Abstract
Purpose
18F-fluoride bone positron emission tomography (PET) has been reported as a useful bone imaging modality. However, no clinical bone PET study had been performed previously in Korea. The authors investigated the usefulness of 18F-fluoride bone PET in Korean patients with malignant or benign bone disease.
Methods
Eighteen consecutive patients (eight women, ten men; mean age, 55 ± 12 years) who had undergone 18F-fluoride bone PET for the evaluation of bone metastasis (n = 13) or benign bone lesions (n = 5) were included. The interpretation of bone lesions on 18F-fluoride bone PET was determined by consensus of two nuclear medicine physicians, and final results were confirmed using combination of all imaging studies and/or clinical follow-up. The analysis was performed on the basis of lesion group.
Results
Thirteen patients with malignant disease had 15 lesion groups, among which seven were confirmed as metastatic bone lesions and eight were confirmed as non-metastatic lesions. 18F-fluoride bone PET correctly identified six of seven metastatic lesions (sensitivity, 86%), and seven of eight non-metastatic lesions (specificity, 88%). On the other hand, five patients with benign conditions had five bone lesion groups; four were confirmed as benign bone diseases and the other one was confirmed as not a bone lesion. 18F-fluoride bone PET showed correct results in all the five lesion groups.
Conclusions
18F-fluoride bone PET showed promising potential for bone imaging in Korean patients with malignant diseases as well as with various benign bone conditions. Therefore, further studies are required on the diagnostic performance and cost-effectiveness of 18F-fluoride bone PET.
Keywords: 18F-fluoride, Bone, Positron emission tomography
Introduction
18F-fluoride has been a clinically useful bone imaging agent since the early 1960s; that is before positron emission tomography (PET) technology was introduced [1]. The main mechanism for bone uptake of 18F-fluoride is chemisorption, which is also the mechanism for the uptake of 99mTc-labeled phosphonate compounds. With this mechanism, the OH− ion of hydroxyapatite crystals is replaced with 18F−. As 18F-fluoride has double the bone uptake and faster blood clearance than 99mTc-labeled phosphonate compounds, it may provide better image quality than 99mTc-labeled phosphonate compounds [2]. The United States Food and Drug Administration approved clinical application of 18F-fluoride in 1972 [3]. However, 18F-fluoride has not been widely used in clinical practice because its high-energy photon (511 keV) is inadequate for Anger-type gamma cameras. On the other hand, 99mTc-labeled phosphonate compounds were introduced in early 1970s, and rapidly spread to be the most widely used bone imaging agents due to the excellent availability of 99mTc and its compatibility with Anger-type gamma cameras [4]. Accordingly, clinicians have paid limited attention to 18F-fluoride as a bone imaging agent.
Since the early 1990s, however, PET scanners and cyclotrons have been disseminated worldwide, provoking renewed interest in 18F-fluoride bone PET, and a number of papers on 18F-fluoride bone PET have been published. 18F-fluoride bone PET has been shown to be useful for evaluating bone diseases, including both malignant [5–7] and benign [8–11] diseases. Moreover, the diagnostic accuracies of 18F-fluoride bone PET for bone diseases have been reported to be superior to those of 99mTc-labeled phosphonate compounds [3]. However, no clinical study on 18F-fluoride bone PET had been reported in Korea. In this study, we investigated the clinical usefulness of 18F-fluoride bone PET in Korean patients with malignant or benign bone diseases.
