Abstract
Since the specific accumulation of iodide in thyroid was found in 1915, radioiodine has been widely applied to diagnose and treat thyroid cancer. Iodide uptake occurs across the membrane of the thyroid follicular cells and cancer cells through an active transporter process mediated by the sodium iodide symporter (NIS). The NIS coding genes were cloned and identified from rat and human in 1996. Evaluation of the NIS gene and protein expression is critical in the management of thyroid cancer, and several approaches have been tried to increase NIS levels. Identification of the NIS gene has provided a means of expanding its role in the radionuclide gene therapy of nonthyroidal cancers as well as thyroid cancer. In this article, we explain the relationship between NIS expression and the treatment of thyroid carcinoma with I-131, and we include a review of the results of our experimental and clinical trials.
Keywords: Sodium iodide symporter (NIS), Thyroid cancer, I-131, Gene therapy
Sodium/Iodide Symporter in Thyroid Cancer
The specific accumulation of iodide in the thyroid gland was discovered in 1915, and its clinical implications were quickly realized. Today, radioiodine is widely used to diagnose and treat thyroid disorders, such as thyroid cancer, and I-131 has been used as the treatment of choice for metastatic thyroid cancer for more than 60 years [1].
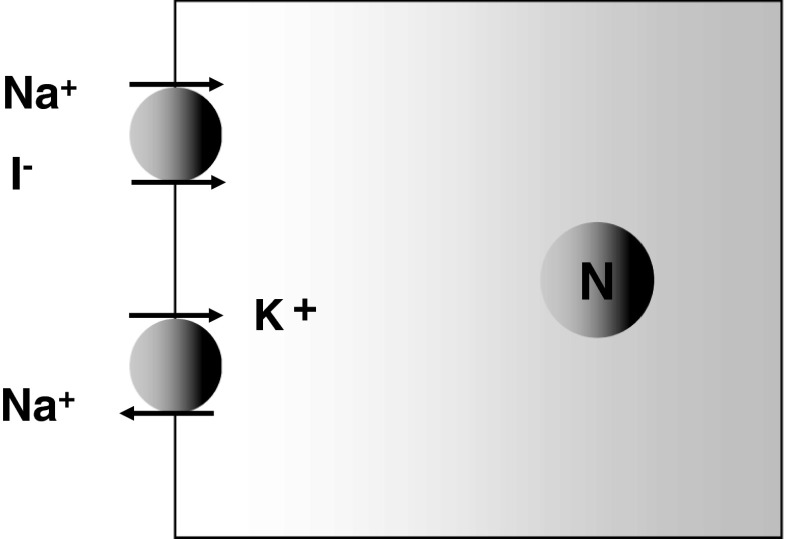
However, the mechanism responsible for this iodine specificity was determined only recently. It is now known that the iodide ion is transported into thyroid cells with a sodium ion by sodium/iodide symporter (NIS). Actually, the force driving iodide uptake is generated by the sodium ion transmembrane concentration gradient, which is produced and maintained by the sodium-potassium pump, ATPase (Fig. 1). The NIS gene was identified in 1996 [2], and its product, hNIS protein, is now known to be an intrinsic membrane protein with 13 transmembrane domains that is composed of 643 amino acids. Furthermore, the recent cloning of the NIS gene enabled the molecular mechanisms underlying iodide transport to be better understood, and has provided a means of expanding its role in the management of thyroid cancer.
Fig. 1.
Sodium iodide symporter (NIS)
I-131 Therapy in Differentiated Thyroid Cancer (DTC)
The incidence of thyroid carcinoma is rapidly increasing in many countries, including Korea, with the result that it is one of the most common cancers in Korean women [3]. DTC accounts for most thyroid cancers, and includes papillary thyroid carcinoma and follicular thyroid carcinoma. DTC is characterized by slow growth and a good prognosis, and improvements in diagnosis and treatment, due to the use of radioiodines, have reduced DTC-associated mortality [4]. Small amounts of NIS are present in thyroid carcinoma cells, and this is responsible for radioiodine uptake by metastatic lesions. However, iodine uptake is impaired in some thyroid carcinomas. In fact, approximately one third of all differentiated thyroid cancers and all anaplastic thyroid cancers do not concentrate radioiodine, and accordingly have a poor prognosis.
Two thousands thirty-six thyroid carcinoma patients have been treated at Seoul National University Hospital over the past 20 years: 1,873 patients with papillary thyroid carcinoma (92%) and 163 with follicular carcinoma (8%). Of these 2,036 patients, 468 (22.9%) had lymph node metastasis and/or distant metastasis according to I-131 post-therapy whole-body scans. In detail, 313 patients (15.4%) had regional lymph node metastasis, 83 (4.1%) mediastinal lymph node metastasis, 109 (5.3%) pulmonary metastasis, and 25 (1.2%) bone metastasis. Ninty-two patients (4.9% of the 1,873) with papillary cancer and 17 (10.4%) of the 163 patients with follicular cancer developed pulmonary metastasis, and 13 patients with papillary cancer (0.7%) and 12 of patients with follicular cancer (7.4%) developed bone metastasis.
In patients who underwent total thyroidectomy, 1.1 GBq of I-131 was administered to ablate remnant thyroid tissue, and when residual cancer tissue remained after surgery or when distant metastasis was suspected, more than 3.7 GBq of I-131 was used as a single therapeutic dose. When a post-therapy whole-body scan showed abnormal I-131 accumulation, I-131 therapy was repeated within 6 months (3.7–5.5 GBq for lung metastasis and 5.5–7.4 GBq for bone metastases), and when a post-therapy whole-body scan did not show abnormal accumulation, patients underwent an I-131 diagnostic scan within 6 months to confirm this finding.
Patients with pulmonary metastasis underwent several courses of I-131 therapy (range 3.7 to 7.4 GBq) over 40 months (range: 6–171 months) and were treated on average 5.1 times (range: 1–14 times). During follow-up, pulmonary metastasis completely disappeared in 38 of the 109 patients (35%), and partial remission occurred in 44 (Table 1, Fig. 2). Of these 109 patients, 45 patients (41%) had a diffuse pattern of I-131 lung uptake on whole-body scans. Furthermore, combined nodular and diffuse uptake was observed in 35 patients (32%), and nodular uptake without diffuse uptake in 29. When response to I-131 therapy was analyzed according to the pattern of I-131 uptake by pulmonary metastatic lesions, lesions with diffuse uptake were found to respond better than lesions with nodular uptake; of 45 patients with diffuse lung uptake, 51% achieved complete remission.
Table 1.
