surgical resection; for these, we utilized the pathologic results of the initial biopsy. All specimens were reviewed by an experienced pathologist and assessed according to the WHO classification (thymoma types A, AB, B1, B2, and B3; and thymic carcinoma). The size of each of the 26 resected tumors, the presence of capsular invasion, and invasion of the surrounding structures were also reviewed.
All thymic epithelial tumors were divided into three subgroups: less-invasive thymomas (types A and AB), more-invasive thymomas (types B1, B2 and B3), and thymic carcinomas. The relationships between 18F-FDG PET or PET/CT findings and these groupings were assessed.
Image analysis
Two nuclear physicians, blinded to the final pathology results of each thymic epithelial tumor, retrospectively reviewed the PET or PET/CT images. The images were independently assessed on the scanner’s dedicated workstation in variable SUV window ranges and three orthogonal views, then the consensus on classification was reached by agreement. Size was determined as the longest diameter of each tumor. All SUVs were normalized to injected dose and patient lean body mass. To measure quantitatively the FDG tumor uptake, the peak SUV was obtained by drawing fixed sphere volume of interest centering around the hottest point in the tumor foci with a diameter of approximately 1.2 cm to produce a 1-cm3 volume [6]. For other image interpretations, we classified the uptake values of each tumor according to a visual grading scale (grade 1, lower than liver; grade 2, similar to liver; grade 3, greater than liver; grade 4, similar to brain) (Fig. 1). For each thymic tumor, the 18F-FDG uptake pattern was described as equivocal, homogeneous, or heterogeneous (Fig. 2). Homogeneous uptake was defined as uniform radiotracer uptake throughout the tumor; heterogeneous uptake was defined as irregular or uneven radiotracer uptake; and equivocal uptake was defined as neither homogeneous nor heterogeneous. The tumor contours were classified on PET-CT fusion images or only PET as round, lobulated, or infiltrative (Fig. 3), with round defined as showing similar width and length, lobulated as showing lobulation with smooth margins, and infiltrative as featuring an irregular external margin. The presence of metastatic lymph nodes, pleural seeding, and distant metastases on PET or PET/CT images was also reviewed.
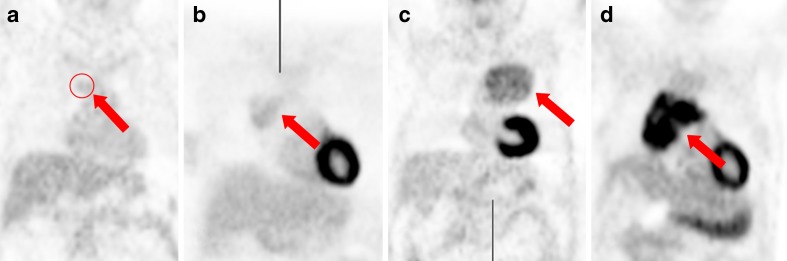
Fig. 1.
PET/CT images show four visual uptake grades of thymic epithelial tumors (arrows) of (a) grade 1 (less than liver), (b) grade 2 (similar to liver), (c) grade 3 (greater than liver), and (d) grade 4 (similar to brain)
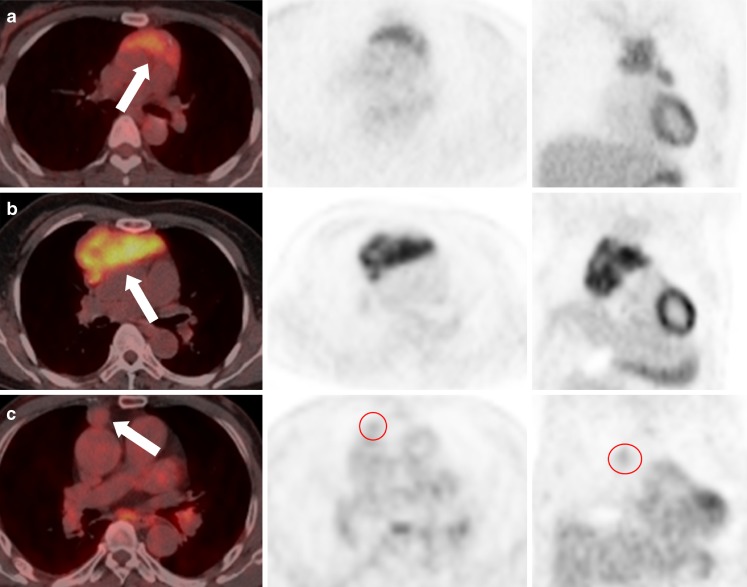
Fig. 2.