Materials and Methods
Patients
Eighteen consecutive patients (eight women, ten men; mean age 55 ± 12 years) who had undergone 18F-fluoride bone PET were enrolled in this study. Underlying diseases were malignant diseases in 13 (two prostate cancers, three thyroid cancers, five breast cancers, one stomach cancer, one colon cancer, and one multiple myeloma) (Table 1) and benign conditions in five patients (one intramuscular hemangioma, one post-traumatic syndrome, one infectious spondylitis, one arthritic joint pain, one non-specific joint pain) (Table 2). The purpose of 18F-fluoride bone PET in the patients with malignant disease was the differential diagnosis of bone lesions that were equivocal on other imaging studies [bone scan, 18F-FDG PET, magnetic resonance imaging (MRI), or computed tomography (CT)] (n = 8) or the re-evaluation of known bone metastasis (n = 5). The purpose of 18F-fluoride bone PET in the patients with benign condition was the evaluation of bone invasion of intramuscular hemangioma (n = 1), evaluation of bone destruction by infectious spondylitis (n = 1), and the identification of bone pain etiology (n = 3).
Table 1.
Characteristics of patients with underlying malignant diseases (M male, F female, BS bone scan using 99mTc-HDP, NC no change, TP true positive, TN true negative, FN false negative, FP false positive, meta (+) metastasis present, meta (–) metastasis absent, F/U follow-up, TP true positive, RT radiotherapy)
| No. | Age | Sex | Underling disease | Issues on bone metastasis | Bone PET finding | No. of lesion group | Final result | Accuracy of bone PET |
|---|---|---|---|---|---|---|---|---|
| 1 | 61 | M | Prostate cancer | Equivocal on BS | Meta (+) | 1 | Meta (+) on MR, start RT | TP |
| 2 | 47 | F | Thyroid cancer | Equivocal on BS | Meta (−) | 1 | Meta (−) on F/U (14 months) | TN |
| 3 | 45 | F | Breast cancer | Equivocal on BS | Meta (−) | 1 | Meta (−) on F/U (17 months) and BS NC | TN |
| 4 | 63 | F | Breast cancer | Equivocal on BS | Meta (−) | 1 | Meta (−) on F/U (17 months) and BS (−) | TN |
| 5 | 63 | M | Thyroid cancer | Equivocal on BS | Meta (−) | 1 | Meta (−) on F/U (12 months) and BS NC | TN |
| 6 | 49 | F | Breast cancer | Equivocal on BS, FDG-PET, and MRI | Meta (+) | 1 | Meta (+) on clinical context | TP |
| 7 | 53 | F | Breast cancer | Equivocal on CT and MRI | Meta (−) | 1 | Meta (+) on clinical context | FN (small lesions) |
| 8 | 47 | F | Breast cancer | Known bone meta, aggravation on MRI, equivocal on BS, humerus | Meta (+), meta (−) humerus | 2 | Meta (+) on chemo change, meta (−) humerus | TP, TN |
| 9 | 57 | M | Multiple myeloma | Known bone meta on MRI, shoulder and elbow pain | Meta (+), meta (−) shoulder and elbow | 2 | Meta (+) on FDG−PET, meta (−) shoulder, elbow on F/U (12 months) | TP, TN |
| 10 | 55 | M | Stomach cancer | Known bone meta on BS, intractable shoulder pain | Meta (+) | 1 | Meta (+) on clinical context | TP (more extensive bone meta than BS |
| 11 | 62 | M | Thyroid cancer | Known bone meta s/p op and RT | Meta (+) | 1 | Meta (−) on I-131 scan | FP (post-op change) |
| 12 | 84 | M | Prostate cancer | Known bone meta on BS s/p hormonal therapy | Meta (+) | 1 | Meta (+) on clinical context | TP |
| 13 | 59 | M | Colon cancer | Equivocal on CT | Meta (−) | 1 | Meta (−) on F/U (6 months) and FDG-PET | TN |
Table 2.
