Abstract
Purpose
Gastric signet ring cell carcinoma (GSRC) is known to have low fluorodeoxyglucose (FDG) uptake. The aim of the study was to investigate the relation between FDG uptake and glucose transporter (GLUT)-1 expression and clinicopathologic parameters in cases of GSRC.
Materials and Methods
Forty patients (28 men, mean age 54 ± 12 years) with histologically confirmed GSRC who underwent pre-operative [18F]FDG PET/CT were enrolled. Maximum standardized uptake values (SUVmax) were compared with clinicopathologic parameters and GLUT-1 expression. Cases were divided based on GLUT-1 expression in tumor tissues into a membranous group (n = 17) and a cytoplasmic group (n = 23).
Results
Mean SUVmax was significantly higher in the membranous group than in the cytoplasmic group (6.06 ± 2.79 vs. 3.67 ± 1.54, P = 0.03). Gastric wall invasion, depth of invasion, extent of LN metastasis, overall stage, and tumor size were found to be related to SUVmax. On the other hand, age, sex, and the presence of distant metastasis were not related to SUVmax. Multivariate analysis revealed that membranous GLUT-1 expression and the extent of LN metastasis independently predicted high FDG uptake.
Conclusions
This study demonstrates that high FDG uptake is mediated by membranous GLUT-1 expression in GSRC.
Keywords: Signet ring cell, Stomach cancer, FDG, Glucose transporter-1
Introduction
Gastric signet ring cell carcinoma (GSRC) is a histological type defined by the microscopic characteristics described by the World Health Organization. The prevalence of GSRC among patients with gastric cancer has been reported to range from 3.4 to 39% [1–3], and, in South Korea, incidences of 12.2 and 8.7% have been reported [4, 5]. One group of researchers reported that GSRC shows a slightly higher predominance among females and younger individuals [1], whereas others have found no gender difference [3, 5–7]. From the prognostic point of view, several authors have demonstrated that GSRC does not have a poorer prognosis than the other types of stomach cancer [1, 2, 5, 7], but the issue remains controversial. Others have suggested that, among advanced gastric cancers, GSRC has a poorer prognosis [4, 6].
Positron emission tomography (PET) with 2-[18F]fluoro-2-deoxy-D-glucose (FDG) has been widely used for detecting primary tumors, staging, planning treatment, monitoring after treatment, and for prognostic evaluations [8, 9]. FDG is actively transported into the intracellular space by glucose transporter (GLUT) and then phosphorylated to FDG-6-phosphate by hexokinase. Because FDG-6-phosphate is not a substrate of the tricarboxylic acid cycle, it accumulates within cells. Eventually, it is dephosphorylated by glucose-6-phosphatase, which allows the efflux of FDG to the extracellular space. Most tumors are known to show increased glucose metabolism, which provides energy for cell growth and produces the precursors required for nucleotide and lipid synthesis [10, 11]. In several cancer types, malignant cells exhibit increased FDG uptake mediated by elevated levels of GLUT and hexokinase [12–15].
Although several previous studies have reported that GSRCs generally show low FDG uptake [16–18], we have experienced several GSRCs showing intense FDG uptake on PET/CT. Furthermore, the cause of this variability in FDG uptake has not been evaluated. Accordingly, the aim of this study was to investigate the significance of GLUT-1 expression and other clinicopathologic parameters that may affect FDG uptake in GSRC.
Materials and Methods
Patients
A total of 42 consecutive GSRC patients underwent preoperative [18F]FDG PET/CT and surgical excision of primary tumors between December 2003 and October 2008. After excluding two diabetic patients, 40 patients were enrolled in this study. All information on patient’s characteristics and clinicopathologic findings (Borrmann’s classification, depth of invasion, tumor size, and TNM stage) was collated retrospectively by medical record review. The mean interval between PET/CT and surgery was 4 days. All [18F]FDG PET/CT images were reviewed blindly on a workstation. Patients were allocated to a membranous group and a cytoplasmic group according to immunohistochemical staining results. This study was approved by the institutional review board (AJIRB-MED-KSP-09-256).
