Abstract
Purpose
We assessed the prognostic value of metabolic tumor volume (MTV) measured using18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) inpatients with locally advanced head and neck squamous cell carcinoma (HNSCC).
Methods
We retrospectively reviewed 56 patients (51 men, five women; mean age 56.0 ± 8.8years) who had locally advanced HNSCC and underwent FDG PET/CT for initial evaluation. All patients had surgical resection and radiotherapy with or without concurrent chemotherapy. The peak standardized uptake value (SUVpeak) and MTV of the target lesion, including primary HNSCC andmetastatic cervical lymph nodes, were measured from FDG PET/CT images. We compared SUVpeak, MTV, and clinicopathologic variables such as age, Eastern Cooperative Oncology Group (ECOG) performance status, pN stage, pT stage, TNM stage, histologic grade and treatment modality to disease-free survival (DFS) and overall survival (OS).
Results
On the initial FDG PET/CT scans, the median SUVpeak was 7.8 (range, 1.8-19.0) and MTV was17.0 cm3 (range, 0.1-131.0 cm3). The estimated 2-year DFS and OS rates were 67.2% and 81.8%. The cutoff points of SUVpeak 6.2 and MTV 20.7 cm3 were the best discriminative values for predicting clinical outcome. MTV and ECOG performance status were significantly related to DFS and OS on univariate and multivariate analyses (p < 0.05).
Conclusion
The MTV obtained from initial FDG PET/CT scan is a significant prognostic factor for disease recurrence and mortality in locally advanced HNSCC treated with surgery and radiotherapy with or without chemotherapy.
Keywords: FDG PET/CT, Head and neck squamous cell carcinoma, Prognostic factor, Metabolic tumor volume, Surgery
Introduction
In head and neck squamous cell carcinoma (HNSCC), identification of prognostic factors may allow the establishment of individualized treatment strategies that lead to improved results. Several clinical and pathological features of the patients with HNSCC have been reported as indicators of clinical outcome and predictors of tumor recurrence. These essentially include tumor size, the stage of tumor at presentation, extent of lymph node involvement, extracapsular lymph node spread, surgical margin involvement and anatomic subsite. However, despite careful evaluation of these factors, it is not possible to reliably predict the outcome of treatment in individual patients [1, 2].
Recently, the degree of 18F-fluorodeoxyglucose (FDG) accumulation assessed by positron emission tomography/computed tomography (PET/CT) has emerged as a new prognostic factor. Due to its biologic feature, FDG uptakes correlate with cell viability and proliferative activities [3–5]. In the past, a few authors have suggested that the degree of FDG uptake quantified as maximum standardized uptake value (SUVmax) is an independent factor predicting clinical outcome after treatment in HNSCC. These articles indicated that higher SUV of the primary tumor predicts a poorer clinical outcome [6–9].
Other authors, however, reported that among the prognostic factors for HNSCC in nonsurgical organ-preservation therapy, the gross tumor volume (GTV) obtained by CT or magnetic resonance imaging (MRI) is the most important parameter for predicting local control, and it is often superior to the pT stage for assessing the probability of cure [10].
Metabolic tumor volume (MTV) is defined as the volume of FDG activity in tumor measured by PET scans. MTV can be readily measured semiautomatically with a dedicated software algorithm. In previous studies, GTV measured from CT was found to correlate well with MTV from PET scans in HNSCC cases [11, 12]. The MTV of malignant tumor reflects not only the tumor burden but also the functional and biologic status of the tumor due to the biologic features of FDG, and MTV can be expected to be a significant novel quantitative biomarker in the management of HNSCC. The value of MTV was already identified in studies performed in patients with HNSCC who received radiotherapy (RT) with or without chemotherapy [11–13]. To our knowledge, there has been no report on MTV as a factor predicting the clinical outcome in HNSCC cases with treatment based on surgery.
The aim of the present study was to determine the significance of MTV as an independent prognostic factor predicting the clinical outcomes characterized by overall survival (OS) and disease-free survival (DFS) in HNSCC patients who received an operation and then radiation therapy with or without chemotherapy.
Materials and Methods
Patients
The population of this retrospective study consisted of patients with locally advanced HNSCC involving oropharynx, hypopharynx, oral cavity, larynx or only metastatic cervical lymph nodes, who had FDG PET/CT for initial staging between December 2003 and January 2009. Exclusion criteria were non squamous cell carcinoma histology, previous treatment, evidence of distant metastasis, and different models of PET/CT scanner. Cancers originating in the salivary gland, paranasal sinus, thyroid and skin were also excluded. A total of 56 patients were determined to be eligible for this study. All treatments consisted of surgical resection of the primary tumor with cervical lymph node dissection, followed by RT with or without concurrent chemotherapy. After the surgical resection, 15 patients (26.8%) underwent RT alone, and 41 patients (73.2%) received concurrent chemoradiation therapy (CCRT). All CCRTs were performed with cisplatin-based concurrent chemoradiation, but there were some case-specific modifications to select the most effective regimen (single-agent treatments with cisplatin in 23 patients, combination treatments with cisplatin and fluorouracil in 15 patients, cisplatin and docetaxel in two patients, and cisplatin and paclitaxel in one patient). RT was done with the total dose range from 50.4 to 60.6 Gy (mean 61.8 ± 3.5) for all 56 patients. The treatment modalities were determined after discussion among surgeons, oncologists, radiation oncologists, pathologists, radiologists and nuclear medicine physicians on the head and neck tumor board at our institution.