Three FDG uptake patterns of thymic epithelial tumors (arrows) are shown: (a) homogeneous, (b) heterogeneous, and (c) equivocal
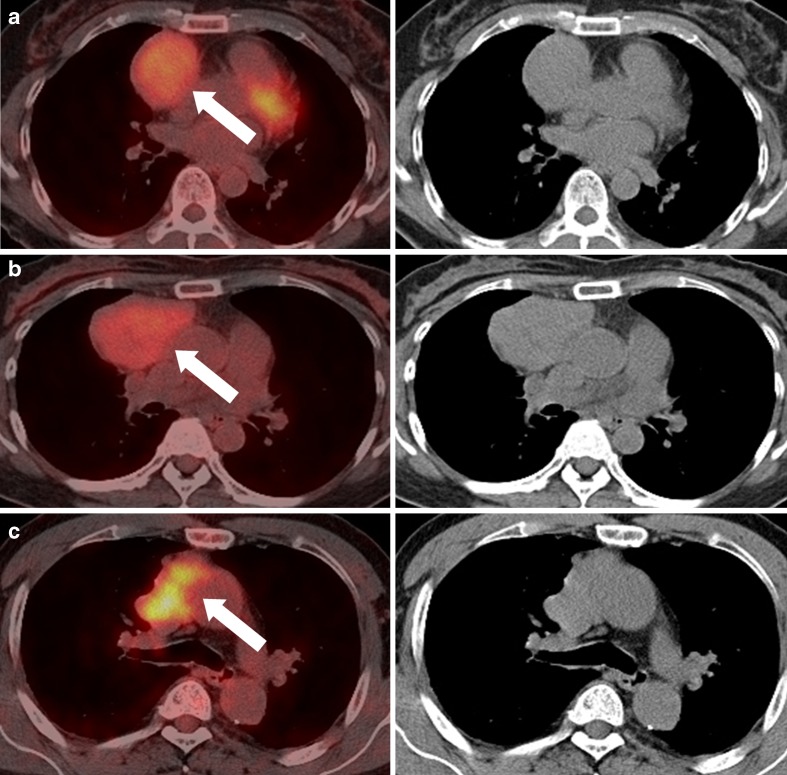
Fig. 3.
Three contours of thymic epithelial tumors (arrows) are classified as (a) round, (b) lobulated, and (c) infiltrative
Statistical analysis
The SUVpeak values of tumors classified into the three simplified WHO histological subgroups were compared using the Kruskal-Wallis test, and Mann-Whitney test with pairwise comparison were used to evaluate significant differences among subgroups. Fisher's exact test was employed to assess associations and tendencies of other categorical data from 18F-FDG PET or PET/CT among the three simplified subgroups. A p value of less than 0.05 was considered statistically significant. All statistical calculations and analyses were performed using the statistical software package SPSS (version 15; Statistical Package for the Social Sciences, Chicago, Ill.).
Results
Analysis of histopathology
Fluoroscopy– or CT-guided needle biopsy of 19 tumors in 19 patients showed that one was a less-invasive thymoma, eight were more-invasive thymomas, and ten were thymic carcinomas. Thymic epithelial tumors were surgically excised, by open thoracotomy or video-assisted thoracoscopic surgery (VATS), from 26 patients; of these, nine were less-invasive thymomas, 15 were more-invasive thymomas, and two were thymic carcinomas.
When we classified the 45 thymic epithelial tumors according to the simplified WHO histological classification, we found that ten were less-invasive thymomas (six type A, four type AB), 23 were more-invasive thymomas (15 type B1, five type B2, three type B3) and 12 were thymic carcinomas. Capsular invasion was observed in eight tumors, one less-invasive thymoma, four more-invasive thymomas, and two thymic carcinomas, and invasion of surrounding structures, such as the adjacent lung parenchyma or pleura, was noted in four tumors, one less-invasive and three more-invasive. Seven thymic epithelial tumors (two less-invasive, four more-invasive thymomas, and one thymic carcinoma) were found during staging workup or routine surveillance using 18F-FDG PET/CT in patients with tumors at other sites, including three with lung tumors, one with a liver tumor, one with a uterine tumor, and one with a germ cell tumor.