I-131 treatment results of lung metastases in patients with differentiated thyroid cancer
| Response | Patient no. |
|---|---|
| Complete remission | 38 (35%) |
| Partial remission | 44 (40%) |
| No change | 16 (15%) |
| Progression | 11 (10%) |
| Total | 109 |
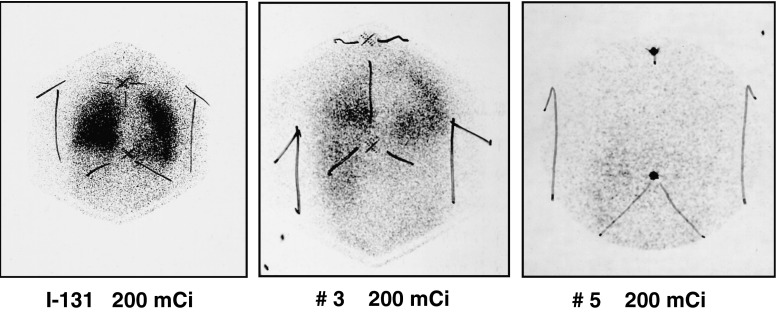
Fig. 2.
A 62-year-old-male post-thyroidectomy papillary cancer patient with lymph nodes and lung metastases. After serial I-131 treatment (200 mCi), metastatic lesions disappeared gradually, resulting in complete remission
Of the 25 patients with bone metastasis from differentiated thyroid carcinoma, 16 (64%) had multiple bone metastases in vertebrae and pelvic bones. A total of 105 bone lesions were detected on post-therapy scans: 40 in vertebra, 24 in pelvic bones, 12 in femurs, and 10 in skulls; others were detected in sternum, ribs, or clavicles. Of these 105 bone lesions, 75 were treated with I-131 alone. Five metastatic lesions were treated with I-131 and external radiotherapy, and 25 metastatic bone lesions were surgically removed because of a pathologic fracture or bone pain. Seventy-five of the 105 bone lesions were treated with I-131 alone, 34 lesions (45%) improved, and 41 showed no response or were aggravated. In particular, 9 of 25 bone lesions treated by surgical resection and I-131 therapy completely disappeared, whereas the other 16 showed I-131 accumulation after combined resection, and remained uncured after repeated I-131 treatment and radiotherapy. The five lesions treated by combined radiotherapy and I-131 achieved partial improvements (Table 2).
Table 2.
I-131 treatment results of bone metastases in patients with differentiated thyroid cancer (number of lesions)
| Treatment modality | Change in I-131 whole-body scan | |||
|---|---|---|---|---|
| Disappeared | Improved | No change or progressed | Total | |
| I-131 only | 0 | 34 | 41 | 75 |
| Op + I-131 | 7 | 12 | 0 | 19 |
| External RT + I-131 | 0 | 5 | 0 | 5 |
| Op + external RT + I-131 | 2 | 4 | 0 | 6 |
| Total | 9 | 55 | 41 | 105 |
Concordant with other reports, we found that I-131 therapy plays a key role in the management of metastatic DTC. Furthermore, our experience is that lung metastases are relatively well controlled by I-131 treatment, and that bone metastases require combined I-131 and surgical resection or external radiation therapy [5–7].
Gene Expression in Differentiated Thyroid Cancer
We evaluated the expressions of thyroid-specific genes, namely, NIS, thyroglobulin, thyroperoxidase, TSH-receptor, and pendrin, and that of glucose transporter-1 (Glut-1) gene using RT-PCR in human DTC tissues, and compared these expressions with the degree of thyroid cancer differentiation. Papillary carcinomas were classified as “relatively well differentiated” or as “relatively less differentiated.” The relatively well-differentiated group (n = 13) had well-formed arborized or broad papillae with a fibrovascular core, and typical cytologic features, i.e., a nuclear groove, pseudo-inclusion, and an empty-appearance [8]. On the other hand, the relatively less differentiated group (n = 11) occasionally contained follicular arrangements or a focal solid pattern, and tumor tissues were characterized by trabecular, diffusely sclerosing, or diffusely follicular patterns rather than well-formed papillae. Tumor cells were frequently columnar or oxyphilic with abundant eosinophilic cytoplasm (that is, they demonstrated ‘oxyphilic change’) accompanied with or without nuclear atypia.
Glut-1 expression was not observed in 1 of the 24 cases and no NIS mRNA expression in 2 cases. The other thyroid-specific genes were expressed in all cases. The relatively well-differentiated group showed higher levels of NIS, pendrin, and thyroglobulin expression, and a lower level of Glut-1 expression than the relatively less differentiated group (Table 3). In particular, a noticeable inter-group difference was observed for the mRNA expressions of NIS and Glut-1, which were found to be expressed at higher and lower levels, respectively, in the well-differentiated group.
Table 3.
The expression ratios of Glut-1 and thyroid-specific genes versus the beta-actin gene
| Well differentiated group (n = 13) | Less differentiated group (n = 11) | p-value (paired T-test) | |
|---|---|---|---|
| NIS | 0.67 (0.20) | 0.36 (0.05) | 0.0001 |
| 0.78 [0.30–0.87] | 0.36 [0.31–0.45] | ||
| Tg | 0.74 (0.16) | 0.60 (0.11) | 0.039 |
| 0.71 [0.56–0.99] | 0.65 [0.42–0.75] | ||
| TPO | 0.56 (0.18) | 0.49 (0.18) | 0.44 |
| 0.58 [0.22–0.89] | 0.47 [0.28–0.87] | ||
| TSH-R | 0.48 (0.27) | 0.39 (0.20) | 0.13 |
| 0.32 [0.16–0.84] | 0.23 [0.13–0.73] | ||
| PD | 0.65 (0.21) | 0.49 (0.08) | 0.036 |
| 0.69 [0.34–0.94] | 0.44 [0.43–0.68] | ||
| Glut-1 | 0.59 (0.07) | 0.66 (0.04) | 0.0027 |
| 0.57 [0.49–0.71] | 0.67 [0.59–0.72] |
Mean (SD); median [min-max]. Results are expressed as ratios versus beta-actin, n = number of samples. NIS, sodium iodide symporter; Tg, thyroglobulin; TPO, thyroperoxidase; TSH-R, thyroid-stimulating hormone receptor; PD, pendrin; Glut-1, glucose transporter-1
Thus, it appears the expressions of NIS and Glut-1 are inversely related, and that they reflect the biological characteristics of cancer differentiation. In particular, it appears that NIS expression decreases, whereas Glut-1 expression increases as dedifferentiation increases.
These findings could be useful for the management of thyroid carcinoma, especially in terms of (1) selecting between F-18-fluorodeoxyglucose positron emission tomography (FDG PET) and a radioiodine whole-body scan, and (2) for decision-making regarding I-131 therapy [9]. These observations are consistent with our experiences, namely that FDG PET is useful for detecting metastatic lesions negative for I-131 on whole-body scans. In our previous studies, we evaluated 108 postoperative patients with a negative radioiodine scan, and found that the sensitivity and specificity of FDG PET for the detection of recurrence were higher than those of serum thyroglobulin measurements [10, 11]. Hooft et al. [12] performed a meta-analysis on 14 articles published between 1996–2000, and concluded that FDG PET achieved a 82% detectability rate for recurrence in patients with an elevated serum thyroglobulin and a negative radioiodine whole-body scan.