Characteristics of the five patients with benign conditions (SD syndrome)
| No. | Age | Sex | Underling disease | Issues on bone lesion | Bone PET finding | No. of lesion group | Final result | Accuracy of bone PET |
|---|---|---|---|---|---|---|---|---|
| 1 | 25 | F | Intramuscular hemangioma | Mild uptake on BSa | Bone lesion (+) | 1 | Bone lesion (+) on X-ray | TP (more extensive bone lesion than BS) |
| 2 | 50 | F | Post-traumatic SD | Bone pain etiology | Bone lesion (+) | 1 | Bone lesion (+) on clinical context | TP |
| 3 | 60 | M | Infectious spondylitis | Mild uptake on BS | Bone lesion (+) | 1 | Bone lesion (+) on MRI and clinical context | TP (more extensive bone lesion than BS) |
| 4 | 50 | M | Lt. hip pain | Bone pain etiology | Bone lesion (−) | 1 | Bone lesion (−) on F/U (10 months) | TN |
| 5 | 56 | M | Low back pain | Bone pain etiology | Bone lesion (+) | 1 | Bone lesion (+) on treatment response | TP |
Acquisition and Interpretation of 18F-Fluoride Bone PET
18F-fluoride bone PET images were acquired using a dedicated PET scanner (Allegro, Philips Medical Systems, USA). Food intake or parenteral infusion of sugar-containing fluids was not prohibited. 18F-fluoride of 0.14 mCi/kg was administered by intravenous injection and image acquisition was started 50 min after administration. Images were acquired from the skull to the upper thigh or from the upper thigh to the feet, to include lesions of interest. Images were acquired over seven to nine beds, and the acquisition time per bed was 2.5 min for emission scan plus 40 s for transmission scan. Images were reconstructed using the three-dimensional row action maximum likelihood algorithm.
18F-fluoride bone PET images were interpreted by consensus between two nuclear medicine physicians. Prominent 18F-fluoride uptake (uptake clearly discriminated from surrounding normal bone) were considered positive bone uptake. For bone metastasis evaluation, a lesion was interpreted based on the intensity and location of uptake. 18F-fluoride uptake in the definite degenerative lesions such as bony spur or known traumatic lesion was excluded from the analysis. Afterwards, interpretation was dichotomized into presence or absence of bone metastasis [metastasis (+) or (−)]. Interpretation of benign conditions was also dichotomized into presence or absence of benign bone lesions [bone lesion (+) or (−)].
Lesion Group Analysis
The diagnostic accuracy of bone PET was investigated on the basis of lesion group. This lesion group analysis is basically similar to a patient-basis analysis, but is more practical. A lesion group was defined as a group of bone lesions that could be regarded as lesions of the same characteristics. For example, if a cancer patient complained of pain in two sites, it was classified as one lesion group (Fig. 3). Similarly, multiple metastatic bone lesions detected in the staging work-up were also classified as one lesion group (Fig. 4). On the other hand, if a cancer patient with known bone metastasis presented new bone lesions (patients 8 and 9 in Table 1), the patient was considered to have two lesion groups.
Fig. 3a, b.
18F-fluoride bone PET confirmed the presence and absence of bone metastasis. a The 57-year-old male patient with multiple myeloma had multiple hypermetabolic bone lesions (long arrows) by 18F-FDG PET (MIP anterior view image). He complained of non-specific pain at the right shoulder and elbow joints. b18F-fluoride bone PET showed multiple metastatic lesions compatible with hypermetabolic lesions, but no other lesions were found. There was no evidence of bone metastasis at right shoulder or elbow joints areas at 12 months after the bone PET study
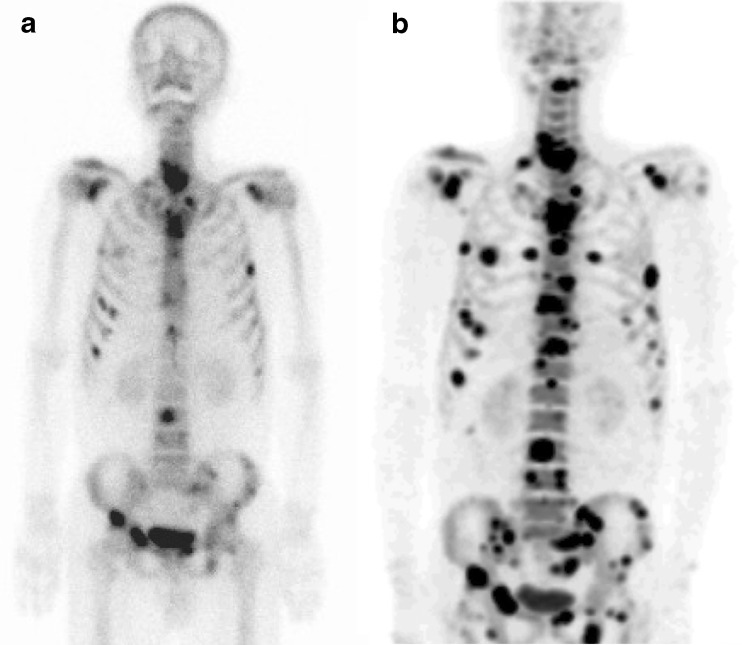
Fig. 4a, b.