FDG PET/CT Acquisition and Image Analysis
After fasting for least 6 h, patients were administered 370 MBq of [18F]FDG intravenously. All patients were instructed to rest comfortably for 60 min and to urinate before scanning. Whole-body PET/CT images were obtained on a Discovery ST scanner (GE Healthcare, Milwaukee, WI, USA). Seven to eight frames (3 min/frame) of emission PET data were acquired in two-dimensional mode after a noncontrast CT scan from the base of the skull to the upper thigh (tube rotation time of 1 s per revolution, 120 kV, 60 mA, 7.5 mm per rotation, and acquisition time of 60.9 s for a scan length of 867 mm). Emission PET images were reconstructed using an iterative method (ordered-subsets expectation maximization with 2 iterations and 30 subsets, field of view = 600 mm, slice thickness = 3.27 mm) and attenuation-corrected with noncontrast CT. Standardized uptake values (SUVs) were calculated based on injected dose and body weight. Two nuclear medicine physicians reviewed PET/CT images blindly on an AW workstation (version 4.4) by consensus. For the semiquantitative analysis of FDG uptake, polygonal regions of interest (ROIs) were placed on primary tumors and maximum SUVs (SUVmax) were recorded. When primary tumors on PET/CT images were indistinguishable because of low FDG uptake, ROIs were copied from noncontrast CT images.
Immunohistochemical Analysis
One representative tumor section containing the highest proportion of malignant cells was chosen for each patient for immunohistochemical staining, which was conducted by a board-certified pathologist (K.J.H). All specimens were fixed with 10% formalin and embedded in a paraffin block. Sections with a thickness of 4 μm were incubated in an oven for 1 h, deparaffinized with xylene for 20 min, and rehydrated. Immunohistochemical staining was performed using an automated staining system (Autostainer, Lab Vision, Fremont, CA, USA). Sections were then subjected to heat-induced antigen retrieval in citrate buffer (pH 6.0) using a microwave oven at 37°C for 15 min, and endogenous peroxidase activity was blocked by treating them with 3% hydrogen peroxidase for 5 min. They were then washed with Tris-buffered saline, reacted with Ultra V Block (Thermo Fisher Scientific, Fremont, CA, USA) for 10 min, incubated with primary polyclonal antibody at a dilution of 1:250 for GLUT-1 (ab652; Abcam, Cambridge, UK) for 40 min at 37°C, and treated with primary antibody enhancer (Thermo Fisher Scientific, Fremont) for 15 min and then with HPR polymer (Thermo Fisher Scientific, Fremont) as secondary antibody for 25 min. After Tris-buffered saline washing, sections were treated with diaminobenzidine to visualize the brown signal. All sections were then counterstained with hematoxylin-eosin, dehydrated, and mounted. Erythrocytes in tissue sections served as positive controls of GLUT-1 expression. An experienced pathologist (J.H.K.) evaluated GLUT-1 staining in the absence of clinical information. According to the location of GLUT-1 staining in signet ring cells, sections were divided into a membranous group and a cytoplasmic group.
Statistical Analysis
Statistical analysis was performed using Medcalc (version 9.6.4). Relations between SUVmax and other parameters were evaluated using the Mann-Whitney U test and the Kruskall-Wallis test. Multivariate regression analysis was used to identify independent predictors of FDG uptake. Also multivariate analysis was performed between SUVmax and significant clinicopathologic factors in univariate analysis. P values less than 0.05 were considered significant.
Results
Patient’s Characteristics
Patients’ characteristics, type of surgery, and preoperative endoscopic findings are summarized in Table 1. The proportion of FDG-avid GSRCs was 42% (17 of 40). Subtotal gastrectomy was the major type of surgery in this study (28/40). Based on preoperative endoscopic findings, most patients (33/40) had advanced gastric cancer (AGC), and Borrmann type III was the commonest endoscopic type.
Table 1.
Characteristics, type of surgery, and endoscopic findings of the patients
| Variables | Values |
|---|---|
| Number of patients | 40 |
| Proportion of FDG-avid GSRC (%) | 42 |
| Age (years) | 54 ± 12 |
| Sex (M:F) | 28:12 |
| Type of surgery (n) | |
| Total gastrectomy | 12 |
| Subtotal gastrectomy | 28 |
| Endoscopic Borrmann type | |
| AGC | 33 |
| I | 1 |
| II | 4 |
| III | 19 |
| IV | 9 |
| EGC | 7 |
FDG Fluorodeoxyglucose, GSRC gastric signet ring carcinoma, AGC advanced gastric cancer, EGC early gastic cancer
Univariate Analysis of the Associations Between FDG Uptake and Clinicopathologic Parameters
Results of univariate analysis between FDG uptake and other clinicopathologic parameters including GLUT-1 expression are summarized in Table 2. Mean SUVmax in the membranous group was significantly higher than in the cytoplasmic group (6.06 ± 2.79 vs. 3.67 ± 1.54, P = 0.03, Fig. 1). Of the clinicopathologic parameters, gastric wall invasion, T stage, extent of LN metastasis, stage, and tumor size were found to be significantly related to FDG uptake. Advanced gastric cancer (AGC) had significantly higher FDG uptake than early gastric cancer (EGC). SUVmax was found to increase significantly with T stage (T3–4 vs. T1–2: 3.60 ± 2.19 vs. 5.58 ± 2.96, P = 0.0019) and N stage (N2–3 vs. N0–1: 6.63 ± 3.40 vs. 3.39 ± 1.13, P = 0.0002). GSRCs >3 cm showed higher FDG uptake than those ≤3 cm. Age, sex, and the presence of distant metastasis were not found to be related to FDG uptake.
Table 2.
Univariate analysis between FDG uptake and clinicopathologic variables in GSRC
| Factor | Number | SUVmax (mean ± SD) | P value |
|---|---|---|---|
| Age (years) | |||
| <55 | 21 | 5.04 ± 3.26 | |
| ≥55 | 19 | 4.38 ± 2.34 | 0.70 |
| Sex | |||
| Male | 28 | 4.66 ± 2.79 | |
| Female | 12 | 4.78 ± 2.44 | 0.77 |
| Gastric wall invasion | |||
| AGC | 30 | 5.35 ± 2.81 | |
| EGC | 10 | 2.71 ± 1.23 | 0.0007† |
| Borrmann type | |||
| II | 1 | 4.1 | |
| III | 17 | 5.28 ± 3.58 | |
| IV | 12 | 5.56 ± 1.95 | 0.4134 |
| Depth of invasion | |||
| T1 | 9 | 2.66 ± 1.23 | |
| T2 | 9 | 4.54 ± 2.79 | |
| T3 | 19 | 5.66 ± 2.81 | |
| T4 | 3 | 5.06 ± 1.10 | 0.004* |
| Extent of LN metastasis | |||
| N0 | 16 | 2.86 ± 1.21 | |
| N1 | 8 | 4.16 ± 1.19 | |
| N2 | 5 | 6.7 ± 2.78 | |
| N3 | 11 | 6.6 ± 2.39 | 0.006* |
| Distant metastasis | |||
| Negative | 36 | 4.78 ± 2.82 | |
| Positive | 4 | 3.95 ± 2.84 | 0.91 |
| Stage | |||
| Ia | 8 | 2.56 ± 1.23 | |
| Ib | 7 | 3.08 ± 1.09 | |
| II | 3 | 3.8 ± 0.49 | |
| IIIa | 5 | 5.02 ± 0.77 | |
| IIIb | 5 | 6.07 ± 2.78 | |
| IV | 12 | 6.3 ± 2.39 | 0.001* |
| Tumor size | |||
| <3 cm | 10 | 2.2 ± 0.48 | |
| ≥3 cm | 30 | 8.03 ± 3.69 | 0.0001† |
| GLUT-1 expression | |||
| Membranous | 17 | 6.06 ± 2.79 | |
| Cytoplasmic | 23 | 3.67 ± 1.54 | 0.03† |
P values were evaluated using *Kruskal-Wallis and †Mann-Whitney U test
FDG Fluorodeoxyglucose, GSRC gastric signet ring cell carcinoma, SUVmax maximum standardized uptake value, AGC advanced gastric cancer, EGC early gastric cancer, GLUT-1 glucose transporter-1
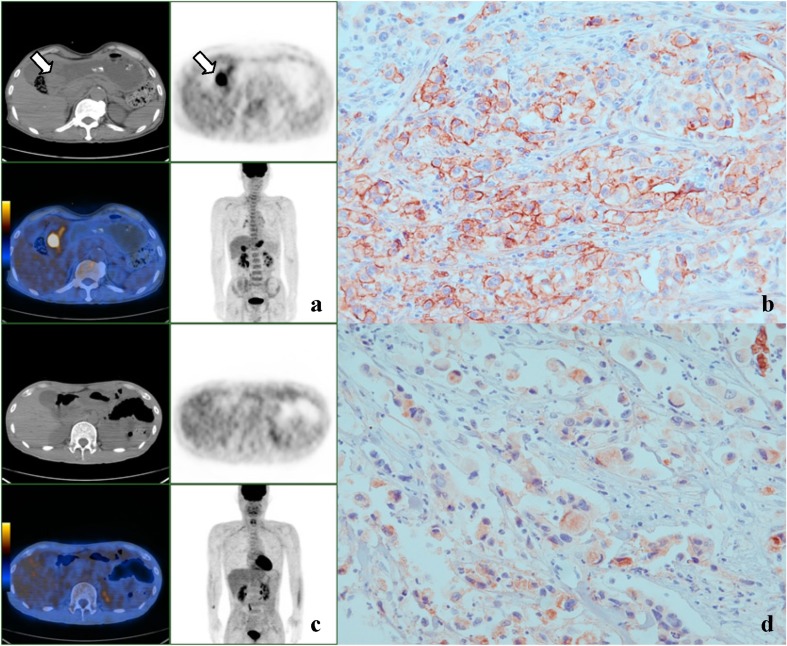
Fig. 1 a–d.
Representative PET and immunohistochemical staining images for GSRC with GLUT-1 expression in membrane and cytoplasm. a, b A case of stage IV GSRC showing intense FDG uptake (SUVmax 8.4) in the gastric antrum (arrow) and membranous GLUT-1 staining. c, d Another case of stage IV GSRC showing no significant FDG uptake in gastric wall and cytoplasmic GLUT-1 staining (×400)
Multivariate Analysis Between FDG Uptake and Clinicopathologic Parameters
As shown in Table 3, multivariate regression analysis between FDG uptake and clinicopathologic variables showed that GLUT-1 staining (P = 0.0056) and N stage (P = 0.0001) were independent predictors for high FDG uptake.
Table 3.
Significant variables affecting FDG uptake in GSRC on multivariate analysis
| Variable | Estimate coefficient | Standard error | P value |
|---|---|---|---|
| GLUT-1 staining | 1.96 | 0.67 | 0.0056 |
| LN metastasis | 1.20 | 0.26 | 0.0001 |
FDG Fluorodeoxyglucose, GSRC gastric signet ring cell carcinoma, GLUT-1 glucose transporter-1
Adjusted R 2 = 0.446
Discussion
In this study, we evaluated the relation between the clinicopathologic findings of GSRC including GLUT-1 expression, and FDG uptake on PET/CT images. It was found that 42% of GSRCs showed membranous GLUT-1 expression, and these GSRCs had significantly higher SUVmax values than those showing cytoplasmic expression. This result suggests that a considerable proportion of GSRCs show elevated FDG uptake due to the membranous expression of GLUT-1. As far as we know, this is the first report to be issued in South Korea of an association between FDG uptake and GLUT-1 expression in GSRC. The majority of previous studies evaluated this association in all types of gastric cancer and not specifically in GSRC.
Degree of GLUT-1 expression has previously been shown to be correlated with increased FDG uptake in a variety of human malignancies [12–15]. Similarly, in gastric carcinoma, GLUT-1 expression has been shown to be significantly correlated with FDG uptake (P = 0.002). Median SUVmax has been reported to be higher in gastric cancers with detectable GLUT-1 expression (6.9, range 2.3–14.1) than in those without GLUT-1 expression (3.1, range 1–8.8) [26]. The above-mentioned results could explain the variability of FDG uptake in gastric cancer during initial staging.
Previous studies reported that nonintestinal gastric cancers, including GSRC, show lower FDG uptake than the intestinal type. Stahl et al. reported that 9 of 22 nonintestinal gastric cancers (42%) and 15 of 18 intestinal cancers (83%) showed detectable FDG uptake by visual analysis [20]. In another study by Yamada et al., noncohesive gastric cancer was found to show significantly lower GLUT-1 expression and FDG uptake than the cohesive type [24]. De Potter et al. also reported that only 25% of GSRCs had FDG avidity in a small group study (n = 8) [21], which concurs with that found in the Korean population [22]. Han et al. concluded that the intestinal type has significantly higher SUVmax values than the nonintestinal type [22]. In addition, Kawamura et al. found that the expression of GLUT-I was higher in papillary and tubular adenocarcinoma than in signet ring cell and mucinous adenocarcinoma [19]. However, in one recent study, 78% of GSRCs (7/9) were included in an FDG-avid group [23]. In the present study, 42% of GSRCs had detectable FDG uptake on PET by visual analysis, and GSRCs with membranous GLUT-1 expression showed significantly higher FDG uptakes than those with cytoplasmic expression, which suggests that the degree of FDG uptake is affected by GLUT-1 expression in signet ring cells. These results are consistent with those of previous studies [19, 24] that examined the relation between FDG uptake and GLUT-1 expression in gastric cancer.
In addition to GLUT-1 expression, a number of clinicopathologic factors were also found to be significantly related to FDG uptake. In particular, primary tumor size of GSRC was found to be significantly associated with SUVmax, which concurs with the results of earlier studies [20, 22–25]. Furthermore, in the present study, depth of invasion (T stage), regional LN involvement (N stage), and overall GSRC stage were found to be significantly related to FDG uptake of the primary tumor, which also closely agrees with the results of previous studies [23, 25] and suggests that FDG uptake is closely associated with tumor progression in GSRC. On the other hand, these results indicate that the diagnostic ability of FDG PET in EGC of the signet ring cell type is likely to be lower than in AGC of the signet ring cell type.
In the present study, multivariate regression analysis showed that GLUT-1 staining and regional lymph node involvement were independently related to FDG uptake, which concurs with a previous report issued by Yamada et al. in which GLUT-1 expression was found to be the factor that influenced FDG uptake in gastric carcinoma [24].
This study has several limitations that require consideration. First, it was based on a retrospective analysis performed at a single institution, and some of the patients with GSRC did not undergo surgery because of advanced stage disease. Accordingly, caution should be exercised when generalizing our findings. Second, gastric mucosa often shows physiologic FDG uptake on PET images. In the present study, we adopted the water-induced gastric distention method to determine FDG uptake in primary gastric tumors in about one-third of our patients, but the analysis was conducted using nondistended PET images for homogeneity reasons. We believe that these limitations warrant a future prospective study incorporating the water distention protocol. Third, as FDG uptake is specifically regulated by type II hexokinase and other glucose transporters, their correlation with FDG uptake in GSRC is also worthy of further investigation.
In conclusion, our findings suggest that FDG uptake in primary GSRC tumors is mediated by membranous expression of GLUT-1.
Acknowledgments
This work was supported by the 2008 grant from Department of Medical Sciences, The Graduate School, Ajou University (J.K. Yoon).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Maehara Y, Sakaguchi Y, Moriguchi S, Orita H, Korenaga D, Kohnoe S, et al. Signet ring cell carcinoma of the stomach. Cancer. 1992;69:1645–1650. doi: 10.1002/1097-0142(19920401)69:7<1645::AID-CNCR2820690702>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 2.Theuer CP, Nastanski F, Brewster WR, Butler JA, Anton-Culver H. Signet ring cell histology is associated with unique clinical features but does not affect gastric cancer survival. Am Surg. 1999;65:915–921. [PubMed] [Google Scholar]
- 3.Antonioli DA, Goldman H. Changes in the location and type of gastric adenocarcinoma. Cancer. 1982;50:775–781. doi: 10.1002/1097-0142(19820815)50:4<775::AID-CNCR2820500425>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 4.Kim JP, Kim SC, Yang HK. Prognostic significance of signet ring cell carcinoma of the stomach. Surg Oncol. 1994;3:221–227. doi: 10.1016/0960-7404(94)90037-X. [DOI] [PubMed] [Google Scholar]
- 5.Kim DY, Park YK, Joo JK, Ryu SY, Kim YJ, Kim SK, et al. Clinicopathological characteristics of signet ring cell carcinoma of the stomach. ANZ J Surg. 2004;74:1060–1064. doi: 10.1111/j.1445-1433.2004.03268.x. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Kim S, Lai JF, Hyung WJ, Choi WH, Choi SH, et al. Advanced gastric carcinoma with signet ring cell histology. Oncology. 2007;72:64–68. doi: 10.1159/000111096. [DOI] [PubMed] [Google Scholar]
- 7.Hyung WJ, Noh SH, Lee JH, Huh JJ, Lah KH, Choi SH, et al. Early gastric carcinoma with signet ring cell histology. Cancer. 2002;94:78–83. doi: 10.1002/cncr.10120. [DOI] [PubMed] [Google Scholar]
- 8.Endo K, Oriuchi N, Higuchi T, Iida Y, Hanaoka H, Miyakubo M, et al. PET and PET/CT using 18F-FDG in the diagnosis and management of cancer patients. Int J Clin Oncol. 2006;11:286–296. doi: 10.1007/s10147-006-0595-0. [DOI] [PubMed] [Google Scholar]
- 9.Schoder H, Larson SM, Yeung HW. PET/CT in oncology: integration into clinical management of lymphoma, melanoma, and gastrointestinal malignancies. J Nucl Med. 2004;45(Suppl 1):72S–81S. [PubMed] [Google Scholar]
- 10.Ong LC, Jin Y, Song IC, Yu S, Zhang K, Chow PK. 2-[18F]-2-deoxy-D-glucose (FDG) uptake in human tumor cells is related to the expression of GLUT-1 and hexokinase II. Acta Radiol. 2008;49:1145–1153. doi: 10.1080/02841850802482486. [DOI] [PubMed] [Google Scholar]
- 11.Southworth R, Parry CR, Parkes HG, Medina RA, Garlick PB. Tissue-specific differences in 2-fluoro-2-deoxyglucose metabolism beyond FDG-6-P: a 19F NMR spectroscopy study in the rat. NMR Biomed. 2003;16:494–502. doi: 10.1002/nbm.856. [DOI] [PubMed] [Google Scholar]
- 12.de Geus-Oei LF, van Krieken JH, Aliredjo RP, Krabbe PF, Frielink C, Verhagen AF, et al. Biological correlates of FDG uptake in non-small cell lung cancer. Lung Cancer. 2007;55:79–87. doi: 10.1016/j.lungcan.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Paudyal B, Oriuchi N, Paudyal P, Higuchi T, Nakajima T, Endo K. Expression of glucose transporters and hexokinase II in cholangiocellular carcinoma compared using [18F]-2-fluro-2-deoxy-D-glucose positron emission tomography. Cancer Sci. 2008;99:260–266. doi: 10.1111/j.1349-7006.2007.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamada K, Tomita Y, Qiu Y, Zhang B, Ueda T, Myoui A, et al. 18F-FDG-PET of musculoskeletal tumors: a correlation with the expression of glucose transporter 1 and hexokinase II. Ann Nucl Med. 2008;22:699–705. doi: 10.1007/s12149-008-0173-9. [DOI] [PubMed] [Google Scholar]