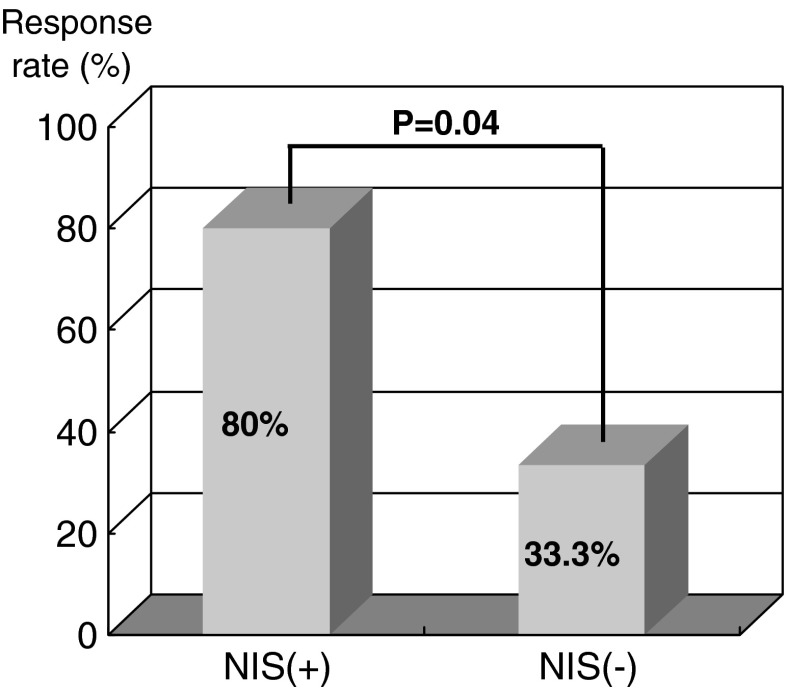
We previously evaluated the expressions of NIS protein in metastatic lesions by immunostaining, and compared these with the outcomes of I-131 therapy [13]. One third of patients negative for NIS immunostaining were found to respond to radioiodine therapy, whereas 80% of patients positive for NIS expression responded to I-131 therapy (Fig. 3), which suggests that thyroid cancer tissues expressing NIS take up more I-131 and that this improves response to radioiodine therapy.
Fig. 3.
Comparison of I-131 therapy effect according to NIS expression measured by immunostaining in metastases of differentiated thyroid cancer
BRAF point mutations are common in thyroid cancer, and their prevalences vary from 23 to 62% [14]. In particular, the V600E mutation is the only BRAF genetic alteration (BRAFV600E) found in papillary thyroid carcinoma, and is associated with low NIS expression and advanced disease at diagnosis. Recurrent thyroid cancers possessing BRAF mutations are almost always undetectable on I-131 scans [15]. Romei et al. [16] reported that the BRAFV600E mutation is significantly correlated with lower levels of NIS and thyroperoxidase at the mRNA and protein levels. Thus, it appears that the BRAFV600E mutation plays an important role in tumor dedifferentiation, which is associated with a loss of radioiodine avidity and treatment failure in recurrent DTC.
Enhancement of NIS in Thyroid Cancer Tissues
In Vitro Studies
The two main methods for increasing NIS levels in tissues involve increasing the expressions of transcription factors to enhance NIS promoter activity, for example, those of redox factor-1 (Ref-1), early growth response 1 (Egr1), paired box-8 (Pax-8), NIS TSH-responsive factor-1 (NTF-1), and thyroid transcription factor-1 (TTF-1) [17, 18], and the use of medications to enhance the expressions of NIS mRNA and protein. Regarding the first method, the NIS upstream promoter region has several regulatory sequences, including NIS upstream enhancer sequences (NUE). NUE is a strong TSH (thyroid-stimulating hormone) responsive enhancer and is highly conserved between species. To achieve full NUE promoter activity, cAMP-responsive element-binding protein (CREB) and Pax-8 are required [19]. In addition, an antioxidant stress nuclear factor, Ref-1, is also known to work with Egr1 and Pax-8 to stimulate NIS expression. Egr-1 is a ubiquitous early growth response transcription factor, and Pax-8 contains a thyroid-selective paired domain containing a transcription factor. TSH increases Ref-1 expression and stimulates its translocation into the nucleus, which promotes the DNA binding of Pax-8 [20, 21]. TTF-1 and NTF-1 have been reported to bind to proximal promoter sites and to contribute to thyroid-specific NIS expression, whereas diamide (an oxidizing agent) reduces NTF-1 binding to the cis-regulatory element, and dithiothreitol (a reducing agent) restores this binding [22]. Furthermore, the involvements of other transcription factors, such as, c-fos, c-jun, and ATF-2 (activating transcription factor-2), may enhance NIS expression by dimerizing with CREB [a basic-leucine zipper (B-ZIP) protein] [23], and the complex mediates DNA binding and dimerization to form homo or heterodimers with other B-ZIP proteins. However, the enhancement of NIS promoter activity by the over-expression of transcription factors has been successfully performed only by external gene therapy.
The second method involves the use of exogenous agents to enhance the expressions of NIS mRNA and protein. Several medications have been investigated as potential redifferentiation agents [24], such as nuclear receptor ligands, inhibitors of epigenetic modulation, and others (Table 4). Aromatic acids like phenylacetate and phenylbutyrate inhibit growth and induce redifferentiation in some cancers [25]. Phenylacetate is a metabolite of phenylbutyrate, and both are viewed as anti-neoplastic drugs with a cytostatic effect in cancer cells [26]. The basic mechanisms involved are unclear, but appear to be related to blocking tumor cells access to free glutamine, the isophenylation of ras family proteins, retinoic acid (RA) receptor activation, histone acetylation, PPAR-gamma activation, and demethylation [27].
Table 4.
Strategy to enhance the expression of NIS
| 1. Overexpression of transcription factors |
| Pax8, TTF-1 |
| 2. Chemicals |
| Retinoids |
| Aromatic fatty acids |
| Peroximal proliferator activated receptor-gamma agonists |
| Histone deacetylase inhibitors |
| DNA methylation inhibitors |
| Hsp90 inhibitors |
Retinoids are most commonly used to promote redifferentiation. Retinoic acids (RAs) are biologically active metabolites of vitamin A, which regulate the growths and differentiations of many cell types by binding specific nuclear receptors [28]. There are two types of retinoic acid receptors, retinoic acid receptor (RAR) and retinoid X receptor (RXR). These form complexes, and these complexes then bind to a specific DNA site, named the retinoic acid responsive element (RARE). After retinoic acid has bound to its receptor complex, RARE is activated and stimulates gene-regulated cell proliferation, differentiation, apoptosis, and homeostasis. Significant inductions of NIS by retinoic acid isomers, such as all trans-retinoic acid (atRA), 9-cis RA, and 13-cis RA, have been reported. atRA binds to all type of RAR isomers, but not to RXRs, whereas 9-cis RA binds to both RAR and RXR with equal affinity, and whereas 13-cis RA has low binding affinity with RARs, it stimulates RARs to bind to RARE. Retinoic acid has been suggested as an effective agent in several cancers like thyroid cancer, promyelocytic leukemia, skin, and lung cancer. Furthermore, in vitro studies using thyroid cancer cells have revealed that retinoic acid increases NIS gene expression, the translational activity of NIS protein, and that it decreases cell proliferation [29].