18F-fluoride bone PET identified more metastatic lesions than 99mTc-HDP bone scan. a A 55-year-old male patient with stomach cancer showed multiple bone lesions suggestive of metastases in his 99mTc-HDP bone scan. b Subsequent 18F-fluoride bone PET identified many more metastatic lesions
Confirmation of 18F-Fluoride Bone PET Findings
18F-fluoride bone PET findings were considered true-positive when subsequent imaging studies demonstrated the presence of the bone lesion; and if not, bone PET findings were considered false-positive. 18F-fluoride bone PET findings were considered true-negative when subsequent imaging studies and follow-up clinical studies demonstrated no evidence of a bone lesion in concordance with bone PET findings; and if not, bone PET findings were considered false-negative.
Results
Malignant Diseases
In 13 patients with malignant disease, 15 lesion groups were identified; seven were metastatic bone lesions and eight were not. The sensitivity of 18F-fluoride bone PET for detecting bone metastasis was 86% (6/7), and its specificity was 88% (7/8) (Table 3). The single false-negative case (patient 7 in Table 1) was multiple spinal metastases, the sizes of which were less than 3 mm. In this case, metastasis was confirmed by subsequent CT and MRI, although tissue biopsy was not confirmed. The single false-positive case (patient 11 in Table 1) showed increased 18F-fluoride uptake around the lumbar spine. In this case, operation and radiotherapy had already been performed for the previously confirmed lumbar metastasis, and the uptake was diagnosed as non-specific uptake after operation and radiotherapy.
Table 3.
Diagnostic accuracy of 18F-fluoride bone PET for detecting bone metastases in 13 patients with underlying malignant disease (15 lesion groups)
| Bone metastasis (+) | Bone metastasis (−) | ||
|---|---|---|---|
| Bone PET (+) | 6 | 1 | 7 |
| Bone PET (−) | 1 | 7 | 8 |
| 7 | 8 | 15 |
Figure 1 shows a true-positive 18F-fluoride PET finding in a prostate cancer patient (patient 1 in Table 1). In this patient, an equivocal lesion on 99mTc-HDP bone scan showed definite uptake on 18F-fluoride bone PET, and confirmed as metastasis. Figure 2 shows a true-negative 18F-fluoride bone PET finding in a breast cancer patient (patient 3 in Table 1). In this patient, bone scan showed an equivocal bone lesion, while 18F-fluoride bone PET clearly demonstrated the location of uptake as facet joint. The patient was diagnosed as facet joint arthritis.
Fig. 1.