18F-FDG PET or PET/CT findings among the three tumor subgroups
Mean longest tumor diameters were 4.5 ± 2.9 cm (range, 1.4–11.0 cm) for less-invasive thymomas, 6.8 ± 3.5 cm (range, 2.6–20.1 cm) for more-invasive thymomas, and 6.6 ± 2.2 cm (range, 4.1–10.2 cm) for thymic carcinomas, with no statistical difference among the three subgroups (p = 0.1238).
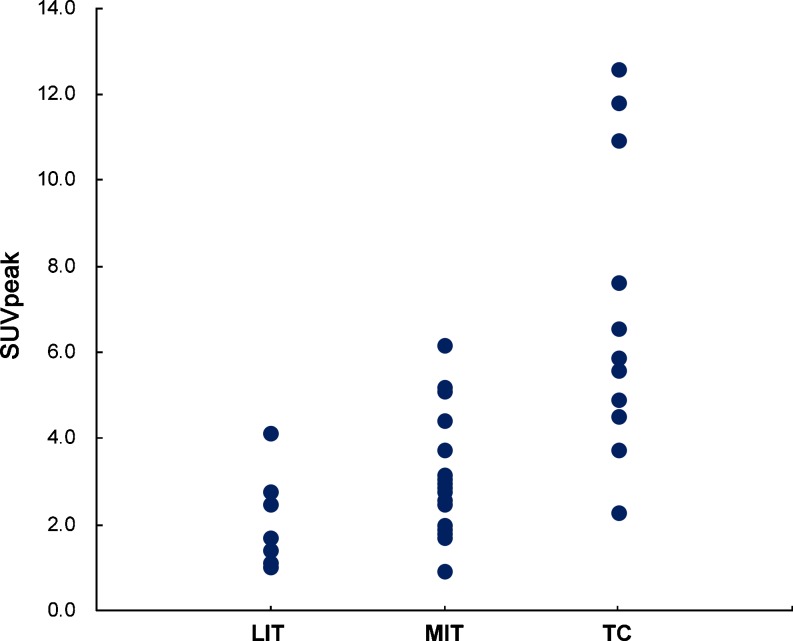
We observed a significant difference in the peak SUVs among the three subgroups. The SUVpeak of less-invasive thymomas (2.1 ± 1.0; range, 1.0–4.2; median, 2.1) was less than that of more-invasive thymomas (3.0 ± 1.3; range, 0.9–6.3; median, 2.6), which was in turn less than that of thymic carcinomas (6.8 ± 3.5; range, 2.3–12.9; median, 5.9) (p < 0.000) (Fig. 4). However, the difference between less—invasive thymomas and more-invasive thymomas was not statistically significant (p = 0.028, p < 0.017 for statistical significance).
Fig. 4.
This plot shows the distribution of the SUVpeak for each patient in the three subgroups of thymic epithelial tumors: LIT less-invasive thymomas, MIT more-invasive thymomas, TC thymic carcinomas
Table 1 shows the results of visual analysis of FDG PET and PET/CT images among the three simplified subgroups of thymic epithelial tumors. The visual uptake grading (p < 0.000), uptake pattern (p = 0.001), and contour of the tumor (p < 0.000) were significantly different among the three subgroups; in addition, there were statistically significant tendencies of visual uptake grading (p < 0.000), uptake pattern (p < 0.000) and contour of the tumor (p < 0.000) among the three subgroups. Furthermore, the differences and tendencies between less-invasive and more-invasive thymomas in visual uptake grading (p = 0.032, p = 0.007), uptake pattern (p = 0.011, p = 0.007), and contour (p = 0.006, p = 0.004) were also statistically significant. The predominant visual grading scale was grade 2 for less-invasive thymomas, observed in five tumors (50%); grade 3 for more-invasive thymomas, observed in 14 tumors (64%); and grade 4 for thymic carcinomas, observed in nine tumors (75%). Homogeneous uptake was observed in a higher proportion of less-invasive thymomas compared with more-invasive thymomas or thymic carcinomas, whereas heterogeneous uptake was observed in a higher proportion of thymic carcinomas compared with more- or less-invasive thymomas. Equivocal patterns were observed for three tumors, all less than 3 cm in size. A lobulated contour was observed in a higher proportion of more-invasive thymomas (83%) than in the other two types, whereas an infiltrative contour was observed in a higher proportion of thymic carcinomas (58%) compared with more- or less-invasive thymomas. Of the less-invasive thymomas, four had round contours and the remainder lobulated contours.
Table 1.