Peroxisome proliferator-activated receptor (PPAR)-gamma is a nuclear hormone receptor transcription factor that regulates cellular growth and differentiation. PPAR-gamma is also capable of binding with RXR, and Pax-8/PPAR-gamma fusion protein (PPFP) is found in almost 50% of follicular thyroid cancer cells. Though PPAR-gamma is expressed at very low levels in the thyroid, its agonists cause growth inhibition, apoptosis, and the redifferentiation of cancer cells. Furthermore, troglitazone has been reported to enhance NIS mRNA expression in follicular and papillary thyroid cancer cells [30]. Both troglitazone and rosiglitazone have been shown to inhibit cancer growth and subjected to clinical trials [31].
In thyroid cancer cells, epigenetic modifications, such as histone deacetylation and DNA hypermethylation, are commonly detected, and these processes are also relevant in the context of de-differentiation and proliferation. Histone deacetylase (HDAC) causes the compaction of nuclear chromatin, which induces the transcriptional repressions of genes that control cellular growth and differentiation. Many HDAC inhibitors are known to induce growth arrest and apoptosis. In thyroid cancer, depsipeptide, suberoylanide hydroxamic acid (SAHA), trichostatin A, and valporic acid have been examined in the context of enhancing NIS. Depsipetide increases NIS mRNA and iodide uptake in follicular, anaplastic, and papillary cancer cells [32], and treatments with SAHA and some nuclear hormone receptors, such as atRA, 9-cis RA, troglitazone, or thyroid hormone, were found to modestly induce the expression of NIS in an anaplastic cancer cell line [33]. Trichostatin A has also been reported to stimulate NIS mRNA expression in follicular, papillary, and Hurthle-cell cancer cell lines [34]. On the other hand, valporic acid was found to increase NIS mRNA and protein levels in papillary and anaplastic cancer cells that showed higher iodide uptakes [35]. Demethylation agents are another epigenetic reagent type, and 5-azacytidine has been reported to increase NIS mRNA and iodide uptake in papillary cancer cells [33, 36].
The post-translational regulation of NIS protein is usually associated with NIS trafficking and prolonging the half-life of NIS. Three-dimensional follicular structures have been reported to play a pivotal role in NIS enhancement [37], and in the same study, cellular polarity was found to be essential for the expression of functional NIS, which may promote NIS translocation to the membrane. In addition, Dohan et al. demonstrated the roles of NIS protein glycosylation and phosphorylation during membrane localization and iodide transport [38]. Cytoplasmic carboxy terminal domains of NIS containing a PDZ motif and a dileucine motif have been reported to be important for the translocalization of NIS to the plasma membrane [39]. Furthermore, TSH is likely to be involved in this post-translational regulation, and agents that regulate glycosylation or phosphorylation are possible candidate enhancers of functional NIS.
Clinical Studies
Clinical pilot studies have also shown that the ability to accumulate iodide may be restored by RA treatment [40–43]. However, the effectiveness and legitimacy of RA implementation in clinical practice remain controversial. For instance, a clinical study in 50 patients with iodide non-accumulating thyroid cancer produced encouraging results with a 75% biological response rate and a 20% overall response rate during follow-up [42]. However, another retrospective study reported only a 20% response rate by I-131 whole-body scans, which was disappointing given the results of the previous study. It appears that the clinical outcomes of RA treatment in advanced thyroid cancer have yet to be established, as do accurate cohort selection criteria.
We administered I-131 plus RA (oral isotretinoin) to 49 patients with metastatic DTC, who did not respond to conventional I-131 therapy. The dosing scheme was started at 1 mg/kg of body weight/day of isotretinoin for 6 weeks before I-131 therapy. After the initial 2 weeks of isotretinoin administration, we searched for side effects, such as dermatological problems and increases in liver enzymes, triglycerides, and cholesterol. When no significant complication was encountered, RA was incremented to 1.5 mg/kg body weight/day.
Clinical assessments were performed 6 months after I-131 plus RA treatment discontinuation. Complete response was achieved by one patient (2.3%) who had completed three courses of I-131 plus RA treatment, and this patient had maintained a complete response status at the last follow-up (at 56.8 months). Stable disease was observed in 19 patients (43.2%) and progressive disease in 24 (54.5%). When seven partial responses were included, the overall clinical response rate was 18.2% (n = 8), and when stable disease cases were included, the overall response rate reached 45.5% (Fig. 4).
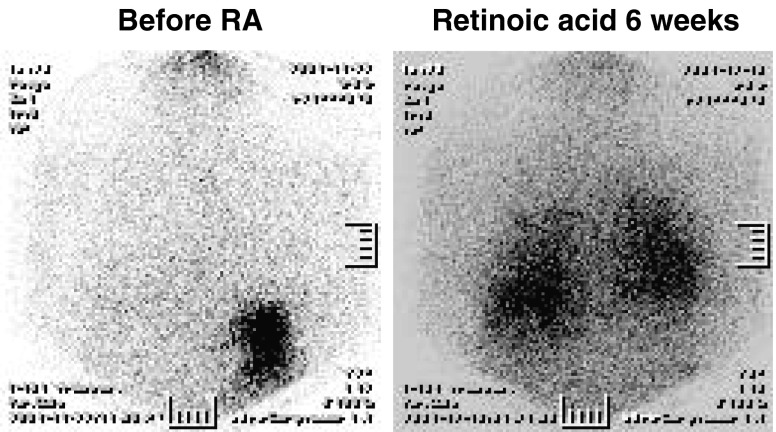
Fig. 4.
Change of post-therapy radioiodine whole-body scan (chest region) before and after retinoic acid (RA) treatment. A 53-year-old female papillary cancer patient with lung metastases initially showed no radioiodine uptake in lung lesions. However, radioiodine uptake increased after the combination therapy with retinoic acid
Another clinical trail is using rosiglitazone, a PPAR-gamma agonist. A phase II trial on rosiglitazone (a PPAR-gamma agonist) was conducted in 2006. Out of ten patients with refractory DTC, I-131 tumor uptake increased in four, and serum Tg decreased in two. Furthermore, in a trial conducted in Thailand in 2008 on 23 patients, it was found that 6 with recurrent/metastatic cancer showed increased I-131 uptake. In addition, it was found that PPAR-gamma receptor status (as determined by immunostaining) was related to I-131 accumulation in tumor tissues.