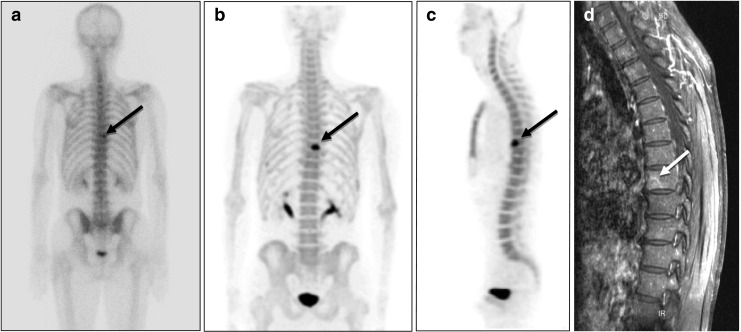
18F-fluoride bone PET of a 61-year-old male patient with prostate cancer. a A suspected to have bone metastasis in the T-8 area (black arrow) in a 99mTc-HDP bone scan (posterior whole body image). b18F-fluoride bone PET readily revealed bone metastasis at the T-8 vertebral body in a maximum intensity projection (MIP) posterior view image, and c in a sagittal image. d T1-weighted gadolinium-enhanced MRI also demonstrated metastasis at the T-8 vertebral body (white arrow). Radiation therapy was applied to the lesion, and a follow-up bone scan revealed improvement
Fig. 2a–d.
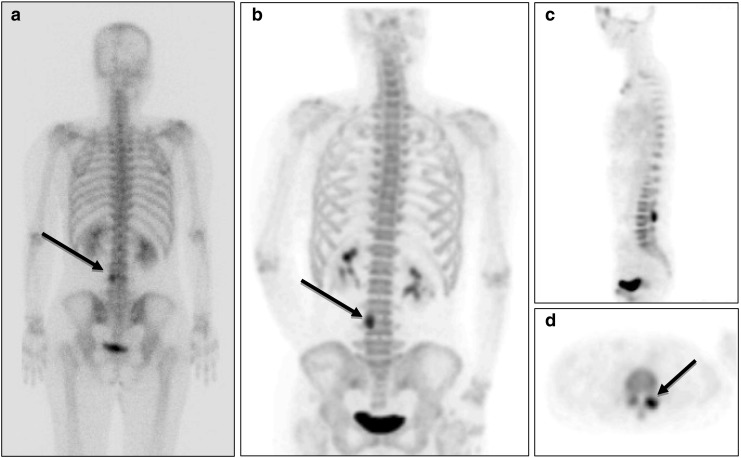
18F-fluoride bone PET ruled out the presence of bone metastasis. a A 45-year-old female patient with breast cancer was suspected of having bone metastasis at L-4 (black arrow) in a 99mTc-HDP bone scan (posterior whole body image). b18F-fluoride bone PET indicated that the lesion was located at left L3-4 facet joint in a MIP posterior view image, c a sagittal image, and d a transaxial image. A follow-up bone scan conducted at 17 months after the bone PET study did not show any change in the lesion, and the patient showed no clinical evidence of bone metastasis
In most cases, 18F-fluoride bone PET showed metastatic bone lesions more definitely than 18F-FDG PET. In a patient with multiple myeloma (patient 9 in Table 1), bone involvement of multiple myeloma was more definitely depicted on 18F-fluoride bone PET than on 18F-FDG PET (Fig. 3). In addition, assessment of number and extent of bone metastasis was more accurate with 18F-fluoride bone PET than with 99mTc-HDP bone scan. In a patient with stomach cancer (patient 10 in Table 1), 18F-fluoride bone PET showed much more metastatic lesions of wider extent than 99mTc-HDP bone scan (Fig. 4).
Benign Conditions
In five patients with benign conditions, five bone lesion groups were identified (Table 2). In four patients, bone lesions were correctly detected on 18F-fluoride bone PET, which was confirmed by other imaging study and follow-up. In another one patient, the absence of bone lesion was also correctly determined on 18F-fluoride bone PET. Therefore, all the five lesion groups were correctly diagnosed on 18F-fluoride bone PET (Table 4).
Table 4.
Diagnostic accuracy of 18F-fluoride bone PET for detecting benign bone lesions in five patients without underlying malignant disease (five lesion groups)
| Bone lesion (+) | Bone lesion (−) | ||
|---|---|---|---|
| Bone PET (+) | 4 | 0 | 4 |
| Bone PET (−) | 0 | 1 | 1 |
| 4 | 1 | 5 |
The extent of a benign bone lesion was more accurately assessed on 18F-fluoride bone PET than on 99mTc-HDP bone scan, as was in malignant diseases. In a patient with intramuscular hemangioma (patient 1 in Table 2), the reactive bone lesions of the right tibia and fibula attributed to intramuscular hemangioma of the right tibialis posterior muscle were more clearly delineated from surrounding normal bone on 18F-fluoride bone PET than on 99mTc-HDP bone scan (Fig. 5). Also in another benign bone disease (patient 3 in Table 2), the extent of bone lesion was greater on bone PET than on bone scan.
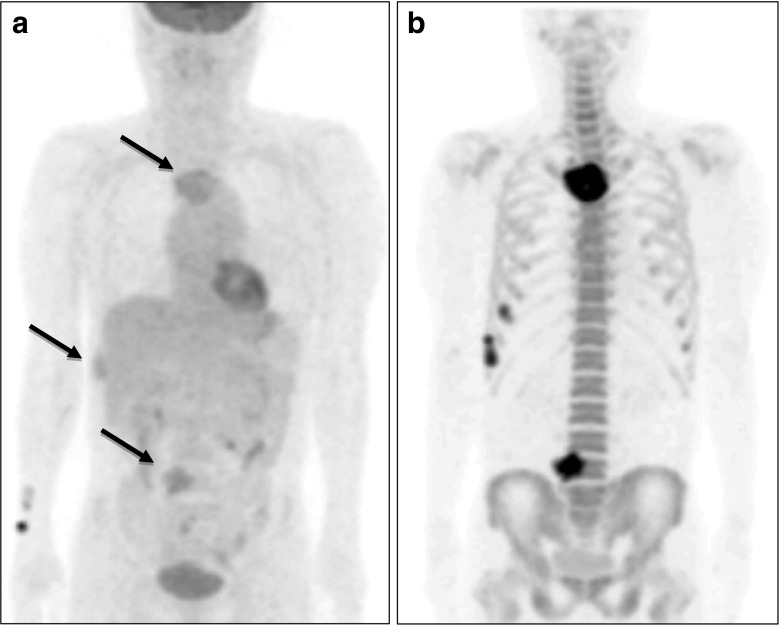
Fig. 5a–c.
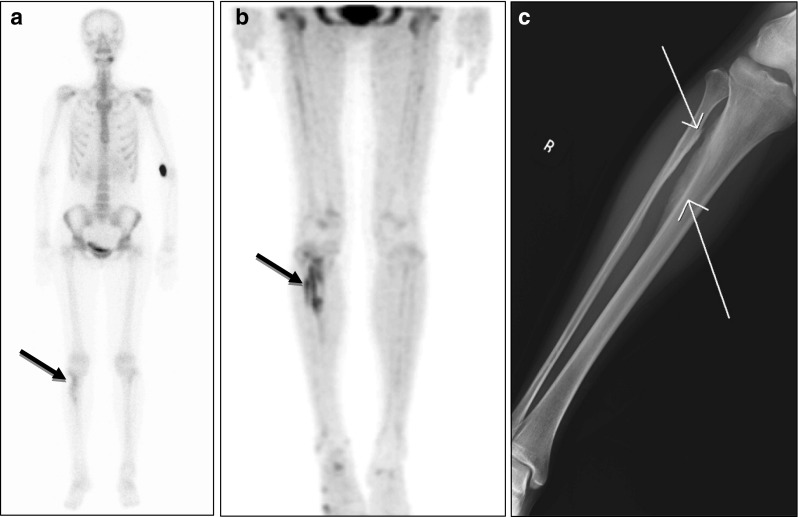
18F-fluoride bone PET depicted more intense and more extensive reactive bone lesions than 99mTc-HDP bone scan. a A 25-year-old female patient with intramuscular hemangioma in the right tibialis posterior muscle was found to have reactive bone lesions in the right tibia and fibular by 99mTc-HDP bone scan (black arrow). b However, 18F-fluoride bone PET demonstrated more intensive and extensive bone lesions. c Simple radiography visualized reactive bone lesions of the right tibia and fibular (white arrows)
Discussion
This is the first clinical report on 18F-fluoride bone PET in Korea. In the present study, patients were divided into two groups: a malignant bone disease group (n = 13) and a benign condition group (n = 5). The diagnostic accuracy of 18F-fluoride bone PET was excellent in both the groups. The single false-negative finding on 18F-fluoride PET was attributable to its low spatial resolution because the lesions were very small, and the single false-positive finding was related to underlying bone destruction or inflammation after operation and radiotherapy. Therefore, when bone metastasis was an issue in the management of malignant disease, 18F-fluoride bone PET could provide the information on the presence of bone metastasis. Benign conditions were also effectively diagnosed using 18F-fluoride bone PET. Of the whole 20 lesion groups evaluated in this study, 18 (90%) were correctly identified by 18F-fluoride bone PET.
In malignant bone diseases, the sensitivity and the specificity were calculated as 86% and 88%, respectively. However, the specificity of 18F-fluoride bone PET in this study should be interpreted with caution because uptake of 18F-fluoride in degenerative lesions was excluded from the analysis. Although uptake of 18F-fluoride in degenerative lesions such as bony spur or spinal endplate is a frequently observed finding and can be easily differentiated on 18F-fluoride bone PET, it is usually not a concern in the evaluation of bone lesions in cancer patients. If such bone lesions had been included, the specificity of 18F-fluoride bone PET would be much higher.
Patients with benign bone diseases are also good candidates for 18F-fluoride bone PET. Bone turnover was successfully evaluated using dynamic 18F-fluoride bone PET [10] in bone graft [8] and in gastrectomy-induced osteopenia [9]. As in malignant diseases, 18F-fluoride bone PET is a promising bone imaging method in benign bone conditions. Because 18F-fluoride has better chemical and physical characteristics than 99mTc-phosphonate compounds, 18F-fluoride PET can provide images of higher quality and better correlation with bone metabolism. Also in this study, 18F-fluoride bone PET showed good results in benign conditions, especially in the assessment of the etiology of bone pain (Table 2), like previous reports [12, 13]. In terms of radiation safety, radiation exposure in 18F-fluoride PET was reported to be similar to that in bone scans using 99mTc-phosphonate compounds [3].
In this study, the analysis was performed on the basis of lesion group. Because the number of bone lesions varied so much among the patients, the diagnostic performance would have been distorted if the analysis was performed on a lesion basis. For example, some patients (patients 7 and 10 in Table 1) had multiple bone lesions, which showed results similar to false-positive or true-positive, while most patients had only one bone lesion. These extreme cases may induce distorted results if the analysis was performed on a lesion basis. On the other hand, analysis on a patient basis also has some limitations. If a cancer patient complains of new bone pain, the pain site should be assessed apart from known bone metastasis. As a result, we adopted analysis on a lesion-group basis, and 20 lesion groups of 18 patients were included in the analysis.
The small number of patients enrolled in this study is a limitation for a more reliable conclusion about the diagnostic accuracy of 18F-fluoride bone PET. Another limitation of this study is that it was not a systematic comparison study between 18F-fluoride bone PET and well-established imaging studies such as bone scan, CT, and MRI. Because 18F-fluoride bone PET was performed at the request of clinicians, matched bone scan or MRI was not strictly acquired.
This is the first clinical study on 18F-fluoride bone PET in Korea, and showed promising potential of 18F-fluoride bone PET in malignant diseases and various benign conditions. Therefore, further studies are requested on the diagnostic performance of 18F-fluoride bone PET in comparison with other imaging modalities, and on the cost-effectiveness of 18F-fluoride bone PET.
