Abstract
Dermatomyositis (DM) or polymyositis (PM) are possibly considered to have an association with malignancies. We describe a case of dermatomyositis in which 18F-fluorodeoxy glucose (FDG)positron emission tomography (PET) was able to detect cancer recurrence earlier than any other modality in a patient with a history of primary pleural lymphoma, a very rare condition of malignancy. Further, a typical finding of dermatomyositis is diffuse hypermetabolism in the bilateral proximal shoulder and pelvic girdle areas was shown on 18F-FDG PET, which can implicate the inflammatory process in the skeletal muscle in dermatomyosistis. This case well illustrates the characteristic 18F-FDG findings of dermatomyositis as well as a capability of 18F-FDG PET in detection of recurrence of lymphoma, even in a rare condition.
Keywords: Dermatomyositis, F-18-fluoro-deoxy glucose, Positiron emission tomography, Primary pleural lymphoma
Introduction
Dermatomyositis is an uncommon idiopathic, inflammatory myopathy characterized by proximal muscle weakness and cutaneous lesions. The association of dematomyositis with malignancy is well established, and there is known to be a variable temporal relationship between the two conditions [1–5]. Malignant tumors originating from lung, breast, ovary, and gastrointestinal track are known to be frequently associated with dermatomyositis [2–4]. However, lymphoma or other hematologic malignancies rarely cause dermatomyositis [6, 7]. Moreover, primary pleural lymphoma, as in this case, is an extremely rare malignancy [8, 9], and no case associated with dermatomyositis has been reported till date.
We describe a case of dermatomyositis that preceded the recurrence of malignancy in a patient with a very rare condition of primary pleural lymphoma. This case intensified the malignancy evaluation when dermatomyositis is diagnosed, even in rare conditions. Furthermore, this case also demonstrates a good capability of 18F-fluorodeoxy glucose (FDG) for detection of malignancy recurrence as well as the involvement of dermatomyositis.
Case Report
A 65-year-old female was admitted with generalized edema and muscle aching, and weight gain of 6 kg for 1 month. She has been followed with a diagnosis of primary pleural lymphoma. The diagnosis was made via incidentally detecting a few scattered atypical cells in the pleural effusion 2 years previously, which was consistent with small lymphocytic lymphoma. No further lymphoma involvement was found at the time of diagnosis and during the follow-up period.
At admission, physical examination detected skin rash in the shoulder, neck, wrist and right thigh areas. Initial laboratory tests revealed a high erythrocyte sedimentation rate (34 mm/h; normal range, 0-20) and elevated C-reactive protein level (2.02 mg/dl; normal range, 0-0.5), and also detected increased levels of creatine kinase (8,680 U/l; normal range, 30-225), lactate dehydrogenase (968 U/l; normal range, 100-220) and aldolase (37.4 U/l; normal range, <7.6) in the serum. Further, the level of myoglobin in the serum (1,253 ng/ml; normal range, 0-65) was markedly increased. Muscle biopsy was performed at the right biceps and first dorsal interosseus muscle. Histopathologic examination demonstrated mild interstitial infiltration of lymphocytes, and ultrastructural examination disclosed microtubulovesicular bodies, which is a useful diagnostic marker of dermatomyositis, in the cytoplasm of endothelial cells (Fig. 1). Sequential electromyographic examination also provided a feature of active myopathy; increased insertion activity, fibrillation potential, and complex repetitive discharge.
Fig. 1.
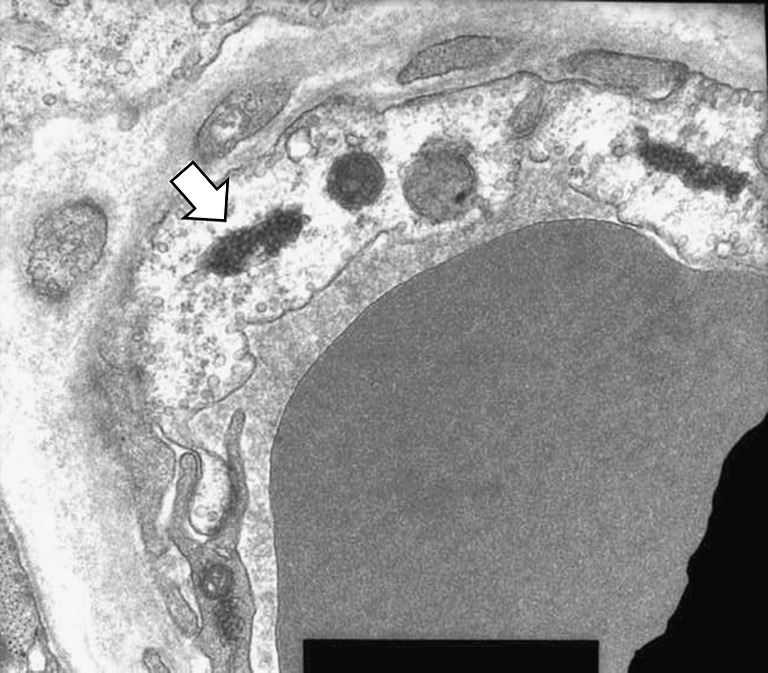
Electomicroscopic finding showing microtubulovesicular bodies (arrowed) in the cytoplasm of endothelial cells, a diagnostic marker of dermatomyositis (transmission electron microscope ×22,400)
The diagnosis of dermatomyositis was finally made based on the typical skin rash, proximal muscle weakness, elevated level of serum muscle enzymes, findings of histopathology of the muscle biopsy and the finding of electromyogram showing a myopathic pattern, which satisfied the criteria for diagnosis of polymyositis and dermatomyositis.
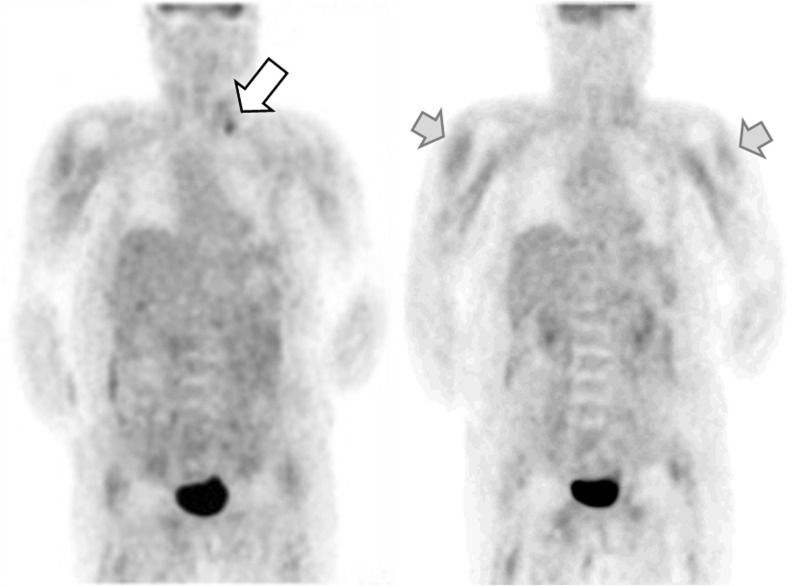
Several studies for the evaluation of residual lymphoma or other occult malignancy were performed, including serum tumor marker, esophago-gastro-dudoenoscopy, colonoscopy, chest computed tomography (CT), and abdomino-pelvic CT, and unremarkable findings were reported. On 18F-FDG covering from upper thigh to the head, diffusely increased FDG uptake was observed in the proximal areas of upper arms and thighs, shoulder and pelvic girdle along the major muscle layers bilaterally, where dermatomyositis pathology is commonly involved (Fig. 2). Additionally, a nodular lesion in the left supraclavicular area and a few suspicious hypermetabolic nodules in the retroperitoneal areas were examined on 18F-FDG positron emission tomography (PET), and recurrence of malignancy was suspected. Bone scintigraphy also showed extraosseous accumulation of Tc-99m HDP in the soft tissue of the both upper arms and both thighs without evidence of bone metastasis. The distribution of radiotracer on bone scan was in semblance of that on 18F-FDG PET. Although the recurrence of lymphoma in the supraclavicular and retroperitoneal area was suspected on the 18F-FDG PET images, the patient wanted to delay further work-up, such as tissue confirmation, after the relief of the symptoms of dermatomyositis. Hence, the patient was treated with high-dose steroid and azathioprin without any further work-up.
Fig. 2.
18F-FDG PET findings. A focal hypermetabolic nodule in the left supraclavicular area on 18F-FDG PET was suspected as a recurred malignancy (white arrow); and 18F-FDG uptake in the skeletal muscle in the upper arms (gray arrows), and upper thigh was demonstrated
Her symptoms of muscle weakness, generalized pain and edema were initially improved with high-dose steroid and azathioprin treatment immediately after diagnosis. Three month later, however, muscle weakness in the lower extremities was worsening despite continuing medication, and generalized edema with abdominal distension had developed. Further imaging examinations, including 18F-FDG PET and contrast-enhanced CT, revealed the massive recurrence of malignant lymphoma in the neck, mediastinum, peritoneum, retroperitoneum and both inguinal area (Fig. 3). Afterward, ultrasonography-guided omental lymph node biopsy was performed, and diffuse large-cell lymphoma was diagnosed, suggesting that lymphoma had recurred with high-grade transformation. The patient subsequently underwent R-CHOP (rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy. However, unfortunately, after one cycle of R-CHOP treatment, the patient died because of tumor lysis syndrome and septicemia.
Fig. 3.
Follow-up 18F-FDG PET (a, b, c) and contrast-enhanced CT (d, e, f) showing that the recurrence of lymphoma was extensively involved in the retroperitoneal lymph nodes, and peritoneum, and pleura. Increased metabolic activity and extent of left supraclavicular lymph node (white arrow) compared with previous scan also implies the progression of lymphoma. Additionally, intense FDG uptake was noticed in the right thigh area, and we speculated this finding as lymphoma involvement or myositis
Discussion
The present case report refers to a patient with dermatomyositis on previously established diagnosis of primary pleural lymphoma. In this case, 18F-FDG PET scan demonstrated the typical finding of dermatomyositis associated with clinically rare primary pleural lymphoma. Furthermore, 18F-FDG PET also suspected the recurrence of the tumors in remote areas by showing focal hypermetabolic lesions, which were diagnosed as recurred lymphoma with high-grade transformation.
Dermatomyositis in malignancy is well established; however, the precise links between malignancy and inflammatory myopathy is not completely understood, and the temporal relationship seems to be variable [2–5]. In most cases, dermatomyositis precedes the development of malignancy. And it can be also simultaneously detected with cancer, or diagnosed while patients with cancer are clinically followed. In our case, dermatomyosis developed without any sign of current malignancy in a patient with previous history, and preceded the recurrence. Therefore, the suspicion of malignancy in dermatomyosis is always considered.
Dermatomyositis can be suspected on the basis of clinical features and confirmed by laboratory tests, serum muscle enzyme concentration, and the presence of auto antibodies, electromyography (EMG) and muscle biopsy [1]. Our patient was in accordance with previous dermatomyositis diagnostic criteria. When diagnosis of dermatomyositis is made, malignancy work-up including serum tumor marker, esophago-gastro-dudoenoscopy, colonoscopy, chest CT, and abdominal CT should be aggressively performed. In particular, whole-body 18F-FDG PET can sensitively detect the malignant lesions with increased cellular metabolism by showing high FDG uptake [10]. Further, the results of a recent study showed that the diagnostic performance of 18F-FDG PET/CT could be comparable with that of broad conventional screening tests for diagnosing hidden malignant lesions in patients with dermatomyositis or polymyositis [11]. In this case, recurrence was suspected in 18F-FDG PET earlier than in any other modalities. Additionally, 18F-FDG uptake can not only be by malignant cells but also by the activated inflammatory cells. This may result as a false-positive finding in some conditions; however, it can provide useful biological information of inflammatory characteristics and the activity in dermatomyositis.
This case well demonstrated that the value of 18F-FDG PET in dermatomyositis lies in not only showing the inflammation in the muscle as hypermetabolism but also detecting malignancy simultaneously. Additionally, even in a rare condition, malignancy should be suspected whenever dermatomyositis is diagnosed.
Acknowledgements
This study was supported by the grant no. 11-2008-039 from SNUBH Research fund.
References
- 1.Briani C, Doria A, Sarzi-Puttini P, Dalakas MC. Update on idiopathic inflammatory myopathies. Autoimmunity. 2006;39:161–70. doi: 10.1080/08916930600622132. [DOI] [PubMed] [Google Scholar]
- 2.Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96–100. doi: 10.1016/S0140-6736(00)03540-6. [DOI] [PubMed] [Google Scholar]
- 3.Fardet L, Rybojad M, Gain M, Kettaneh A, Cherin P, Bachelez H, et al. Incidence, risk factors, and severity of herpesvirus infections in a cohort of 121 patients with primary dermatomyositis and dermatomyositis associated with a malignant neoplasm. Arch Dermatol. 2009;145:889–93. doi: 10.1001/archdermatol.2009.152. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Jiang SP, Huang L. Dermatomyositis and malignancy: a retrospective study of 115 cases. Eur Rev Med Pharmacol Sci. 2009;13:77–80. [PubMed] [Google Scholar]
- 5.Huang YL, Chen YJ, Lin MW, Wu CY, Liu PC, Chen TJ, et al. Malignancies associated with dermatomyositis and polymyositis in Taiwan: a nationwide population-based study. Br J Dermatol. 2009;161:854–60. doi: 10.1111/j.1365-2133.2009.09274.x. [DOI] [PubMed] [Google Scholar]
- 6.Shen JK, Ding YM, Zhou WJ, Jin J. Polymyositis/dermatomyositis associated with acute myelocytic leukemia. Rheumatol Int. 2008;12:1265–7. doi: 10.1007/s00296-008-0600-1. [DOI] [PubMed] [Google Scholar]
- 7.Ledwich LJ, Olenginski TP. A rare lymphoma in a patient with amyopathic dermatomyositis. Am J Clin Dermatol. 2010;11:151–5. doi: 10.2165/11530170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Parnell AP, Frew I. Case report: non-Hodgkin’s lymphoma presenting as an encasing pleural mass. Br J Radiol. 1995;68:926–7. doi: 10.1259/0007-1285-68-812-926. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad H, Pawade J, Falk S, Morgan JA, Balacumaraswami L. Primary pleural lymphomas. Thorax. 2003;58:908–9. doi: 10.1136/thorax.58.10.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munoz MA, Conejo-Mir JS, Congregado-Loscertales M, Holgado C, Moya F, Loscertales J. The utility of positron emission tomography to find an occult neoplasm in a patient with dermatomyositis. J Eur Acad Dermatol Venereol. 2007;21:1418–9. doi: 10.1111/j.1468-3083.2007.02220.x. [DOI] [PubMed] [Google Scholar]
- 11.Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, Vidaller-Palacín A, Martínez-Gómez X, Trallero-Araguás E, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123:558–62. doi: 10.1016/j.amjmed.2009.11.012. [DOI] [PubMed] [Google Scholar]