Radionuclide Gene Therapy Using NIS
Strategy
Approximately one-third of differentiated thyroid cancers and all anaplastic thyroid cancers do not concentrate radioiodine, which prevents the use of radioiodine for the diagnosis and treatment of metastatic thyroid cancer. However, the recent cloning and characterization of the NIS gene have paved the way for the development of a novel radionuclide gene therapy, because the targeted expression of functional NIS in cancer cells enables these cells to concentrate iodide from plasma, and therefore, offers the possibility of radioiodine therapy [1, 9, 44]. This strategy could also be applied to non-thyroid cancers. Several investigators have shown that the NIS gene can be transfected into a variety of cell types using nonviral or viral vectors, and that it increases radioiodine uptake by up to several hundred-fold [44, 45]. In addition, some authors have demonstrated that NIS gene transfer renders a variety of cells susceptible to I-131 [46–50, 52–56] (Table 5). Furthermore, combination therapies based on the targeting and the expression of the NIS gene plus radioiodine treatment could also be used to treat non-thyroid malignant diseases, such as melanoma, cervical, breast, liver, and colon cancer.
Table 5.
Summary of published data on NIS-expressing cancer cells and the therapeutic effects of NIS
| Researcher | Cell | Vector | Iodide uptake | Therapeutic effect |
|---|---|---|---|---|
| Spitzweg [47] | LNCap (human prostate cancer) | Adenovirus | 50 | In vivo (+) |
| Schipper [48] | QGP (pancreatic neuroendocrine tumor) | Transfection (electroporation) | 50 | In vitro (+) |
| Dingli [54] | ARH-77 (human myeloma) | Lentivirus | 18 | In vivo (+) |
| Nakamoto [45] | MCF-7 (human breast cacner) | Transfection (liposome) | 9 | NA |
| Haberkorn [44] | MH3924H (rat Morris hepatoma) | Retrovirus | 17 | NA |
| Boland [46] | SiHac (human cervix cancer) | Adenovirus | 4–25 | In vitro (+) |
| In vivo (-) | ||||
| Kang [49] | SK-Hep1 (human hepatocellular carcinoma) | Transfection (liposome) | 150 | In vitro (+) |
| Kim [53] | CT-26 (mouse colon cancer cell) | Lentivirus | 10 | In vivo (+) |
| Dwyer [52] | Capan-2 (pancreatic cancer cell line) | Adenovirus | 43 | In vivo (+) |
| Petrich [51] | K1 (papillary thyroid carcinoma) | Transfection (FuGENE) | 350 | In vivo (+) |
| Scholz [50] | HCT 116 (colon carcinoma cells) | Transfection (liposome) | 10 | In vitro (+) |
We previously transferred the NIS gene into human thyroid anaplastic cancer cells (ARO) and human hepatocellular carcinoma cells (SK-Hep1) by liposome transfection, and into mouse colon cancer cells (CT-26) by using a lentiviral vector [49, 53, 57]. The resulting NIS-expressing stable cell lines accumulated radioiodine between 10- to 120-fold more than controls. Furthermore, when we transplanted NIS-expressing cells into nude mice, xenografted NIS-expressing tumors were found to accumulate more I-125 than control tumors, and were clearly visualized by scintigraphy using Tc-99m, Re-188, or I-131. Because Re-188 and Tc-99m can be accumulated by the same transporter [58], radioiodine, Re-188 and Tc-99m are quite useful in therapeutic and imaging radionuclides. When we examined the possibility of performing radionuclide gene therapy using SK-Hep1 cells, it was found that the in vitro survival rate of NIS expressing hepatoma cells was reduced by 54% and 71% after 1 mCi/ml of I-131 or Re-188 treatment, respectively, as compared with parental cells [49]. In the case of CT-26 cells, tumor growth was inhibited in NIS-expressing cells by I-131, as determined by caliper measurements and Tc-99m scintigraphic imaging for up to 46 days post-tumor challenge [53].
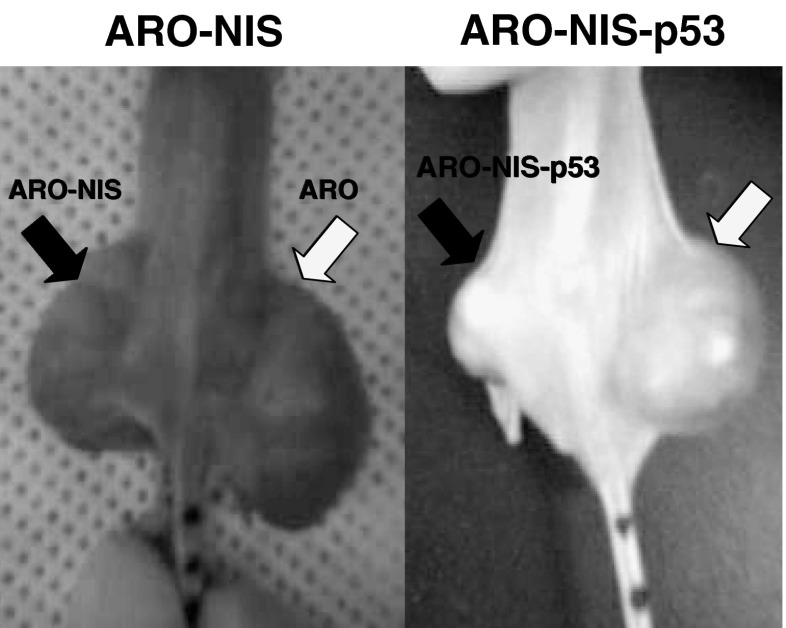
However, I-131 and Re-188 were found to have no cytotoxic effect on NIS-transfected ARO (ARO-N) cells. We found that SK-Hep1 cells express a wild-type functional p53 gene and that ARO cells express a mutated nonfunctional p53 gene. Subsequent intratumoral adenovirus infection with the p53 gene reduced ARO-N tumor sizes after Re-188 treatment in a tumor-xenograft model (Fig. 5). But ARO tumors with saline-treated mice and p53 gene-transferred mice showed tumor progression. In fact, only ARO-N tumors with p53 gene transfer exhibited tumor shrinkage after Re-188 treatment.
Fig. 5.
Radionuclide gene therapy in ARO and ARO-NIS tumor-bearing mice. At 10 days post-transplantation, adenovirus plus the wt-p53 gene was administered intratumorally. At 2 days post-transplantation, Re-188 (185 MBq) was injected intraperitoneally. At 4 weeks post-transplantation, mice were photographed using a digital camera. After Re-188 treatment, ARO-NIS tumors transfected with Ad-p53 showed tumor shrinkage
Modification
Although the future of the radionuclide gene therapy approach using NIS appears promising, some problems remain. The major problem concerns rapid efflux of radioiodides from anaplastic thyroid cancer cells and from non-thyroidal cells that lack the organification system required to trap radioiodides. Thyroperoxidase (TPO) gene cotransfection with NIS might provide a means of retaining radioiodine in cells. TPO iodinates the tyrosine residues of thyroglobulin and induces organification, which results in iodide accumulation within thyrocytes. In an attempt to organify iodide that has been taken up, Boland et al. [59] constructed a recombinant adenovirus encoding the human TPO gene under the control of cytomegalovirus early promoter (AdTPO), and observed significant increases in iodide organification in nonthyroidal tumor cells (human cervical tumor cells) coinfected with both AdTPO and AdNIS in the presence of exogenous hydrogen peroxide.
Another strategy to retain radioiodine that has been taken up involves transferring the genes of transcription factors. In malignant cells, structural and functional alterations of these factors play key roles during oncogenesis and induce different cancer phenotypes. Thyroid-specific transcription factors have been investigated in this context, and Pax8, TTF (thyroid transcription factor-1), and TTF-2 have been found among them. Furthermore, the expression of these factors caused the dedifferentiation of the thyroid cancer phenotype. To reactivate thyroid functions in thyroid cancer cells, Furuya et al. [60] constructed a TTF-1 expressing adenovirus (AdTTF-1). The infection of NIS gene-transfected thyroid cancer cells with AdTTF-1 was found to increase the amount of protein-bound radioiodide significantly and to prolong radioiodide efflux. Furthermore, in NIS-transfected thyroid cancer cell-xenografted nude mice, AdTTF-1 infections significantly induced iodide retention and organification in tumors. These results indicate that to retain radioiodides, TPO or TTF-1 expressions can specifically induce iodide organification and retard radioiodide uptake on non-thyroidal or thyroidal cancer cells.
The use of Re-188 or At-211, instead of I-131, offers another means of improving radionuclide NIS gene therapy. Re-188 and At-211 also accumulate in NIS expressing cells and xenografted tumors. Furthermore, the emission characteristics and physical properties of Re-188 are superior to those of I-131 (it has a shorter physical half-life and a higher beta energy than I-131; 16.9 h vs. 8 days and 0.764 vs. 0.134 MeV, respectively) [61]. One dosimetric calculation showed that the radiation dose administered by Re-188 is 4.5 times that of I-131 [61]. Alpha particle radionuclides are attractive radiopharmaceuticals for targeted cancer therapy because they are extremely cytotoxic due to a high LET (linear energy transfer of ∼100 keV/μm) and are potentially highly specific due to their short particle paths. The average alpha particle energy of At-211 is 6.8 MeV with a range of 55–80 μm, and it has a mean LET of 99 keV/μm [62]. In a study on the potential use of Re-188 as an alternative radionuclide, it was concluded that it had a therapeutic effect on NIS-expressing prostate cancer cells in an in vitro clonogenic assay [63]. After treating NIS-expressing prostate cancer xenografted mice with 55.5 MBq of I-131 or Re-188, smaller tumors were found to be reduced to similar extents, whereas larger tumors were significantly more reduced by Re-188. Furthermore, when we compared the therapeutic efficiencies of I-131 and Re-188 on NIS expressing hepatoma cells, the cytotoxicity of Re-188 was found to be superior to that of I-131 in an in vitro clonogenic assay [49]. In NIS gene transferred papillary thyroid cancer cells, At-211 induced complete tumor eradication and no tumor recurrence over a follow-up of 1 year in tumor-bearing nude mice [51]. Willhauck et al. [64] reported that At-211 had a significant therapeutic effect on NIS gene transferred prostate cancer cells in vivo and in vitro and suggested a potential role for At-211 as an attractive radionuclide for the NIS-targeted therapy of particularly small tumors.
Tumor-targeted gene expression has also been used to enhance radionuclide gene therapy using NIS. One example is provided by prostate-specific promoters, which can selectively target the NIS gene in prostate cancer cells. Spitzweg et al. established NP-1 tumors by stably transfecting LNCaP cells (a human prostatic adenocarcinoma cell line) with the NIS gene controlled by prostate-specific antigen promoter. Xenografted NP-1 tumors accumulated 25–30% of I-123 (biological T1/2 45 h). After a single injection of a therapeutic dose of 1-131 (3 mCi), significant tumor reductions were achieved (average volume reduction of 90%), and complete tumor regression was observed in up to 60% of tumors [65]. To target hepatoma, we prepared DNA constructs containing human α-fetoprotein (AFP) enhancer/promoter in the upstream region of the NIS gene, and transferred these into AFP-producing hepatocellular carcinoma (HCC) tumor cells (HuH-7) and AFP-nonproducing tumor cell line (C6). I-125 uptake was found to increase only in AFP-secreting HCC cells, and this uptake was completely blocked by treating cells with potassium perchlorate. AFP-NIS transferred HuH-7 tumors in nude mice were clearly visualized by scintigraphy after injecting Tc-99m, and their growths were significantly inhibited by 3 mCi of I-131 as compared with wild-type (HuH-7) and non-AFP producing (AFP-NIS transferred C6) tumors [66]. Willhauck et al. [67] examined the feasibility of HCC radioiodine therapy following hNIS gene transfer using a mouse AFP promoter construct to target NIS expression to HCC cells using liposomes. Stably transfected AFP-producing HCCs showed a 10- to 60-fold increase in iodide accumulation. Furthermore, in an in vitro clonogenic assay up to 78% and 93% of NIS-transfected Hepa 1–6 and HepG2 cells were found to be killed by I-131, whereas up to 96% of control cells survived. In addition, in NIS-expressing HepG2 xenografts, tumor growth was significantly inhibited after administering 1.5 mCi of I-131. Accordingly, HCC-targeted NIS gene expression using AFP enhancer/promoter appears to be a useful innovative treatment strategy for HCC. Telomerase reverse transcriptase (TERT) is active in highly proliferative cells like most cancer cells, germ cells, and stem cells. In one study to express NIS selectively in tumors, NIS gene expression driven by hTERT promoter was used [68]. A hNIS expressing stable cell line (HepG2:hTERT-positve HCC) under the control of 5mmTERT promoter was generated using a retroviral system. The radioiodine uptakes of 5mmTERT-NIS-transfected Hep3B cells were found to be 22 times greater than those of Hep3B cells, and survival rates were significantly lower than those of Hep3B cells after incubation with I-131. In addition, in a xenograft model, 5mmTERT-NIS-transfected Hep3B tumors were clearly visualized by Tc-99m scintigraphy. Furthermore, the tumor growth of Hep3B-5mmTERT-NIS was retarded by 3 mCi of I-131, whereas Hep3B tumor growth progressed.
Several factors reduce iodide uptake by thyroid cancer, and in addition to reduced NIS expression, TSH receptor, and transcription factors, a signal transduction failure and defects in the translocation of NIS protein to the cell membrane may all reduce radioiodide uptake. Therefore, to achieve successful radionuclide gene therapy, further detailed research on the mechanisms of NIS function and its regulation is required.
Conclusion
NIS activity provides the molecular basis for radioiodine therapy in thyroid cancer, and I-131 has been used effectively to treat metastatic thyroid carcinoma in the presence of the NIS gene and its protein. However, anaplastic thyroid cancers and some differentiated thyroid cancers do not accumulate sufficient radioiodine for treatment purposes. Several medications have been investigated in the context of increasing the expression of the NIS gene and protein, and of these retinoic acid and rosiglitazone, which have both been subjected to clinical trials, are the most promising. Currently, there is much research interest in the possibility of NIS gene transfer to facilitate the radioiodine therapy of thyroidal and of non-thyroidal cancers.
NIS offers a means of using radioiodine to manage metastatic thyroid cancer. Furthermore, we believe NIS could expand the role of molecular nuclear medicine in the management of thyroid carcinoma.
References
- 1.Chung JK. Sodium iodide symporter: its role in nuclear medicine. J Nucl Med. 2002;43:1188–1200. [PubMed] [Google Scholar]
- 2.Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- 3.Ministry of health and welfare (2002) Annual report of the Korea central cancer registry, 2003
- 4.Fernandes JK, Day TA, Richardson MS, Sharma AK. Overview of the management of differentiated thyroid cancer. Curr Treat Options Oncol. 2005;6:47–57. doi: 10.1007/s11864-005-0012-3. [DOI] [PubMed] [Google Scholar]
- 5.Bernier MO, Leenhardt L, Hoang C, Aurengo A, Mary JY, Menegaux F, et al. Survival and therapeutic modalities in patients with bone metastases of differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2001;86:1568–1573. doi: 10.1210/jc.86.4.1568. [DOI] [PubMed] [Google Scholar]
- 6.Stojadinovic A, Shoup M, Ghossein RA, Nissan A, Brennan MF, Shah JP, et al. The role of operations for distant metastatic well-differentiated thyroid carcinoma. Surgery. 2002;131:636–643. doi: 10.1067/msy.2002.124732. [DOI] [PubMed] [Google Scholar]
- 7.Shoup M, Stojadinovic A, Nissan A, Ghossein RA, Frredman S, Brennan MF, et al. Prognostic indicators of outcomes in patients with distant metastases from differentiated thyroid carcinoma. J Am Coll Surg. 2003;197:191–197. doi: 10.1016/S1072-7515(03)00332-6. [DOI] [PubMed] [Google Scholar]
- 8.Hedinger C, Williams ED, Sobin LH, et al. Histological typing of thyroid tumours. World Health Organization International Histological Classification of Tumours. Berlin: Springer Verlag; 1988. [Google Scholar]
- 9.Chung JK, Kang JH. Translational research using the sodium/iodide symporter in imaging and therapy. Eur J Nucl Med Mol Imaging. 2004;31:799–802. doi: 10.1007/s00259-004-1475-3. [DOI] [PubMed] [Google Scholar]
- 10.Chung JK, So Y, Lee JS, Choi CW, Lim SM, Hong SW, et al. Value of FDG PET in papillary thyroid carcinoma with negative 131I whole-body scan. J Nucl Med. 1999;40:986–992. [PubMed] [Google Scholar]
- 11.Wang W, Macapinlac H, Larson SM, Yeh SD, Akhurst T, Finn RD, et al. [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography localizes residual thyroid cancer in patients with negative diagnostic 131I whole body scans and elevated serum thyroglobulin levels. J Clin Endocrinol Metab. 1999;84:2291–2302. doi: 10.1210/jc.84.7.2291. [DOI] [PubMed] [Google Scholar]
- 12.Hooft L, Hoekstra OS, Deville W, Lips P, Tulder GJ, Boers M, et al. Diagnostic accuracy of F-18 fluorodeoxyglucose positron emission tomography in the follow-up of papillary or follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86:3779–3786. doi: 10.1210/jc.86.8.3779. [DOI] [PubMed] [Google Scholar]
- 13.Min JJ, Chung JK, Lee YJ, Jeong JM, Lee DS, Jang JJ, et al. Relationship between expression of the sodium/iodide symporter and I-131 uptake in recurrent lesions of differentiated thyroid carcinoma. Eur J Nucl Med. 2001;28:639–645. doi: 10.1007/s002590100509. [DOI] [PubMed] [Google Scholar]
- 14.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 15.Riesco-Eizaguirre G, Gutiérrez-Martínez P, García-Cabezas MA, Nistal M, Santisteban P. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I-targeting to the membrane. Endocr Relat Cancer. 2006;13:257–269. doi: 10.1677/erc.1.01119. [DOI] [PubMed] [Google Scholar]
- 16.Romei C, Ciampi R, Faviana P, Agate L, Molinaro E, Bottici V, et al. BRAFV600E mutation, but not RET/PTC rearrangements, is correlated with a lower expression of both thyroperoxidase and sodium iodide symporter genes in papillary thyroid cancer. Endocr Relat Cancer. 2008;15:511–520. doi: 10.1677/ERC-07-0130. [DOI] [PubMed] [Google Scholar]
- 17.Puppin C, Arturi F, Ferretti E, Russo D, Sacco R, Tell G, et al. Transcriptional regulation of human sodium/iodide symporter gene: a role for redox factor-1. Endocrinology. 2004;145:1290–1293. doi: 10.1210/en.2003-1250. [DOI] [PubMed] [Google Scholar]
- 18.De Felice M, Di Lauro R. Thyroid development and its disorders: genetics and molecular mechanisms. Endocrine Reviews. 2004;25:722–746. doi: 10.1210/er.2003-0028. [DOI] [PubMed] [Google Scholar]
- 19.Taki K, Kogai T, Kanamoto Y, Hershman JM, Brent GA. A thyroid-specific far-upstream enhancer in the human sodium/iodide symporter gene requires Pax-8 binding and cyclic adenosine 3’, 5’-monophosphate response element-like sequence binding proteins for full activity and is differentially regulated in normal and thyroid cancer cells. Mol Endocrinol. 2002;16:2266–2282. doi: 10.1210/me.2002-0109. [DOI] [PubMed] [Google Scholar]
- 20.Tell G, Pellizzari L, Pucillo C, Puglisi F, Cesselli D, Kelley MR, et al. TSH controls Ref-1 nuclear translocation in thyroid cells. J Mol Endocrinol. 2000;24:383–390. doi: 10.1677/jme.0.0240383. [DOI] [PubMed] [Google Scholar]
- 21.Tell G, Pellizzari L, Cimarosti D, Pucillo C, Damante G. Ref-1 controls pax-8 DNA-binding activity. Biochem Biophys Res Commun. 1998;252:178–183. doi: 10.1006/bbrc.1998.9548. [DOI] [PubMed] [Google Scholar]
- 22.Ohmori M, Endo T, Harii N, Onaya T. A novel thyroid transcription factor is essential for thyrotropin-induced up-regulation of Na+/I-symporter gene expression. Mol Endocrinol. 1998;12:727–736. doi: 10.1210/me.12.5.727. [DOI] [PubMed] [Google Scholar]
- 23.Chun JT, Di Dato V, D’Andrea B, Zannini M, Di Lauro R. The CRE-like element inside the 50-upstream region of the rat sodium/iodide symporter gene interacts with diverse classes of b-Zip molecules that regulate transcriptional activities through strong synergy with Pax-8. Mol Endocrinol. 2004;18:2817–2829. doi: 10.1210/me.2004-0020. [DOI] [PubMed] [Google Scholar]
- 24.Haugen BR. Redifferentiation therapy in advanced thyroid cancer. Curr Drug Targets Immune Endor Metabol Disord. 2004;4:175–180. doi: 10.2174/1568008043339811. [DOI] [PubMed] [Google Scholar]
- 25.Camacho LH, Olson J, Tong WP, Young CW, Spriggs DR, Malkin MG. Phase I dose escalation clinical trial of phenylbutyrate sodium administered twice daily to patients with advanced solid tumors. Invest New Drugs. 2007;25:131–138. doi: 10.1007/s10637-006-9017-4. [DOI] [PubMed] [Google Scholar]
- 26.Coelho SM, De Carvalho DP, Vaisman M. New Perspective on the treatment of differentiated thyroid cancer. Arq Bras Endocrinol Metabol. 2007;51:612–624. doi: 10.1590/s0004-27302007000400017. [DOI] [PubMed] [Google Scholar]
- 27.Kogai T, Taki K, Brent GA. Enhancement of sodium/iodide symporter expression in thyroid and breast cancer. Endocr Relat Cancer. 2006;13:797–826. doi: 10.1677/erc.1.01143. [DOI] [PubMed] [Google Scholar]
- 28.Marcus M, Coulton (2000) Fat-soluble vitamins A, K, E. In: Hadman JG, Limbird LE, Gilman AG (eds) Goodman and Gilmamn’s the pharmacologic basis of therapeutics. Mc Graw Hill, pp 1773–1792
- 29.Schmutzler C, Winzer R, Meissner-Weigl J, Kohrle J. Retinoic acid increases sodium/iodide symporter mRNA levels in human thyroid cancer cell lines and suppresses expression of functional symporter in nontransformed FRTL-5 rat thyroid cells. Biochem Biophys Res Commun. 1997;240:832–838. doi: 10.1006/bbrc.1997.7715. [DOI] [PubMed] [Google Scholar]
- 30.Park JW, Zarnegar R, Kanauchi H, Wong MG, Hyun WC, Ginzinger DG, et al. Troglitazone, the peroxisome proliferator-activated receptor-gamma agonist, induces antiproliferation and redifferentiation in human thyroid cancer cell lines. Thyroid. 2005;15:222–231. doi: 10.1089/thy.2005.15.222. [DOI] [PubMed] [Google Scholar]
- 31.Ohta K, Endo T, Haraguchi K, Hershman JM, Onaya T. Ligands for peroxisome proliferator-activated receptor gamma inhibit growth and induce apoptosis of human papillary thyroid carcinoma cells. J Clin Endocrinol Metab. 2001;86:2170–2177. doi: 10.1210/jc.86.5.2170. [DOI] [PubMed] [Google Scholar]
- 32.Kitazono M, Robey R, Zhan Z, Sarlis NJ, Skarulis MC, Aikou T, et al. Low concentrations of the histone deacetylase inhibitor, depsipeptide (FR901228), increase expression of the Na(+)/I(-) symporter and iodine accumulation in poorly differentiated thyroid carcinoma cells. J Clin Endocrinol Metab. 2001;86:3430–3435. doi: 10.1210/jc.86.7.3430. [DOI] [PubMed] [Google Scholar]
- 33.Akagi T, Luong QT, Gui D, Said J, Selektar J, Yung A, et al. Induction of sodium iodide symporter gene and molecular characterisation of HNF3 beta/FoxA2, TTF-1 and C/EBP beta in thyroid carcinoma cells. Br J Cancer. 2008;99:781–788. doi: 10.1038/sj.bjc.6604544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furuya F, Shimura H, Suzuki H, Taki K, Ohta K, Haraguchi K, et al. Histone deacetylase inhibitors restore radioiodide uptake and retention in poorly differentiated and anaplastic thyroid cancer cells by expression of the sodium/iodide symporter thyroperoxidase and thyroglobulin. Endocrinology. 2004;145:2865–2875. doi: 10.1210/en.2003-1258. [DOI] [PubMed] [Google Scholar]
- 35.Fortunati N, Catalano MG, Arena K, Brignardello E, Piovesan A, Boccuzzi G. Valproic acid induces the expression of the NaC/I-symporter and iodine uptake in poorly differentiated thyroid cancer cells. J Clin Endocrinol Metab. 2004;89:1006–1009. doi: 10.1210/jc.2003-031407. [DOI] [PubMed] [Google Scholar]
- 36.Venkataraman GM, Yatin M, Ain KB. Cloning of the human sodium-iodide symporter promoter and characterization in a differentiated human thyroid cell line, KAT-50. Thyroid. 1998;8:63–69. doi: 10.1089/thy.1998.8.63. [DOI] [PubMed] [Google Scholar]
- 37.Kogai T, Curcio F, Hyman S, Cornford EM, Brent GA, Hershman JM. Induction of follicle formation in long-term cultured normal human thyroid cells treated with thyrotropin stimulates iodide uptake but not sodium/iodide symporter messenger RNA and protein expression. J Endocrinol. 2000;167:125–135. doi: 10.1677/joe.0.1670125. [DOI] [PubMed] [Google Scholar]
- 38.Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, et al. The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. Endocr Rev. 2003;24:48–77. doi: 10.1210/er.2001-0029. [DOI] [PubMed] [Google Scholar]
- 39.De la Vieja A, Ginter CS, Carrasco N. Molecular analysis of a congenital iodide transport defect: G543E impairs maturation and trafficking of the Na+/I-symporter. Mol Endocrinol. 2005;19:2847–2858. doi: 10.1210/me.2005-0162. [DOI] [PubMed] [Google Scholar]
- 40.Kurebayashi J, Tanaka K, Otsuki T, Moriya T, Kunisue H, Uno M, et al. All-trans retinoic acid modulates expression levels of thyroglobulin and cytokines in a new human poorly differentiated papillary thyroid carcinoma cell line, KTC-1. J Clin Endocrinol Metab. 2000;85:2889–2896. doi: 10.1210/jc.85.8.2889. [DOI] [PubMed] [Google Scholar]
- 41.Simon D, Koehrle J, Reiners C, Boerner AR, Schmutzler C, Mainz K, et al. Redifferentiation therapy with retinoids: therapeutic option for advanced follicular and papillary thyroid carcinoma. World J Surg. 1998;22:569–574. doi: 10.1007/s002689900436. [DOI] [PubMed] [Google Scholar]
- 42.Gruenwald F, Menzel C, Bender H, Palmedo H, Otte R, et al. Redifferentiation therapy-induced radioiodine uptake in thyroid cancer. J Nucl Med. 1998;39:1903–1906. [PubMed] [Google Scholar]
- 43.Gruenwald F, Pakos E, Bender H, Menzel C, Otte R, