Abstract
Purpose
The aim of this study was to assess the diagnostic efficacy of PET/CT using various parameters for the characterization of adrenal nodules in lung cancer patients.
Methods
Sixty-one adrenal nodules in 51 lung cancer patients were evaluated. The final diagnosis was based on histology (n = 2) or imaging follow-up (n = 59, range of follow-up: 7–57 months, median 27 months). Each adrenal nodule was analyzed using four parameters of PET/CT: the maximum standardized uptake value (SUVmax), the adrenal nodule/liver ratio of the SUV (SUV ratio), Hounsfield units (HU) and size. The optimal cutoff of each parameter for the identification of metastatic nodule was determined by ROC analysis and then the diagnostic efficacy was compared among the parameters.
Results
Of the 61 adrenal nodules, 45 (73%) were considered metastasis. The optimal cutoff values of the parameters were SUVmax >2.7, SUV ratio >1.3, HU >18 and size >20 mm, respectively. The sensitivity, specificity and accuracy by SUVmax >2.7 were 88.9%, 87.5% and 88.5%, and those by SUV ratio >1.3 were 84.4%, 100% and 88.5%, respectively. The combination of SUV ratio >1.3 and HU >18 had sensitivity of 97.7%, specificity of 81.2% and accuracy of 93.4% to predict adrenal metastasis in patients with lung cancer.
Conclusion
SUV ratio from F-18 FDG PET/CT could identify the adrenal metastasis in lung cancer patients. The combination of SUV ratio and HU can improve the accuracy of differentiating benign and metastatic adrenal lesions in lung cancer patients.
Keywords: Adrenal metastasis, FDG, PET/CT, Lung cancer
Introduction
Incidental adrenal masses are detected in approximately 5% of the patients who undergo routine abdominal computed tomography (CT) [1]. Most of these lesions are regarded as benign adenomas in patients without any history of malignancy [2]. However, adrenal metastases are frequently noted in patients with carcinoma. In a review of 1,000 consecutive autopsies, adrenal metastases were noted in 27% of the carcinoma patients; the incidence of adrenal metastasis in the lung cancer patients included on that study was 35% [3]. Therefore, accurately differentiating benign from metastatic adrenal masses is important for the optimal management of patients with lung cancer. Percutaneous biopsy remains the “gold standard” for confirming the status of adrenal lesion, but this is an invasive procedure that is often associated with complications [4].
Noninvasive imaging methods, such as CT or magnetic resonance imaging (MRI), have been used to differentiate metastases from benign adrenal adenoma. CT has shown usefulness for differentiating benign from malignant adrenal tumors by measuring the attenuation [Hounsfield units (HU)] [5–7]. MRI is often performed to further characterize the indeterminate masses seen on CT [8, 9]. 18Fluorine-fluorodeoxyglucose (F-18 FDG) positron emission tomography (PET) and PET/CT have shown encouraging results for evaluating adrenal metastasis in patients with lung cancer [10–14]. In contrast to CT, F-18 FDG PET imaging provides metabolic information, based on the increased glucose metabolism in malignant lesions. The CT component on integrated PET/CT had a limited role for correcting the attenuation and for anatomic localization in the past. Most PET/CT scans do not routinely include the contrast studies. Therefore, the metabolic parameters on PET and the HU on unenhanced CT implemented on the integrated PET/CT scan can be used together for the identification of adrenal mass. But there is no consensus on the accurate diagnostic criteria for the evaluation of adrenal masses with using these two parameters.
The purpose of this study was to assess the diagnostic efficacy of F-18 FDG PET/CT for the evaluation of adrenal metastasis in lung cancer patients and to develop the optimal diagnostic algorithm using the PET/CT parameters.
Materials and Methods
Patients
We initially identified patients who underwent a diagnostic work-up for primary lung cancer by both diagnostic CT and F-18 FDG PET/CT scans from July 2005 to February 2008. Fifty-one consecutive patients who had lung cancer and abnormal adrenal lesions diagnosed at our institute were enrolled. The eligibility requirements included: (1) primary lung cancer proven by pathology, (2) abnormal adrenal lesion(s) detected on either diagnostic CT scans (nodule or enhanced nodularity) or FDG PET scans (focal FDG uptake greater than background), (3) the time between FDG PET/CT scan and diagnostic CT scans was no more than 2 weeks, and (4) adrenal lesions proven by pathology or imaging follow-up for more than 6 months.
FDG PET/CT Imaging
F-18 FDG PET/CT scans were acquired using an integrated PET/CT scanner (Discovery LS; GE Medical Systems, Milwaukee, Wis.), which consisted of a PET scanner (Advanced NXi; GE medical Systems) and an eight-slice helical CT scanner (LightSpeed Plus; GE Medical Systems). All patients were instructed to fast for at least 6 h before the scans. Blood glucose levels in all 51 patients were <6.6 mmol/l. Truncal PET scans were performed in two-dimensional (2D) mode using five to seven bed positions to ensure adequate coverage from head to pelvic floor. Emission scans (5 min/frame; 128 × 128 matrix) were performed 50 min after intravenously injecting 370 MBq of F-18 FDG. CT scans were performed immediately before PET scans using a multidetector helical CT scanner. The imaging parameters were as follows: 140 kVp, 80 mA, 0.8 s per CT rotation, a pitch of 6 and a 22.5-mm/s table speed. No contrast material was administered. CT images were created using a 512 × 512 matrix, but were reduced this to a 128 × 128 matrix to correspond to PET emission images. PET/CT images were reconstructed using CT scans for attenuation correction and the OSEM algorithm (two iterations, 16 subsets), as previously described [15]. The images were co-registered using dedicated software (eNTEGRA; GE medical Systems).
FDG PET/CT Image Interpretation
Abnormal FDG uptake was defined as uptake greater than the background uptake in the surrounding tissues that did not exhibit tracer uptake. The areas of abnormal FDG uptake were identified, and the intensities of FDG uptake were quantified by calculating standardized uptake values (SUVs) from the amounts of FDG that was injected, the total body weight and the regional uptake on the attenuation-corrected regional image. Specifically, SUV was defined as the maximum SUV of the region of interest (ROI) and it was calculated by the following equation: (activity/unit volume)/(injected dose/total body weight). All PET/CT scans were reviewed and interpreted by two experienced nuclear medicine physicians.
The ROIs were drawn manually around the adrenal lesions on the transaxial sections of the PET images. In order to minimize the variation according to the size of the ROIs and to assure reproducibility, we obtained the maximum SUV, which was defined as the peak SUV of the pixel with the highest counts in the sequential transaxial scans throughout the adrenal lesions. For comparing the adrenal FDG uptake, we also drew more than three circular ROIs of 3-cm diameter in the livers on the transaxial sections that did not contain metastatic lesions or other parenchymal abnormalities, and we calculated the maximum SUVs of the liver (SUVL).
On the diagnostic CT scans, we measured the size of the adrenal lesions at their long axis. With the help of a radiologist and with the diagnostic CT scans, we drew circular ROIs of 5-mm diameter in the adrenal lesions on more than two sequential transaxial CT sections of the PET/CT scans that did not contain X-ray artifacts but they sufficiently covered the lesion sites, and then we calculated the mean HU.
Assessment of Adrenal Metastases
Adrenal metastases were confirmed on the histopathologic specimens in two patients who underwent adrenal resection. For the other cases, we considered those lesions as adrenal metastases when the adrenal lesions exhibited sequential aggravation on the following CT scans or FDG PET/CT scans after 2 months or they showed a decrease of more than 30% of diameter on the following CT scans after chemotherapy in 2 months. Adrenal lesions were regarded as benign when the sequential CT or FDG PET/CT scans did not exhibit any change during the follow-up period that was at least more than 6 months.
Definitions and Calculations of the PET/CT Parameters
We defined the maximum SUV of adrenal lesions as SUVmax, the adrenal-to-liver ratio of the maximum SUV as SUV ratio, and the Hounsfield units of the adrenal lesion as HU. The size of the adrenal lesion was measured at the long axis on CT scans.
Statistics
We compared size, HU, SUVmax and SUV ratio between the adrenal metastasis and the benign lesions by independent t-tests. We analyzed the diagnostic performance of each parameter and the optimal cut-offs in terms of their abilities to discriminate adrenal metastases from benign lesions. For this purpose, we used the receiver operating characteristic (ROC) curves and calculated the areas under the curves (AUC) for each parameter. Comparison of the AUC for each parameter was analyzed using MedCalc version 7.4 (MedCalc Software, Mariakerke, Belgium). We chose the parameters that best predicted adrenal metastases and determined the practical cut-offs that showed the highest accuracy. We then grouped the patients using these cut-offs, and calculated sensitivity, specificity and accuracy. Finally, we devised a decision tree for diagnosing adrenal metastases based on the imaging parameters and the calculated predictive values using this model. All the calculations were performed using SPSS version 13.0 (SPSS, Chicago, Ill.). All the p values were derived from two-sided tests, and p values <0.05 were considered significant.
Results
Characteristics of the Patients and the Adrenal Lesions
The patients’ characteristics are detailed in Table 1. Their median age was 62 years (range: 38–78 years) and 78% of the patients were male. Forty-three patients (84.3%) had non-small cell lung cancer (NSCLC) and eight (15.6%) had small cell lung cancer (SCLC). Based on the revised AJCC staging system without considering the adrenal lesions, seven patients (13.7%) were Stage I, four patients (7.8%) were a Stage II, seven (13.7%) were a Stage III and 33 (64.7%) were a Stage IV. Ten (19.6%) of 51 patients showed bilateral adrenal lesions and so 61 adrenal lesions were assessed in this study.
Table 1.
Patients’ characteristics
| Characteristic | Value (%) | |
|---|---|---|
| Total number of patients | 51 | |
| Age (year) | Mean ± SD | 60 ± 9.5 |
| Range | 38–78 | |
| Gender | Male | 40 (78.4) |
| Female | 11 (21.5) | |
| AJCC stage | I | 7 (13.7) |
| II | 4 (7.8) | |
| III | 7 (13.7) | |
| IV | 33 (64.7) | |
| Histopathology | NSCLC | 43 (84.3) |
| SCLC | 8 (15.6) | |
| Number of adrenal lesions | Total | 61 |
| Unilateral | 41 | |
| Bilateral | 10 | |
The median size of the adrenal lesions on the diagnostic CT scans was 20 mm (range: 8–90 mm). The median HU of the adrenal lesions on the PET/CT scans was 28 (range: 10–42). The median values of SUVmax and SUV ratio were 4.1 and 1.8, respectively. Forty-five adrenal lesions (73.7%) were defined as metastases. Two adrenal lesions of two patients were resected and these were histopathologically proven to be metastases. The other 43 adrenal lesions were regarded as metastatic ones due to aggravation that was seen on the follow-up sequential imaging studies or they had a decrease in diameter of more than 30% after chemotherapy. Sixteen adrenal lesions (26.2%) were considered as non-metastatic benign ones because there was no interval change on the follow-up sequential imaging studies for at least 6 months (median period: 27 months, range: 7–57.5 months).
Comparison of Size and the PET/CT Parameters Between the Metastatic and Non-metastatic Adrenal Lesions
The size and PET-CT parameters according to the adrenal lesions are plotted in Fig. 1. There were significant differences in size and the PET/CT parameters between the metastatic and non-metastatic adrenal lesions (Table 2). The sizes of the metastatic and non-metastatic adrenal lesions were 27 ± 17 mm and 17 ± 5 mm, respectively, and the difference was significant (p = 0.001). The HU (29 ± 8 vs 9 ± 13, respectively) as well as both the SUVmax (6.5 ± 3.8 vs 2.0 ± 0.7, respectively) and the SUV ratio (3.0 ± 2.0 vs 0.8 ± 0.2, respectively), were all significantly different (p < 0.001).
Fig. 1.