References
- 1.Blau M, Ganatra R, Bender MA. 18F-fluoride for bone imaging. Semin Nucl Med. 1972;2:31–37. doi: 10.1016/S0001-2998(72)80005-9. [DOI] [PubMed] [Google Scholar]
- 2.Lee WW, So Y. Skeletal system. In: Chung JK, Lee MC, editors. Nuclear medicine. Seoul: Korea Medical Book Publisher; 2008. pp. 509–571. [Google Scholar]
- 3.Grant FD, Fahey FH, Packard AB, Davis RT, Alavi A, Treves ST. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med. 2008;49:68–78. doi: 10.2967/jnumed.106.037200. [DOI] [PubMed] [Google Scholar]
- 4.Thrall JH. Technetium-99 m labeled agents for skeletal imaging. CRC Crit Rev Clin Radiol Nucl Med. 1976;8:1–31. [PubMed] [Google Scholar]
- 5.Schirrmeister H, Guhlmann A, Kotzerke J, Santjohanser C, Kuhn T, Kreienberg R, et al. Early detection and accurate description of extent of metastatic bone disease in breast cancer with fluoride ion and positron emission tomography. J Clin Oncol. 1999;17:2381–2389. doi: 10.1200/JCO.1999.17.8.2381. [DOI] [PubMed] [Google Scholar]
- 6.Schirrmeister H, Glatting G, Hetzel J, Nussle K, Arslandemir C, Buck AK, et al. Prospective evaluation of the clinical value of planar bone scans, SPECT, and 18F-labeled NaF PET in newly diagnosed lung cancer. J Nucl Med. 2001;42:1800–1804. [PubMed] [Google Scholar]
- 7.Even-Sapir E, Metser U, Flusser G, Zuriel L, Kollender Y, Lerman H, et al. Assessment of malignant skeletal disease: initial experience with 18F-fluoride PET/CT and comparison between 18F-fluoride PET and 18F-fluoride PET/CT. J Nucl Med. 2004;45:272–278. [PubMed] [Google Scholar]
- 8.Piert M, Winter E, Becker GA, Bilger K, Machulla H, Muller-Schauenburg W, et al. Allogenic bone graft viability after hip revision arthroplasty assessed by dynamic [18F]fluoride ion positron emission tomography. Eur J Nucl Med. 1999;26:615–624. doi: 10.1007/s002590050429. [DOI] [PubMed] [Google Scholar]
- 9.Piert M, Zittel TT, Jahn M, Stahlschmidt A, Becker GA, Machulla HJ. Increased sensitivity in detection of a porcine high-turnover osteopenia after total gastrectomy by dynamic 18F-fluoride ion PET and quantitative CT. J Nucl Med. 2003;44:117–124. [PubMed] [Google Scholar]
- 10.Brenner W, Vernon C, Muzi M, Mankoff DA, Link JM, Conrad EU, et al. Comparison of different quantitative approaches to 18F-fluoride PET scans. J Nucl Med. 2004;45:1493–1500. [PubMed] [Google Scholar]
- 11.Brenner W, Vernon C, Conrad EU, Eary JF. Assessment of the metabolic activity of bone grafts with 18F-fluoride PET. Eur J Nucl Med Mol Imaging. 2004;31:1291–1298. doi: 10.1007/s00259-004-1568-z. [DOI] [PubMed] [Google Scholar]
- 12.Lim R, Fahey FH, Drubach LA, Connolly LP, Treves ST. Early experience with fluorine-18 sodium fluoride bone PET in young patients with back pain. J Pediatr Orthop. 2007;27:277–282. doi: 10.1097/BPO.0b013e31803409ba. [DOI] [PubMed] [Google Scholar]
- 13.Ovadia D, Metser U, Lievshitz G, Yaniv M, Wientroub S, Even-Sapir E. Back pain in adolescents: assessment with integrated 18F-fluoride positron-emission tomography-computed tomography. J Pediatr Orthop. 2007;27:90–93. doi: 10.1097/01.bpo.0000242438.11682.10. [DOI] [PubMed] [Google Scholar]