Visual analysis of FDG-PET or PET/CT images among three simplified subgroups of thymic epithelial tumors (LIT less-invasive thymoma, MIT more-invasive thymoma, TC thymic carcinoma)
| LIT (n = 10) | MIT (n = 23) | TC (n = 12) | Total (n = 45) | |
|---|---|---|---|---|
| Visual gradinga | ||||
| Grade 1 | 3 | 1 | 0 | 4 |
| Grade 2 | 5 | 6 | 0 | 11 |
| Grade 3 | 2 | 14 | 3 | 19 |
| Grade 4 | 0 | 2 | 9 | 11 |
| Uptake pattern | ||||
| Equivocal | 3 | 0 | 0 | 3 |
| Homogeneous | 5 | 9 | 1 | 15 |
| Heterogeneous | 2 | 14 | 11 | 27 |
| Contour | ||||
| Round | 4 | 0 | 0 | 4 |
| Lobulated | 6 | 19 | 5 | 30 |
| Infiltrative | 0 | 4 | 7 | 11 |
aGrade 1, lower than liver; grade 2, similar to liver; grade 3, greater than liver; grade 4, similar to brain
Based on the results of 18F-FDG PET or PET/CT scans, lymph node metastasis was suspected in eight patients (one with a more-invasive thymoma and seven with thymic carcinomas), pleural seeding in nine patients (one with a less-invasive thymoma, four with more-invasive thymomas, and four with thymic carcinomas) and distant metastasis in six patients (two with more-invasive thymomas and four with thymic carcinomas).
Discussion
Our results suggest that findings on 18F-FDG PET or PET/CT scans differed significantly among the histologic subgroups of thymic epithelial tumor. The SUVpeak of thymic carcinomas was significantly higher than that of both less- and more-invasive thymomas. Although SUVpeak did not differ significantly between less– and more-invasive thymomas, the visual uptake grading, uptake pattern, and contour differed significantly. Thus, imaging findings may help to distinguish less– from more-invasive thymomas, as well as thymic carcinomas from thymomas.
Pretreatment evaluation of the invasiveness of a thymic epithelial tumor is important in determining a treatment plan and the extent of surgery, if needed. Types A and AB generally behave like benign tumors, whereas type B1 is a low-grade malignant tumor, type B2 has a greater degree of malignancy, and type B3 has a poor prognosis, similar to that of thymic carcinoma [5]. Most medullary (WHO A) and mixed histology (WHO AB) tumors appear noninvasive, corresponding to Masaoka stages I and II, whereas cortical (WHO B1, 2 and 3) thymomas appear more invasive and occur more commonly as stage III and IV lesions [1]. No significant difference in survival has been observed between patients with stage I and II disease [7]. Furthermore, type A and AB thymomas were shown to be less frequently associated with myasthenia gravis than tumors of type B [8]. Whenever possible, more-invasive thymomas and invasive carcinomas should be completely resected, as ectopic thymic tissue may have a negative impact on local control and survival [9, 10]. In contrast, less-invasive thymomas may be excised using VATS technology, which combines minimal invasiveness and an acceptable extent of resection [2, 11]. Fine-needle aspiration (FNA) biopsy can differentiate among mediastinal lesions and can be used to diagnose or classify thymomas histopathologically [12–14]. FNA, however, carries risks of needle track seeding of malignant cells; moreover, the small tissue volumes obtained may make histologic differentiation between thymomas and other anterior mediastinal masses difficult [10]. If a needle biopsy is not possible or is non-diagnostic, several invasive surgical methods can be utilized for further diagnostic evaluation [1]. 18F-FDG PET/CT scanning may be a noninvasive method of predicting WHO histological subtypes and invasiveness of thymic epithelial tumors.
Similar to this study, previous reports have attempted to use SUVmax to differentiate among histologic types of thymic epithelial tumors. For example, SUVmax of thymomas (low- and high-risk) was found to be significantly lower than that of thymic carcinomas, but SUVmax could not differentiate between high risk and low risk thymomas [4]. Moreover, the tumor/lung ratio obtained from fixed ROI did not differ between thymomas of different stages [15], and no difference in SUV obtained from fixed ROI was observed between invasive and noninvasive thymomas [16]. We used SUVpeak because the SUVmax may vary considerably according to scanner performance, matrix size, slice thickness, scanner diameter and reconstruction methods. Like SUVmax, a hottest point within the tumor was included in a fixed small volume of interest for the prediction of histologic type, because the histologic type of each thymoma is determined by its most malignant component [5]. Therefore, the results of SUVpeak in this study concur with SUVmax. In addition to assessing SUV, like previous reports, we utilized visual uptake grading for assessing the degree of FDG tumor uptake. Additionally, other findings, such as uptake pattern and contour, were used to further characterize subgroups of thymic epithelial tumors. Among the three subgroups, there were coherent tendencies in these findings.
Visual grading of the degree of 18F-FDG uptake has been used for tumor characterization. To the best of our knowledge, the application of visual uptake grading has not been reported previously in thymic epithelial tumors; thus, our results are the first to show a significant difference on 18F-FDG PET/CT between less- and more-invasive thymomas, not merely between thymic carcinomas and thymomas, even though visual grading is more simple than measuring SUVs. Sung et al. [4] reported that homogeneous 18F-FDG uptake pattern was observed more frequently in thymic carcinomas than in high-risk or low-risk thymomas. However, in our study an increasing tendency of heterogeneous 18F-FDG uptake pattern was seen in the order: less-invasive to more-invasive thymoma to thymic carcinoma. Our results were similar to that of Kumar et al. [17], who observed that a higher proportion of patch uptake was seen in thymic carcinoma than in high-risk thymomas or low-risk thymomas. Moreover, CT scans of thymic epithelial tumors showed that types A and AB thymomas were round, whereas most type B thymomas were flat with an irregular surface or lobulation [18]. Similarly, we found that all four round tumors were type A or AB thymomas. Thus, a round or oval thymoma is more likely to be type A or AB, suggesting that round tumors are less invasive.
Although our study population was relatively larger than in previous reports, our study had several limitations. First, it was retrospective in design and carried a selection bias, in that not all patients with thymic epithelial tumors underwent 18F-FDG PET or PET/CT scanning before treatment. Prospective studies, including patients with all types of anterior mediastinal masses in addition to thymic epithelial tumors, are needed to evaluate the accuracy and effectiveness of 18F-FDG PET/CT in pretreatment assessment. Second, some patients with presumed invasive subtypes underwent needle biopsy only, without final surgical resection. Considering the heterogeneity of thymic epithelial tumors, some of these patients may have had more invasive tumors, as assessed from surgically resected specimens. However, any discrepancy between the results of needle biopsy and surgical resection would have had only a small effect on treatment assessment because most of these tumors were advanced.
Pretreatment diagnoses based on the WHO classification system can assist during the assessment and treatment planning for patients with thymic epithelial tumors [19, 20]. We found that findings on 18F-FDG PET or PET/CT scans differed significantly among the three simplified tumor subgroups based on the WHO classification. Among thymic epithelial tumors, thymic carcinomas had a higher proportion with visual uptake grade 4, heterogeneous uptake pattern, lobulated or infiltrative contour and high metabolic activity, whereas less-invasive thymomas had a higher proportion with visual uptake grade 1 or 2, homogeneous uptake pattern, round or lobulated contour and low metabolic activity. The SUVpeak, along with other 18F-FDG PET or PET/CT findings such as visual uptake grading, uptake pattern, and overall contour, might be helpful in differentiating subgroups of thymic epithelial tumors before treatment, thus determining the best surgical method and indicating the need for preoperative induction therapy.
Acknowledgements
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A084136).
References
- 1.Wright CD. Management of thymomas. Crit Rev Oncol Hematol. 2008;65:109–120. doi: 10.1016/j.critrevonc.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Cheng YJ, Kao EL, Chou SH. Videothoracoscopic resection of stage II thymoma: prospective comparison of the results between thoracoscopy and open methods. Chest. 2005;128:3010–3012. doi: 10.1378/chest.128.4.3010. [DOI] [PubMed] [Google Scholar]
- 3.Jeong YJ, Lee KS, Kim J, Shim YM, Han J, Kwon OJ. Does CT of thymic epithelial tumors enable us to differentiate histologic subtypes and predict prognosis? AJR Am J Roentgenol. 2004;183:283–289. doi: 10.2214/ajr.183.2.1830283. [DOI] [PubMed] [Google Scholar]
- 4.Sung YM, Lee KS, Kim BT, Choi JY, Shim YM, Yi CA. 18F-FDG PET/CT of thymic epithelial tumors: usefulness for distinguishing and staging tumor subgroups. J Nucl Med. 2006;47:1628–1634. [PubMed] [Google Scholar]
- 5.Shibata H, Nomori H, Uno K, Sakaguchi K, Nakashima R, Iyama K, et al. 18F-fluorodeoxyglucose and 11C-acetate positron emission tomography are useful modalities for diagnosing the histologic type of thymoma. Cancer. 2009;115:2531–2538. doi: 10.1002/cncr.24278. [DOI] [PubMed] [Google Scholar]
- 6.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50:122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim DJ, Yang WI, Choi SS, Kim KD, Chung KY. Prognostic and clinical relevance of the World Health Organization schema for the classification of thymic epithelial tumors: a clinicopathologic study of 108 patients and literature review. Chest. 2005;127:755–761. doi: 10.1378/chest.127.3.755. [DOI] [PubMed] [Google Scholar]
- 8.Okumura M, Shiono H, Minami M, Inoue M, Utsumi T, Kadota Y, et al. Clinical and pathological aspects of thymic epithelial tumors. Gen Thorac Cardiovasc Surg. 2008;56:10–16. doi: 10.1007/s11748-007-0177-8. [DOI] [PubMed] [Google Scholar]
- 9.Augustin F, Schmid T, Sieb M, Lucciarini P, Bodner J. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery thymectomy. Ann Thorac Surg. 2008;85:S768–S771. doi: 10.1016/j.athoracsur.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 10.Tomaszek S, Wigle DA, Keshavjee S, Fischer S. Thymomas: review of current clinical practice. Ann Thorac Surg. 2009;87:1973–1980. doi: 10.1016/j.athoracsur.2008.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casey EM, Kiel PJ, Loehrer PJ., Sr Clinical management of thymoma patients. Hematol Oncol Clin N Am. 2008;22:457–473. doi: 10.1016/j.hoc.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Ali SZ, Erozan YS. Thymoma. Cytopathologic features and differential diagnosis on fine needle aspiration. Acta Cytol. 1998;42:845–854. doi: 10.1159/000331958. [DOI] [PubMed] [Google Scholar]
- 13.Chhieng DC, Rose D, Ludwig ME, Zakowski MF. Cytology of thymomas: emphasis on morphology and correlation with histologic subtypes. Cancer. 2000;90:24–32. doi: 10.1002/(SICI)1097-0142(20000225)90:1<24::AID-CNCR4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 14.Shin HJ, Katz RL. Thymic neoplasia as represented by fine needle aspiration biopsy of anterior mediastinal masses. A practical approach to the differential diagnosis. Acta Cytol. 1998;42:855–864. doi: 10.1159/000331959. [DOI] [PubMed] [Google Scholar]
- 15.Liu RS, Yeh SH, Huang MH, Wang LS, Chu LS, Chang CP, et al. Use of fluorine-18 fluorodeoxyglucose positron emission tomography in the detection of thymoma: a preliminary report. Eur J Nucl Med. 1995;22:1402–1407. doi: 10.1007/BF01791148. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki M, Kuwabara Y, Ichiya Y, Akashi Y, Yoshida T, Nakagawa M, et al. Differential diagnosis of thymic tumors using a combination of 11C-methionine PET and FDG PET. J Nucl Med. 1999;40:1595–1601. [PubMed] [Google Scholar]
- 17.Kumar A, Regmi SK, Dutta R, Kumar R, Gupta SD, Das P, et al. Characterization of thymic masses using 18F-FDG PET-CT. Ann Nucl Med. 2009;23:569–577. doi: 10.1007/s12149-009-0283-z. [DOI] [PubMed] [Google Scholar]
- 18.Tomiyama N, Johkoh T, Mihara N, Honda O, Kozuka T, Koyama M, et al. Using the World Health Organization Classification of thymic epithelial neoplasms to describe CT findings. AJR Am J Roentgenol. 2002;179:881–886. doi: 10.2214/ajr.179.4.1790881. [DOI] [PubMed] [Google Scholar]
- 19.Okumura M, Miyoshi S, Fujii Y, Takeuchi Y, Shiono H, Inoue M, et al. Clinical and functional significance of WHO classification on human thymic epithelial neoplasms: a study of 146 consecutive tumors. Am J Surg Pathol. 2001;25:103–110. doi: 10.1097/00000478-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Okumura M, Ohta M, Tateyama H, Nakagawa K, Matsumura A, Maeda H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer. 2002;94:624–632. doi: 10.1002/cncr.10226. [DOI] [PubMed] [Google Scholar]