For follow-up, all patients were evaluated by physical examination, including endoscopy and imaging studies (CT, MRI, ultrasound scan, and/or FDG PET/CT), for follow-up twice or three times in the first year and then once or twice every subsequent year. Locoregional and distant failures were identified by these methods. Biopsy was performed when pathologic confirmation was needed.
This study was approved by the institutional review board at our institution. Informed consent was waived due to the retrospective design of this study.
FDG PET/CT
All patients fasted for at least 6 h before the FDG PET/CT study. An amount of 370-555 MBq of FDG was injected intravenously, and scanning began 60 min later. None of the patient had blood glucose level greater than 130 mg/dl before the injection. No intravenous contrast agent was administered. Studies were acquired on combined PET/CT in-line system, Biograph Duo (Siemens Medical Solutions, Knoxville, Tenn.). The acquisition time was 2-3 min per bed position. All patients were in supine position during PET/CT scanning. Precontrast CT began at the orbitomeatal line and progressed to the proximal thigh (130 kVp, 80 mAs, and 5 mm slice thickness). PET scan followed immediately over the same body region. The CT data were used for attenuation correction, and images were reconstructed using a standard ordered-subset expectation maximization (OSEM) algorithm (two iterations, eight subsets). The axial spatial intrinsic resolution of the system was 6.5 mm at the center of the field of view.
Measurement of SUV and MTV in FDG PET/CT
All FDG PET/CT images were reviewed at a workstation with fusion software (Syngo; Siemens Medical Solutions, Knoxville, Tenn.) that provided multiplanar reformatted images and displayed PET images with attenuation correction, CT images, and PET/CT fusion images. The images were closely reviewed for the detection of the region showing increased FDG uptake in head and neck by two nuclear medicine physicians who are board certified in both nuclear medicine and radiology. The peak SUV (SUVpeak) was defined as the highest FDG uptake value within the primary tumor and metastatic cervical lymphadenopathy in both sides of the neck, and was obtained by measuring SUVmax in transaxial views. PET positive cervical lymph nodes were defined as metastatic lymph nodes in the measurement of MTV and SUVpeak. As the primary tumor and metastatic lymph nodes were conglomerated and their boundary was indistinguishable in many cases, the SUVpeak could be either from primary tumor or PET positive lymph node.
MTV was defined as the summed volume in cubic centimeters (cm3) including the primary tumor and cervical metastatic lymphadenopathy on the bilateral neck, when present. The MTV was measured using a semiautomated contouring program on a Leonardo workstation (Siemens Medical Solutions, Knoxville, Tenn.), based on the tumor-to-background intensity ratio. In the measurement of targeted MTV, we set a fixed SUV cutoff value of 2.5, which was the most frequently used threshold value in previous studies measuring the MTV [14–16]. Each tumor identified was then segmented semiautomatically in three dimensions. The tumor boundaries were drawn large enough to incorporate target lesions, and in transaxial, coronal and sagittal planes to reduce the confounding influence by physiologically glucose-avid tissues such as salivary glands and benign conditions such as sinusitis (Fig. 1). Then, an isocontour connecting the outline of the target lesion showing SUV of 2.5 was set automatically, and all voxels with SUV over 2.5 within the isocontour were included in the MTV calculation by the software. After all of the hypermetabolic lesions were segmented, the software quantified the final MTV in cubic centimeters.
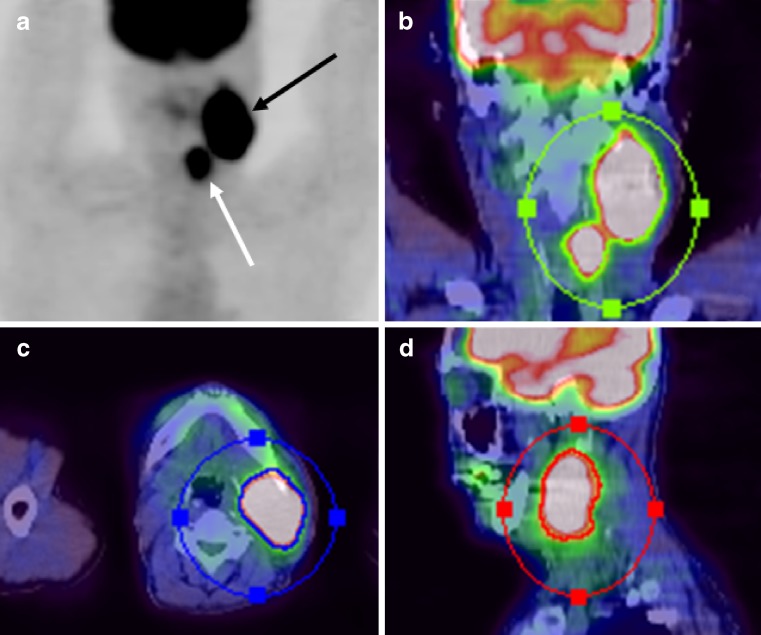
Fig. 1.
Metabolic tumor volume including primary site and regional metastatic lymphadenopathy of a 60-year-old man with hypopharyngeal squamous cell carcinoma (T2N3M0). a Maximal intensity projection (MIP) image of FDG PET/CT shows localized intense FDG uptake areas of primary site (white arrow) and metastatic lymph node (black arrow). The contour surrounding the target lesions inside the region of interest was semiautomatically produced in coronal (b), axial (c), and sagittal (d) planes from fusion images of FDG PET/CT. All voxels presenting SUV >2.5 within the contouring margin were incorporated to define the metabolic tumor volume
Statistical Analysis
All statistical analysis was performed using the SPSS windows 13.0 software (SPSS, Chicago, Ill.), except for the maximal chi-square method which was done by software R 2.12.0 (www.r-project.org). We used the Kaplan-Meier method for the estimation of DFS (with event defined as relapse at any site or death) and OS (with event defined as any cancer-related death) curves. The time interval for the end point was calculated from the date of operation to the date of death or the first clinical examination or imaging study that indicated locoregional recurrence or distant metastasis. The maximal chi-square method of Miller and Siegmund [17], and Halpern [18] was applied to determine the cutoff value of MTV which best dichotomized all patients into two subgroups with poor or good prognosis in terms of DFS. The same method was also adapted for SUVpeak to determine the cutoff point. As a result, SUVpeak 6.2 and MTV 20.7 cm3 were determined to be the cutoff points, which were used to categorize MTV and SUVpeak into two subgroups. Age was also divided into two subgroups by the same method resulting in 58 as a cutoff point. Univariate analysis using the log-rank test was applied to assess the differences of DFS and OS curves across SUVpeak, MTV, clinical and pathological variables such as age, Eastern Cooperative Oncology Group (ECOG) performance status, pT stage, pN stage, TNM stage, histologic grade and treatment modality. Cox proportional hazards model using forward conditional stepwise selection was performed for the multivariate analysis with variables that showed statistical significance in the univariate analysis. A value of p less than 0.05 was considered significant.
Result
Patient Characteristics
The clinical and pathological characteristics and treatment modality of total 56 patients are summarized in Table 1. The study group was composed of 51 male and five female patients with the mean age of 56.0 ± 8.8 years (range, 26-72 years). All patients included in this study were diagnosed with locally advanced HNSCC, including 11 TNM stage III (19.6%) and 45 IV (80.4%) cases. Distribution of the primary tumor sites was 16 in oropharynx (28.6%), 14 in oral cavity (25.0%), 12 in larynx (21.4%) and 11 in hypopharynx (19.6%). There were three patients (5.4%) with only cervical lymph node metastases which were histologically confirmed as squamous cell carcinoma and removed by lymph node dissection, but no definite primary tumor site identified on physical examination or imaging studies including CT, MRI and PET/CT. These lesions were also treated with RT or CCRT after the surgery. Median follow-up from the time of operation for all patients was 33.2 months (range, 3.0-65.3 months).
Table 1.
Clinical and pathological characteristics (n = 56)
| Characteristics | No. of patients (%) |
|---|---|
| Gender | |
| Male | 51 (91.1) |
| Female | 5 (8.9) |
| Patient age (years) | |
| Range | 26-72 |
| Mean | 56.0 ± 8.8 |
| ECOG performance status | |
| ECOG 0-1 | 44 (78.6) |
| ECOG 2-3 | 12 (21.4) |
| Primary tumor sites | |
| Oropharynx | 16 (28.6) |
| Oral cavity | 14 (25.0) |
| Larynx | 12 (21.4) |
| Hypopharynx | 11 (19.6) |
| MUO | 3 (5.4) |
| pT stage | |
| TX-2 | 27 (48.2) |
| T3-4 | 29 (51.8) |
| pN stage | |
| N0-1 | 15 (26.8) |
| N2-3 | 41 (73.2) |
| TNM stage | |
| III | 11 (19.6) |
| IV | 45 (80.4) |
| Histologic grade | |
| Well-differentiated | 15 (26.8) |
| Moderately differentiated | 36 (64.3) |
| Poorly differentiated | 5 (8.9) |
| Treatment modality | |
| Surgery with RT | 15 (26.8) |
| Surgery with CCRT | 41 (73.2) |
MUO metastasis of unknown origin (histologically confirmed as squamous cell carcinoma)
Among 56 patients, there were 15 patients with locoregional failure (26.8%), and ten patients with distant failure (17.9%), of whom six were also accompanied by locoregional failure. When last checked, 44 patients were alive and 12 patients had died. Five (33.3%) of the 15 cases with locoregional failure were manifested as cervical lymph node metastases. The distribution of distant metastases were: one case in mediastinal lymph node (10.0%), one in axillary lymph node (10.0), seven in lung (70.0%), and one in bone (10.0%). The 12 deaths were due to disease progression in five cases (41.6%), two from pneumonia (16.8%), and five of unspecified causes (41.6%). Locoregional failures were found in seven of 12 deaths, distant failures in seven deaths, and three deaths had both locoregional and distant failures.
Prognostic Values
The median MTV and SUVpeak of the target lesions in all 56 patients were 17.0 cm3 (range, 0.1-131.0 cm3) and 7.8 (range, 1.8-19.0). The median time from operation to locoregional or distant failure was 30.0 months (range 3.0-65.3 months). The median time from operation to death was 9.6 months (range 3.0-33.2 months). The Kaplan-Meier estimations of 2-year DFS and OS were 67.2% and 81.8%, respectively.
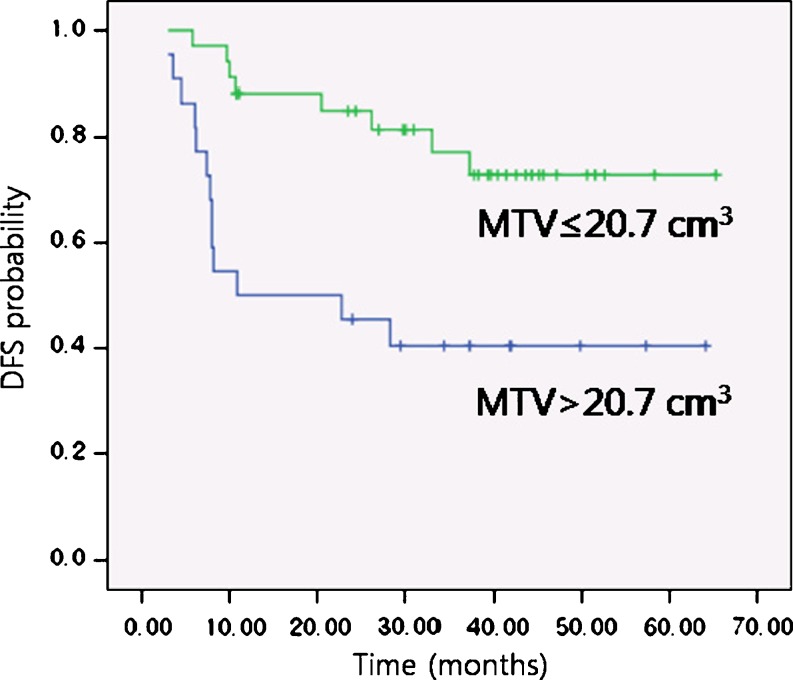
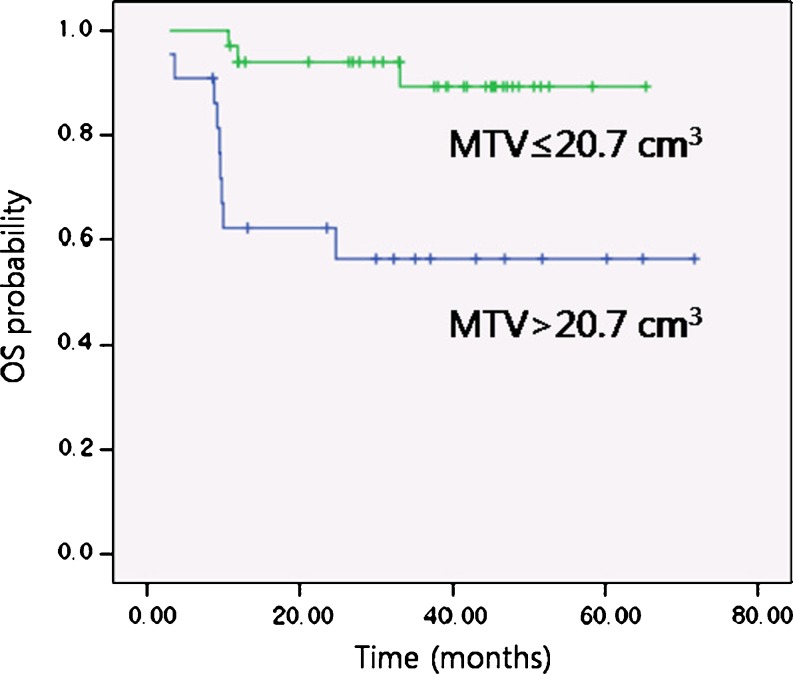
In the log-rank test, patients with lower MTV (≤20.7 cm3) showed significantly higher DFS (85.0% vs 45.5%; p = 0.002; Fig. 2) and OS (94.0% vs 62.2%; p = 0.001; Fig. 3) at 2 years compared with patients with higher MTV (>20.7 cm3) (Table 2). The patients with lower SUVpeak (≤6.2), however, did not demonstrate statistically significant difference in DFS (80.8% vs 64.7%; p value = 0.721) and OS (93.8% vs 77.0%; p = 0.238) at 2 years compared with those with higher SUVpeak (>6.2). The patients with better ECOG performance status (0-1) also demonstrated significantly higher DFS (79.1% vs 33.3%; p value < 0.001) and OS (93.1% vs 41.7%; p value < 0.001) at 2 years than those with worse ECOG performance status (2-3). Among the other clinicopathological variables, statistically significant difference in 2-year DFS was identified for age, whereas histologic grade, pT stage, pN stage, TNM stage, and treatment modality did not have a statistical significance in the prediction of disease outcome in 2-year DFS and OS. On multivariate analysis performed with Cox proportional hazards model, we selected age, MTV, and ECOG as covariates due to their statistical significance on log-rank test. We also added SUVpeak which was the variable of interest in this study. After adjustments were made for treatment modality as a significant confounding factor, MTV and ECOG performance status were identified as significant prognostic factors for DFS and OS at 2 years (p < 0.05) (Table 3).
Fig. 2.
Kaplan-Meier DFS probabilities for an MTV of 20.7 cm3 or less (n = 34) and an MTV higher than 20.7 cm3 (n = 22) (p = 0.002)
Fig. 3.
Kaplan-Meier OS probabilities for an MTV of 20.7 cm3 or less (n = 34) and an MTV higher than 20.7 cm3 (n = 22) (p = 0.001)
Table 2.
Log-rank test of clinical and therapeutic factors
| Factor | No. of patients | 2-year DFS (%) | p value | 2-year OS (%) | p value |
|---|---|---|---|---|---|
| Age | 0.017 | 0.072 | |||
| ≥58 | 30 | 60.6 | 72.1 | ||
| <58 | 26 | 76.5 | 89.9 | ||
| Histologic grade | 0.905 | 0.587 | |||
| WD | 15 | 64.3 | 78.6 | ||
| MD | 36 | 71.7 | 85.9 | ||
| PD | 5 | 80.0 | 80.0 | ||
| pT stage | 0.572 | 0.208 | |||
| TX-T2 | 27 | 74.1 | 88.5 | ||
| T3-T4 | 29 | 65.1 | 75.9 | ||
| pN stage | 0.183 | 0.373 | |||
| N0-N1 | 15 | 80.0 | 86.7 | ||
| N2-N3 | 41 | 65.4 | 80.1 | ||
| TNM stage | 0.572 | 0.208 | |||
| III | 11 | 74.1 | 88.5 | ||
| IV | 45 | 65.1 | 75.9 | ||
| ECOG | < 0.001 | < 0.001 | |||
| 0-1 | 44 | 79.1 | 93.1 | ||
| 2-3 | 12 | 33.3 | 41.7 | ||
| Treatment modality | 0.355 | 0.345 | |||
| Surgery with RT | 15 | 80.0 | 86.7 | ||
| Surgery with CCRT | 41 | 65.4 | 80.0 | ||
| SUVpeak | 0.721 | 0.238 | |||
| ≤6.2 | 16 | 80.8 | 93.8 | ||
| >6.2 | 40 | 64.7 | 77.0 | ||
| MTV (cm3) | 0.002 | 0.001 | |||
| ≤20.7 | 34 | 85.0 | 94.0 | ||
| >20.7 | 22 | 45.5 | 62.2 |
WD well differentiated, MD moderately differentiated, PD poorly differentiated, ECOG Eastern Cooperative Oncology Group performance status
Table 3.
Cox proportional hazards models for DFS and OS
| Parameter | DFS | OS | ||
|---|---|---|---|---|
| Hazard ratio | p | Hazard ratio | p | |
| MTVa | 0.251 | 0.003 | 0.174 | 0.011 |
| ECOGb | 0.197 | 0.001 | 0.116 | 0.001 |
aMTV ≤20.7 cm3 vs >20.7 cm3
bECOG performance status 0-1 vs 2-3
Discussion
The treatment for HNSCC depends on various factors. Physicians and patients often have the option of both surgical and nonsurgical treatments for tumors of similar stage. The final decision is based on an overall assessment of the risk of local recurrence, 5-year survival rates, treatment-associated morbidity, and institutional and patient preference [10]. All patients included in this study underwent surgical resection of primary tumor and metastatic cervical lymph nodes, and RT with or without concurrent chemotherapy. Treatment methods were determined after considering conventional prognostic factors by a multi-disciplinary cancer management team.
The well-established prognostic factors for lower survival rate in HNSCC are the presence of lymph node metastases, positive resection margins and poorly differentiated appearance with perineural or perivascular invasion [19]. In 2008, Le Tourneau et al. [20] performed a retrospective study on 308 consecutive patients who had HNSCC treated with surgery and postoperative RT, and found that only pT and pN stages were significant predictive factors for clinical outcome, and these results were compatible with those from another study on 420 patients done in 2001 [21]. On the other hand, other studies [22, 23] showed different results and pT and pN stages did not predict treatment outcome and survival. In the present study, pT and pN stage did not demonstrate a statistical significance either (Tables 2 and 3). However, the lack of significance in these studies and our study may be due to small number of cases.
FDG PET/CT has been widely used to evaluate patients with variable malignant tumors, including HNSCC. FDG PET/CT simultaneously providing anatomical and functional information is useful for the initial detection of primary tumor, metastases, synchronous primary tumor, RT planning, assessment of treatment response, and surveillance for tumor recurrence and metastases after treatment [1, 24]. FDG PET/CT imaging has shown high sensitivity, ranging from 82% to 90%, and performance superior to CT or MRI in the detection of primary tumor, regional nodal involvement and distant metastases for the evaluation of HNSCC [25, 26]. Furthermore, many recent studies have focused on the risk stratification of HNSCC using biologic parameters such as SUV and MTV assessed by FDG PET/CT. Several studies have shown that patients with HNSCC who had high SUVmax were associated with worse local control and poor survival rates, and should be considered for a more aggressive treatment approach [6–9]. Allal et al. [7] and Roh et al. [8] found that high FDG uptake correlated with advanced pT and pN stages. However, in several studies, the SUVmax of primary tumor or all lesions, including regional metastatic lymph nodes, was not confirmed as a significant factor predicting clinical outcome in the patients with HNSCC undergoing radiation therapy with or without chemotherapy [11–13, 26–28]. In our results, the lower SUVpeak of tumor tissue (≤6.2) did not demonstrate higher rates of DFS (p = 0.721) and OS (p = 0.238) with a statistical significance.
The pT stage is defined as the extent of primary tumor in terms of size and invasion into the adjacent structure. Although pT stage was used to reflect tumor extent, it is not representative of the three-dimensional tumor volume. Therefore, we can expect that tumor burden, a three-dimensional volumetric measurement, may be more reliable for the assessment of tumor extent, and serve as a better independent prognostic factor than pT stage [14]. Plataniotis et al. [29] found that GTV correlated with treatment outcomes for patients with locally advanced HNSCC treated with radiation therapy or radiation therapy plus chemotherapy. Several authors also reported that GTV obtained from CT scan was a significant predictor for clinical outcome in the treatment of HNSCC [10, 30–32]. A few articles suggested that the larger MR-derived GTV was associated with higher recurrence and poorer survival rates [33, 34]. In 2005, Chong et al. [35] demonstrated that MRI was superior to CT for depicting the gross extent of tumor infiltration, and positive correlation between MR-derived tumor volume and pT stage was demonstrated.
MTV is obtained by FDG PET/CT using dedicated software which measures the volume of FDG accumulation based on the high tumor-to-background intensity ratio after attenuation correction [12]. Because of the good correlation between the MTV and the GTV assessed by other imaging tools such as CT [11, 12], we can expected that MTV may be another important factor to be considered in the management of HNSCC. A study to compare CT, MRI, and FDG PET for delineation of tumor volume in pharyngolaryngeal squamous cell carcinoma with the results validated by surgical specimen indicated that FDG PET was the most accurate modality for measuring tumor volume [36].
There have been relatively few reports on MTV as an independent prognostic factor in the treatment of HNSCCs, compared with the many studies on GTV performed using CT or MRI. In the study of La et al. [12], with 85 HNSCC patients undergoing definitive radiation therapy with or without concurrent chemotherapy, univariate analysis showed a significant inverse relationship between MTV and DFS [likelihood ratio (LR) = 13.6, p < 0.001], and OS (LR = 11.7, p < 0.001). Furthermore, an increase in MTV of 17.4 ml was significantly associated with an increased hazard of recurrence or death. Seol et al. [11] demonstrated that an MTV of 9.3 cm3 or higher was significantly associated with an increased risk of recurrence (2.19-fold, p = 0.0006) and death (1.62-fold, p = 0.051) in 59 patients with HNSCC treated with chemoradiation therapy. Our study differs from these previous studies in that all 56 patients included in this study received surgical resection of primary tumor with lymph node dissection. Our results also indicated that MTV (cutoff volume = 20.7 cm3) is a significant independent predicting factor for 2-year DFS (85.0% vs 45.5%, p = 0.002) and OS (94.0% vs 62.2%, p = 0.001), whereas pT stage did not have a statistical significance for DFS and OS on univariate analysis (Table 2). However, we are cautious to say that MTV is more valuable than pT stage, as our study population was not large.
Performance status is a global assessment of the patient’s actual level of function and ability for self-care. It is a major prognostic factor, a predictor of the benefit and toxicity of treatments, as well as an indicator of comorbidity and other host factors [37]. Among several methods of assessment, Karnofsky’s Scale Performance Status (KPS) and ECOG Scale of Performance Status are widely used. A study by Lee et al. [38] demonstrated that KPS is an independent prognostic factor in the treatment of HNSCC. In the study of La et al. [12], KPS was also found to be a predictive factor for DFS and OS. In our study, ECOG performance status was also confirmed as a significant predictor for the clinical outcome on univariate and multivariate analyses (Tables 2 and 3). This is compatible with the conclusion that ECOG performance status is an important prognostic factor in HNSCC in the study by Rades et al. [39].
In this study, the limitations which have to be taken into consideration are the relatively small number of subjects, the retrospective nature of study design, the narrow window of tumor severity that included only TNM stages III and IV, the heterogeneity of primary tumor sites, the heterogeneity of radiation dose and chemotherapy regimen, and relatively short follow-up period. The small population was due to the strict inclusion criteria of the present study. We plan to perform a further study on the MTV of HNSCC as a prognostic factor with larger population in the future.
In addition to these limitations, there is the controversy about measuring MTV. A threshold SUV of 2.5 for segmentation of the target lesion in FDG PET/CT has been commonly applied to non small cell lung cancer, but the most appropriate threshold in HNSCC is yet to be determined. Further study is needed on accurate measurement of MTV and ideal threshold SUV. Nevertheless, the results from the present study clearly showed that MTV is associated with clinical outcome in patients with HNSCC following treatment with surgery and RT or CCRT. As mentioned above, the significance of MTV as an independent prognostic factor has been confirmed in recent studies on patients receiving nonsurgical methods [11–13]. The results from the patients who underwent surgery in this study were compatible with prior studies on nonsurgically treated patients. As a result, we believe that MTV should be seriously considered not only as the predictor of clinical outcome but also as a critical parameter when we determine the suitable treatment method for HNSCC.
Conclusion
The MTV of primary tumor and regional metastatic lymph nodes obtained from FDG PET/CT is a significant prognostic factor for disease recurrence and mortality in locally advanced HNSCC treated with surgery and RT with or without chemotherapy.
Acknowledgements
The research was supported by the Converging Research Center Program through the Ministry of Education, Science and Technology (2010 K001055).
References
- 1.Subramaniam RM, Truong M, Peller P, Sakai O, Mercier G. Fluorodeoxyglucose-positron-emission tomography imaging of head and neck squamous cell cancer. AJNR Am J Neuroradiol. 2009 doi: 10.3174/ajnr.A1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allal AS, Dulguerov P, Allaoua M, Haenggeli CA, el El-Ghazi A, Lehmann W, et al. Standardized uptake value of 2-[(18)F] fluoro-2-deoxy-D-glucose in predicting outcome in head and neck carcinomas treated by radiotherapy with or without chemotherapy. J Clin Oncol. 2002;20:1398–1404. doi: 10.1200/JCO.20.5.1398. [DOI] [PubMed] [Google Scholar]
- 3.Minn H, Clavo AC, Grénman R, Wahl RL. In vitro comparison of cell proliferation kinetics and uptake of tritiated fluorodeoxyglucose and L-methionine in squamous cell carcinoma of the head and neck. J Nucl Med. 1995;36:252–258. [PubMed] [Google Scholar]
- 4.Minn H, Joensuu H, Ahonen A, Klemi P. Fluorodeoxyglucose imaging: a method to assess the proliferative activity of human cancer in vivo. Comparison with DNA flow cytometry in head and neck tumors. Cancer. 1988;61:1776–1781. doi: 10.1002/1097-0142(19880501)61:9<1776::AID-CNCR2820610909>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Jacob R, Welkoborsky HJ, Mann WJ, Jauch M, Amedee R. [Fluorine-18]fluorodeoxyglucose positron emission tomography, DNA ploidy and growth fraction in squamous cell carcinoma of the head and neck. ORL J Otorhinolaryngol Relat Spec. 2001;63:307–313. doi: 10.1159/000055764. [DOI] [PubMed] [Google Scholar]
- 6.Minn H, Lapela M, Klemi PJ, Grénman R, Leskinen S, Lindholm P, et al. Prediction of survival with fluorine-18-fluoro-deoxyglucose and PET in head and neck cancer. J Nucl Med. 1997;38:1907–1911. [PubMed] [Google Scholar]
- 7.Allal AS, Slosman DO, Kebdani T, Allaoua M, Lehmann W, Dulguerov P. Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-[18F]fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys. 2004;59:1295–1300. doi: 10.1016/j.ijrobp.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 8.Roh JL, Pae KH, Choi SH, Kim JS, Lee S, Kim SB, et al. 2-[18F]-Fluoro-2-deoxy-D-glucose positron emission tomography as guidance for primary treatment in patients with advanced-stage resectable squamous cell carcinoma of the larynx and hypopharynx. Eur J Surg Oncol. 2007;33:790–795. doi: 10.1016/j.ejso.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Torizuka T, Tanizaki Y, Kanno T, Futatsubashi M, Naitou K, Ueda Y, et al. Prognostic value of 18F-FDG PET in patients with head and neck squamous cell cancer. AJR Am J Roentgenol. 2009;192:156–160. doi: 10.2214/AJR.08.1429. [DOI] [PubMed] [Google Scholar]
- 10.Mukherji SK, Schmalfuss IM, Castelijns J, Mancuso AA. Clinical applications of tumor volume measurements for predicting outcome in patients with squamous cell carcinoma of the upper aerodigestive tract. AJNR Am J Neuroradiology. 2004;25:1425–1432. [PMC free article] [PubMed] [Google Scholar]
- 11.Seol YM, Kwon BR, Song MK, Choi YJ, Shin HJ, Chung JS, et al. Measurement of tumor volume by PET to evaluate prognosis in patients with heal and neck cancer treated by chemo-radiation therapy. Acta Oncol. 2010;49:201–208. doi: 10.3109/02841860903440270. [DOI] [PubMed] [Google Scholar]
- 12.La TH, Filion EJ, Turnbull BB, Chu JN, Lee P, Nguyen K, et al. Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;74:1335–1341. doi: 10.1016/j.ijrobp.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung MK, Jeong HS, Park SG, Jang JY, Son YI, Choi JY, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res. 2009;15:5861–5868. doi: 10.1158/1078-0432.CCR-08-3290. [DOI] [PubMed] [Google Scholar]
- 14.Nestle U, Kremp S, Schaefer-Schuler A, Sebastian-Welsch C, Hellwig D, Rübe C, et al. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-small cell lung cancer. J Nucl Med. 2005;46:1342–1348. [PubMed] [Google Scholar]
- 15.Konski A, Doss M, Milestone B, Haluszka O, Hanlon A, Freedman G, et al. The integration of 18-fluoro-deoxy-glucose positron emission tomography and endoscopic ultrasound in the treatment-planning process for esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1123–1128. doi: 10.1016/j.ijrobp.2004.07.717. [DOI] [PubMed] [Google Scholar]
- 16.Greco C, Rosenzweig K, Cascini GL, Tamburrini O. Current status of PET/CT for tumour volume definition in radiotherapy treatment planning for non-small cell lung cancer (NSCLC) Lung Cancer. 2007;57:125–134. doi: 10.1016/j.lungcan.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Miller R, Siegmund D. Maximally selected x2 statistics. Biometrics. 1982;38:1011–1016. doi: 10.2307/2529881. [DOI] [Google Scholar]
- 18.Halpern J. Maximally selected x2 statistics for small samples. Biometrics. 1982;38:1017–1023. doi: 10.2307/2529882. [DOI] [Google Scholar]
- 19.Sanderson RJ, Ironside JA. Squamous cell carcinomas of the head and neck. BMJ. 2002;325:822–827. doi: 10.1136/bmj.325.7368.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Tourneau C, Jung GM, Borel C, Bronner G, Flesch H, Velten M. Prognostic factors of survival in head and neck cancer patients treated with surgery and postoperative radiation therapy. Acta Otolaryngol. 2008;128:706–712. doi: 10.1080/00016480701675668. [DOI] [PubMed] [Google Scholar]
- 21.Bastit L, Blot E, Debourdeau P, Menard J, Bastit P, Le Fur R. Influence of the delay of adjuvant postoperative radiation therapy on relapse and survival in oropharyngeal and hypopharyngeal cancers. Int J Radiat Oncol Biol Phys. 2001;49:139–146. doi: 10.1016/S0360-3016(00)01376-6. [DOI] [PubMed] [Google Scholar]
- 22.Langendijk JA, de Jong MA, Leemans CR, de Bree R, Smeele LE, Doornaert P, et al. Postoperative radiotherapy in squamous cell carcinoma of the oral cavity: the importance of the overall treatment time. Int J Radiat Oncol Biol Phys. 2003;57:693–700. doi: 10.1016/S0360-3016(03)00624-2. [DOI] [PubMed] [Google Scholar]
- 23.Suwinski R, Sowa A, Rutkowski T, Wydmanski J, Tarnawski R, Maciejewski B. Time factor in postoperative radiotherapy: a multivariate locoregional control analysis in 868 patients. Int J Radiat Oncol Biol Phys. 2003;56:399–412. doi: 10.1016/S0360-3016(02)04469-3. [DOI] [PubMed] [Google Scholar]
- 24.Al-Ibraheem A, Buck A, Krause BJ, Scheidhauer K, Schwaiger M (2009) Clinical Applications of FDG PET and PET/CT in Head and Neck Cancer. J Oncol 2009:208725. Epub 2009 Aug 20 [DOI] [PMC free article] [PubMed]
- 25.Laubenbacher C, Saumweber D, Wagner-Manslau C, Kau RJ, Herz M, Avril N, et al. Comparison of fluorine-18-fluorodeoxyglucose PET, MRI and endoscopy for staging head and neck squamous-cell carcinomas. J Nucl Med. 1995;36:1747–1757. [PubMed] [Google Scholar]
- 26.Schwartz DL, Ford E, Rajendran J, Yueh B, Coltrera MD, Virgin J, et al. FDG-PET/CT imaging for preradiotherapy staging of head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:129–136. doi: 10.1016/j.ijrobp.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 27.Greven KM, Williams DW, 3rd, McGuirt WF, Sr, Harkness BA, D’Agostino RB, Jr, Keyes JW, Jr, et al. Serial positron emission tomography scans following radiation therapy of patients with head and neck cancer. Head Neck. 2001;23:942–946. doi: 10.1002/hed.1136. [DOI] [PubMed] [Google Scholar]
- 28.Vernon MR, Maheshwari M, Schultz CJ, Michel MA, Wong SJ, Campbell BH. Clinical outcomes of patients receiving integrated PET/CT-guided radiotherapy for head and neck carcinoma. Int J Radiat Oncol Biol Phys. 2008;70:678–684. doi: 10.1016/j.ijrobp.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 29.Plataniotis GA, Theofanopoulou ME, Kalogera-Fountzila A, Haritanti A, Ciuleanou E, Ghilezan N, et al. Prognostic impact of tumor volumetry in patients with locally advanced head-and-neck carcinoma (non-nasopharyngeal) treated by radiotherapy alone or combined radiochemotherapy in a randomized trial. Int J Radiat Oncol Biol Phys. 2004;59:1018–1026. doi: 10.1016/j.ijrobp.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Chao KS, Ozyigit G, Blanco AI, Thorstad WL, Deasy JO, Haughey BH, et al. Intensity-modulated radiation therapy for oropharyngeal carcinoma: impact of tumor volume. Int J Radiat Oncol Biol Phys. 2004;59:43–50. doi: 10.1016/j.ijrobp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Mancuso AA, Mukherji SK, Schmalfuss I, Mendenhall W, Parsons J, Pameijer F, et al. Preradiotherapy computed tomography as a predictor of local control in supraglottic carcinoma. J Clin Oncol. 1999;17:631–637. doi: 10.1200/JCO.1999.17.2.631. [DOI] [PubMed] [Google Scholar]
- 32.Chang CC, Chen MK, Liu MT, Wu HK. The effect of primary tumor volumes in advanced T-staged nasopharyngeal tumors. Head Neck. 2002;24:940–946. doi: 10.1002/hed.10151. [DOI] [PubMed] [Google Scholar]
- 33.Chu ST, Wu PH, Hou YY, Chang KP, Chi CC, Lee CC, et al. Primary tumor volume of nasopharyngeal carcinoma: significance for recurrence and survival. J Chin Med Assoc. 2008;71:461–466. doi: 10.1016/S1726-4901(08)70149-7. [DOI] [PubMed] [Google Scholar]
- 34.Knegjens JL, Hauptmann M, Pameijer FA, Balm AJ, Hoebers FJ, de Bois JA, et al. Tumor volume as outcome predictor in chemoradiation for advanced head and neck cancer. Head Neck. 2010 doi: 10.1002/hed.21459. [DOI] [PubMed] [Google Scholar]
- 35.Chong VF, Zhou JY, Khoo JB, Chan KL, Huang J. Correlation between MR imaging-derived nasopharyngeal carcinoma tumor volume and TNM system. Int J Radiat Oncol Biol Phys. 2006;64:72–76. doi: 10.1016/j.ijrobp.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 36.Daisne J-F, Duprez T, Weynand B, Lonneux M, Hamoir M, Reychler H, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology. 2004;233:93–100. doi: 10.1148/radiol.2331030660. [DOI] [PubMed] [Google Scholar]
- 37.Minna JD, Higgins GA, Glatstein EJ. Cancer of lung. In: De Vita VT, Jr HS, Rosenberg SA, editors. Cancer: Principles and practice of oncology. Philadelphia: Lippincott; 1985. pp. 507–597. [Google Scholar]
- 38.Lee WR, Berkey B, Marcial V, Fu KK, Cooper JS, Vikram B, et al. Anemia is associated with decreased survival and increased locoregional failure in patients with locally advanced head and neck carcinoma: a secondary analysis of RTOG 85-27. Int J Radiat Oncol Biol Phys. 1998;42:1069–1075. doi: 10.1016/S0360-3016(98)00348-4. [DOI] [PubMed] [Google Scholar]
- 39.Rades D, Fehlauer F, Wroblesky J, Albers D, Schild SE, Schmidt R. Prognostic factors in head-and-neck cancer patients treated with surgery followed by intensity-modulated radiotherapy (IMRT), 3D-conformal radiotherapy, or conventional radiotherapy. Oral Oncol. 2007;43:535–543. doi: 10.1016/j.oraloncology.2006.05.006. [DOI] [PubMed] [Google Scholar]