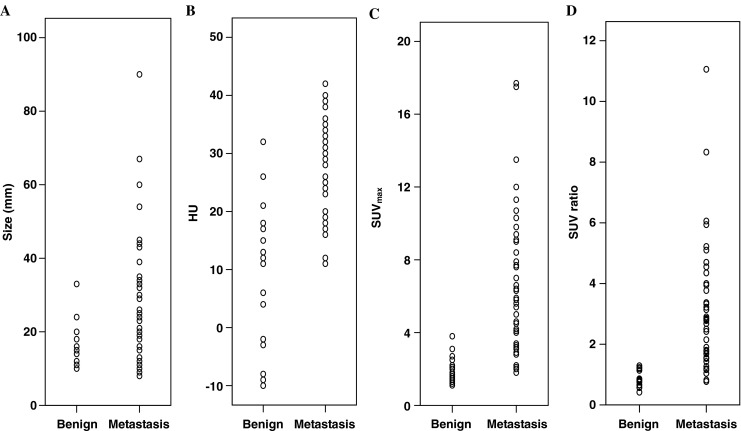
Differences in distribution between benign and metastatic adrenal nodules for size (a), HU (b), SUVmax (c), and SUV ratio (d)
Table 2.
Comparison of parameters between benign and metastatic adrenal lesions
| Parameters | Benign (n = 16)a | Metastasis (n = 45)a | P value |
|---|---|---|---|
| Size (mm) | 17 ± 5 | 27 ± 17 | 0.001 |
| HU | 9 ± 13 | 29 ± 8 | <0.001 |
| SUVmax | 2.0 ± 0.7 | 6.5 ± 3.8 | <0.001 |
| SUV ratio | 0.8 ± 0.2 | 3.0 ± 2.0 | <0.001 |
aData are means ± standard deviations
ROC Curve Analysis of Adrenal Metastasis
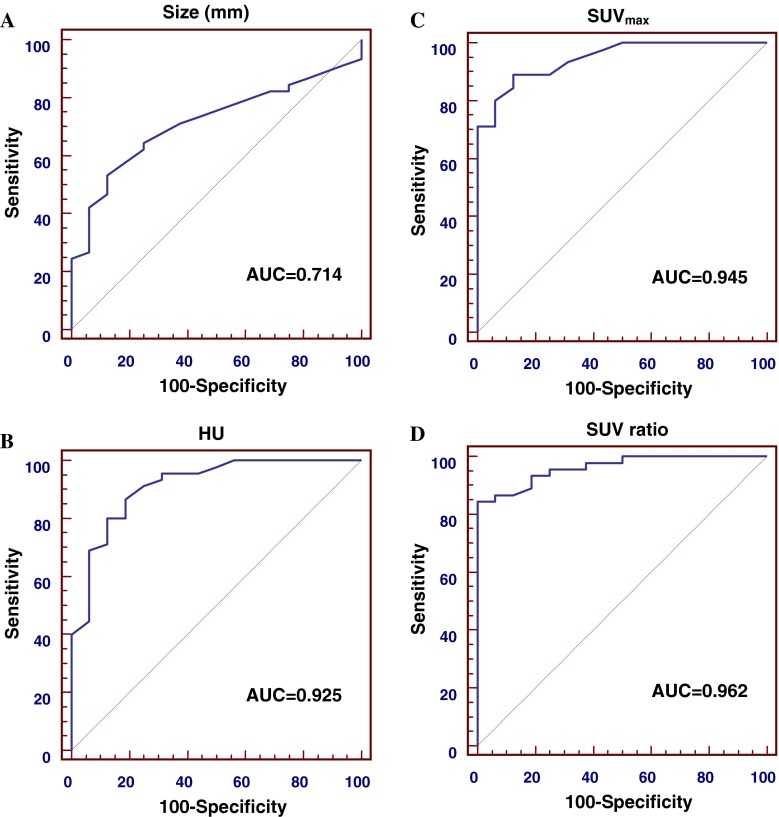
The ROC curves of size, HU, SUVmax and SUV ratio were plotted to predict adrenal metastasis (Fig. 2). SUV ratio showed the largest AUC (0.962), followed by SUVmax (0.945), HU (0.925) and size (0.714). Based on the AUC, SUV ratio exhibited the highest diagnostic performance to predict adrenal metastasis. The optimal cut-offs of the parameters were SUVmax >2.7, SUV ratio >1.3, HU >18 and size >20 mm, respectively. The sensitivity, specificity and accuracy by SUVmax >2.7 were 88.9%, 87.5% and 88.5%, and those by SUV ratio >1.3 were 84.4%, 100% and 88.5%, respectively. The sensitivity, specificity and accuracy by HU >18 were 86.7%, 81.2% and 85.2%, whereas those by size >20 mm were 53.3%, 87.5% and 62.3%, respectively (Table 3). We found that high SUVmax (>2.7) or SUV ratio (>1.3) values predicted adrenal metastasis, and therefore, we could set the cut-off values for either SUVmax or SUV ratio. Although both of the two parameters reflect the degree of FDG uptake, they have close correlation with each other by their nature and SUV ratio value exhibited a larger AUC than SUVmax, so we chose the cut-off of SUV ratio. Based on this cut-off, we divided the adrenal lesions into two groups: group I (SUV ratio >1.3; n = 38) and group II (SUV ratio ≤1.3; n = 23). All 38 adrenal lesions in group I had metastasis. Of the 23 adrenal lesions in group II, 16 (69.5%) were benign and seven (30.4%) were adrenal metastasis.
Fig. 2.
ROC curve analysis of adrenal metastasis. ROC curves of size (a), HU (b), SUVmax (c), and SUV ratio (d) were plotted to predict adrenal metastasis. Based on the AUC, SUV ratio shows highest diagnostic performance to predict adrenal metastasis
Table 3.
Diagnostic performance by each parameter and combined model
| Optimal cut-off | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|---|---|---|
| SUVmax >2.7 | 88.9 | 87.5 | 88.5 |
| SUV ratio >1.3 | 84.4 | 100 | 88.5 |
| HU >18 | 86.7 | 81.2 | 85.2 |
| Size >20 mm | 53.3 | 87.5 | 62.3 |
| SUV ratio >1.3 and HU >18 | 97.7 | 81.2 | 93.4 |
ROC Curve Analysis for the Group II Patients
The size of group II tumors was smaller than the size of group I tumors (mean ± SD: 14 ± 5 vs 29 ± 17 mm, respectively, p < 0.05). We thought that metabolic underestimation had occurred due to the small volume effect, and so another criterion besides metabolic parameters was required. In order to predict adrenal metastasis for the group II patients, we drew ROC curves and calculated the AUCs of size and HU. HU was found to better predict adrenal metastasis among the group II patients (AUC = 0.888) than size (AUC = 0.612). Six of the nine adrenal lesions with an HU of >18 were metastasis, while most adrenal lesions (13 of 14) with an HU of ≤18 were benign lesions.
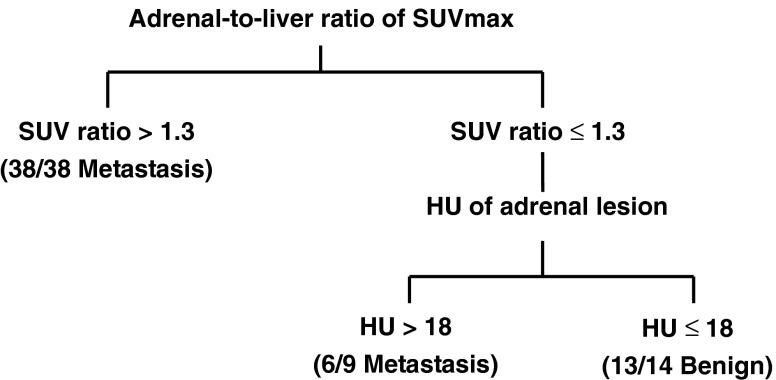
Decision Tree for the Evaluation of Adrenal Metastasis
A decision tree was devised to predict adrenal metastasis based on the SUV ratio and HU values (Fig. 3). According to our model, 47 adrenal lesions were predicted to have metastasis, and the remaining 14 were predicted to be benign. The sensitivity, specificity and accuracy to predict adrenal metastasis in the patients with lung cancer and who had abnormal adrenal lesions were 97.7%, 81.2% and 93.4%, respectively. This diagnostic performance was compared with the single parameters of PET/CT (Table 3).
Fig. 3.
Proposed decision tree for prediction of adrenal metastasis. Combination of SUV ratio greater than 1.3 and HU greater than 18 can improve the sensitivity and accuracy. Numbers in parentheses represent individual nodules satisfying the criteria
Discussion
Detection of adrenal masses on imaging studies can be problematic when staging or restaging lung cancer. If an adrenal mass is considered to be metastasis, then the management and prognosis of the patient will be changed. However, isolated ipsilateral adrenal metastasis in a patient with otherwise resectable NSCLC is considered to be localized disease [16]. In some studies, resection of isolated adrenal metastases has been shown to improve the survival of these patients [17, 18]. Therefore, accurate differentiation of benign from metastatic adrenal masses is important for the optimal management of patients with lung cancer.
CT has been widely used as a conventional imaging modality for the detection and evaluation of adrenal masses. A benign adrenal mass tends to be smaller than a malignant mass, but, a small-sized adrenal malignancy should be considered. No size threshold has yielded both high sensitivity and specificity [2]. Adrenal adenomas frequently contain a large amount of intracytoplasmic lipid, and so they show low attenuation on CT, which allows quantitative evaluation by measuring the HU [7, 19]. Many researchers have reported that attenuation thresholds on unenhanced CT have shown a better performance than size to diagnose adrenal malignancy and nonadenomas [5–7]. An adrenal lesion of 10 HU or less on unenhanced CT suggests the presence of intracytoplasmic lipid and therefore an adenoma with a sensitivity of 71% and specificity of 98% [5]. However, approximately 30% of adenomas are lipid poor, and these adenomas show higher attenuation values of greater than 10 HU [2]. Thus, not all adrenal adenomas can be characterized using unenhanced CT alone. Attenuation values (HU) are influenced by the CT technique. The CT component of integrated PET/CT in our study was obtained using a lower kilovoltage and milliamperage compared with the conventional CT settings. Therefore, overestimation of HU should be considered in small-sized lesions such as adrenal nodules [13]. Contrast washout studies have recently enabled differentiating adrenal metastases from lipid-poor adenomas [20, 21]. Adenomas demonstrate a more rapid washout of contrast medium, whether they are lipid-rich or not. But this is often not feasible in clinical practice because unenhanced CT scans are not routinely obtained, and patients frequently leave the department before their CT scans are reviewed.
Previous studies with PET or PET/CT have proposed variable thresholds of FDG avidity that are highly sensitive for detecting metastatic adrenal lesions [10–14, 22–25]. Two recent studies [14, 22] using a cut-off of the SUVmax >3.1 reported sensitivities of 98.5 and 97.3%, respectively. Other studies using visual assessment (an adrenal lesion was considered to be metastasis if its FDG uptake was greater than that of liver) also reported excellent sensitivities of 93–100% [10, 12, 25]. However, adrenal adenoma can also show FDG uptake greater than that of liver, thus decreasing the specificity of the PET interpretation.
In our study, various parameters were used to improve the diagnostic efficacy of F-18 FDG PET/CT for differentiating benign and metastatic adrenal nodules in lung cancer patients. SUV ratio, SUVmax and HU were more sensitive and accurate than size for identifying metastatic adrenal lesions. In terms of specificity, the SUV ratio criterion was superior to the others (100 vs 87.5%). We propose a diagnostic approach for the evaluation of adrenal metastasis by a combination of SUV ratio >1.3 and HU >18. The sensitivity, specificity and accuracy of this model to predict adrenal metastasis were 97.7%, 81.2% and 93.4%, respectively. These diagnostic performance exhibited higher sensitivity and accuracy than those of the single parameters such as SUVmax and SUV ratio, and equal specificity compared with HU.
This is a retrospective study, and so there may have been a sampling bias. Our study had a small population and only a small number of adrenal lesions (two of 61, 3%) were proved with histopathologic confirmation. Additional large studies are needed to confirm and expand on our results for more accurate diagnosis of adrenal metastasis.
In conclusion, the SUV ratio from F-18 FDG PET/CT could identify adrenal metastasis in lung cancer patients. Our combined criteria of SUV ratio >1.3 and HU >18 can improve the accuracy of differentiating benign and metastatic adrenal lesions in lung cancer patients.
References
- 1.Kloos RT, Gross MD, Francis IR, Korobkin M, Shaprio B. Incidentally discovered adrenal masses. Endocr Rev. 1995;16:460–84. doi: 10.1210/edrv-16-4-460. [DOI] [PubMed] [Google Scholar]
- 2.Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309–40. doi: 10.1210/er.2002-0031. [DOI] [PubMed] [Google Scholar]
- 3.Abrams HL, Siro R, Goldstein N. Metastases in carcinoma: analysis of 1, 000 autopsied cases. Cancer. 1950;3:74–85. doi: 10.1002/1097-0142(1950)3:1<74::AID-CNCR2820030111>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Mody MK, Kazerooni EA, Korobkin M. Percutaneous CT-guided biopsy of adrenal masses: immediate and delayed complications. J Comput Assist Tomogr. 1995;19:434–9. doi: 10.1097/00004728-199505000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Boland GW, Lee MJ, Gazelle GS, Halpern EF, McNicholas MM, Mueller PR. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201–4. doi: 10.2214/ajr.171.1.9648789. [DOI] [PubMed] [Google Scholar]
- 6.Lee MJ, Hahn PF, Papanicolaou N, Egglin TK, Saini S, Mueller PR, et al. Benign and malignant adrenal masses: CT distinction with attenuation coefficients, size, and observer analysis. Radiology. 1991;179:415–8. doi: 10.1148/radiology.179.2.2014283. [DOI] [PubMed] [Google Scholar]
- 7.Korobkin M, Giordano T, Brodeur F, Francis IR, Siegelman ES, Quint LE, et al. Adrenal adenomas: relationship between histologic lipid and CT and MR findings. Radiology. 1996;200:743–7. doi: 10.1148/radiology.200.3.8756925. [DOI] [PubMed] [Google Scholar]
- 8.Haider MA, Ghai S, Jhaveri K, Lockwood G. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711–6. doi: 10.1148/radiol.2313030676. [DOI] [PubMed] [Google Scholar]
- 9.Reinig JW, Doppman JL, Dwyer AJ, Frank J. MRI of indeterminate adrenal masses. AJR Am J Roentgenol. 1986;147:493–6. doi: 10.2214/ajr.147.3.493. [DOI] [PubMed] [Google Scholar]
- 10.Kumar R, Xiu Y, Yu JQ, Takalkar A, El-Haddad G, Potenta S, et al. 18F-FDG PET in evaluation of adrenal lesions in patients with lung cancer. J Nucl Med. 2004;45:2058–62. [PubMed] [Google Scholar]
- 11.Erasmus JJ, Patz EF, Jr, McAdams HP, Murray JG, Herndon J, Coleman RE, et al. Evaluation of adrenal masses in patients with bronchogenic carcinoma using 18F-fluorodeoxyglucose positron emission tomography. AJR Am J Roentgenol. 1997;168:1357–60. doi: 10.2214/ajr.168.5.9129444. [DOI] [PubMed] [Google Scholar]
- 12.Gupta NC, Graeber GM, Tamim WJ, Rogers JS, Irisari L, Bishop HA. Clinical utility of PET-FDG imaging in differentiation of benign from malignant adrenal masses in lung cancer. Clin Lung Cancer. 2001;3:59–64. doi: 10.3816/CLC.2001.n.019. [DOI] [PubMed] [Google Scholar]
- 13.Sung YM, Lee KS, Kim BT, Choi JY, Chung MJ, Shim YM, et al. 18F- FDG PET versus 18F- FDG PET/CT for adrenal gland lesions characterization: a comparison of diagnostic efficacy in lung cancer patients. Korean J Radiol. 2008;9:19–28. doi: 10.3348/kjr.2008.9.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brady MJ, Thomas K, Wong TZ, Franklin KM, Ho LM, Paulson EK. Adrenal nodules at FDG PET/CT in patients known to have or suspected of having lung cancer: a proposal for an efficient diagnostic algorithm. Radiology. 2009;250:523–30. doi: 10.1148/radiol.2502080219. [DOI] [PubMed] [Google Scholar]
- 15.Na II, Cheon GJ, Choe DH, Byun BH, Kang HJ, Koh JS, et al. Clinical significance of (18)F-FDG uptake by N2 lymph nodes in patients with resected stage IIIA N2 non-small-cell lung cancer: a retrospective study. Lung Cancer. 2008;60:69–74. doi: 10.1016/j.lungcan.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Kocijancic I, Vidmar K, Zwitter M, Snoj M. The significance of adrenal metastases from lung carcinoma. Eur J Surg Oncol. 2003;29:87–8. doi: 10.1053/ejso.2002.1372. [DOI] [PubMed] [Google Scholar]
- 17.Luketich JD, Burt ME. Does resection of adrenal metastases from non small cell lung cancer improve survival? Ann Thorac Surg. 1996;62:1614–6. doi: 10.1016/S0003-4975(96)00611-X. [DOI] [PubMed] [Google Scholar]
- 18.Porte H, Siat J, Guibert B, Lepimpec-Barthes F, Jancovici R, Bernard A, et al. Resection of adrenal metastases from non-small cell lung cancer: a multicenter study. Ann Thorac Surg. 2001;71:981–5. doi: 10.1016/S0003-4975(00)02509-1. [DOI] [PubMed] [Google Scholar]
- 19.Cirillo RL, Jr, Bennett WF, Vitellas KM, Poulos AG, Bova JG. Pathology of the adrenal gland: imaging features. AJR Am J Roentgenol. 1998;170:429–35. doi: 10.2214/ajr.170.2.9456959. [DOI] [PubMed] [Google Scholar]
- 20.Caoili EM, Korobkin M, Francis IR, Cohan RH, Dunnick NR. Delayed enhanced CT of lipid-poor adrenal adenomas. AJR Am J Roentgenol. 2000;175:1411–5. doi: 10.2214/ajr.175.5.1751411. [DOI] [PubMed] [Google Scholar]
- 21.Pena CS, Boland GW, Hahn PF, Lee MJ, Mueller PR. Characterization of indeterminate (lipid-poor) adrenal masses: use of washout characteristics at contrast-enhanced CT. Radiology. 2000;217:798–802. doi: 10.1148/radiology.217.3.r00dc29798. [DOI] [PubMed] [Google Scholar]
- 22.Blake MA, Slattery JM, Kalra MK, Halpern EF, Fischman AJ, Mueller PR, et al. Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy—initial experience. Radiology. 2006;238:970–7. doi: 10.1148/radiol.2383042164. [DOI] [PubMed] [Google Scholar]
- 23.Metser U, Miller E, Lerman H, Lievshitz G, Avital S, Even-Sapir E. 18F-FDG PET/CT in the evaluation of adrenal masses. J Nucl Med. 2006;47:32–7. [PubMed] [Google Scholar]
- 24.Park BK, Kim CK, Kim B, Choi JY. Comparison of delayed enhanced CT and 18F-FDG PET/CT in the evaluation of adrenal masses in oncology patients. J Comput Assist Tomogr. 2007;31:550–6. doi: 10.1097/rct.0b013e31802fa8e1. [DOI] [PubMed] [Google Scholar]
- 25.Yun M, Kim W, Alnafisi N, Lacorte L, Jang S, Alavi A. 18F-FDG PET in characterizing adrenal lesions detected on CT or MRI. J Nucl Med. 2001;42:1795–9. [PubMed] [Google Scholar]