- 15.Shim HK, Lee WW, Park SY, Kim H, So Y, Kim SE. Expressions of glucose transporter types 1 and 3 and hexokinase-II in diffuse large B-cell lymphoma and other B-cell non-Hodgkin’s lymphomas. Nucl Med Biol. 2009;36:191–197. doi: 10.1016/j.nucmedbio.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Bhure U, Schmitt AM, Pestalozzi BC, Hany TF, Strobel K. FDG-negative signet ring cell cancer of the stomach with FDG-positive skin metastases. Clin Nucl Med. 2007;32:226–228. doi: 10.1097/01.rlu.0000255241.97811.4d. [DOI] [PubMed] [Google Scholar]
- 17.Buyyounouski MK, Klump WJ, Konski A, Wu H, Adler LP. FDG PET imaging of signet-ring cell adenocarcinoma of the stomach. Clin Nucl Med. 2005;30:118–119. doi: 10.1097/00003072-200502000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Yoshioka T, Yamaguchi K, Kubota K, Saginoya T, Yamazaki T, Ido T, et al. Evaluation of 18F-FDG PET in patients with advanced, metastatic, or recurrent gastric cancer. J Nucl Med. 2003;44:690–699. [PubMed] [Google Scholar]
- 19.Kawamura T, Kusakabe T, Sugino T, Watanabe K, Fukuda T, Nashimoto A, et al. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer. 2001;92:634–641. doi: 10.1002/1097-0142(20010801)92:3<634::AID-CNCR1364>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 20.Stahl A, Ott K, Weber WA, Becker K, Link T, Siewert JR, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging. 2003;30:288–295. doi: 10.1007/s00259-002-1029-5. [DOI] [PubMed] [Google Scholar]
- 21.De Potter T, Flamen P, Van Cutsem E, Penninckx F, Filez L, Bormans G, et al. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging. 2002;29:525–529. doi: 10.1007/s00259-001-0743-8. [DOI] [PubMed] [Google Scholar]
- 22.Han EJ, Choi WH, Chung YA, Kim KJ, Maeng LS, Sohn KM, et al. Comparison between FDG uptake and clinicopathologic and immunohistochemical parameters in pre-operative PET/CT scan of primary gastric carcinoma. Nucl Med Mol Imaging. 2009;43:26–34. [Google Scholar]
- 23.Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg. 2004;28:247–253. doi: 10.1007/s00268-003-7191-5. [DOI] [PubMed] [Google Scholar]
- 24.Yamada A, Oguchi K, Fukushima M, Imai Y, Kadoya M. Evaluation of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography in gastric carcinoma: relation to histological subtypes, depth of tumor invasion, and glucose transporter-1 expression. Ann Nucl Med. 2006;20:597–604. doi: 10.1007/BF02984657. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Cheong JH, Yun MJ, Kim J, Lim JS, Hyung WJ, et al. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer. 2005;103:2383–2390. doi: 10.1002/cncr.21074. [DOI] [PubMed] [Google Scholar]
- 26.Alakus H, Batur M, Schmidt M, Drebber U, Baldus SE, Vallböhmer D, Prenzel KL, Metzger R, Bollschweiler E, Hölscher AH, Mönig SP. Variable 18F-fluorodeoxyglucose uptake in gastric cancer is associated with different levels of GLUT-1 expression. Nucl Med Commun. 2010;31(6):532–538. doi: 10.1097/MNM.0b013e32833823ac. [DOI] [PubMed] [Google Scholar]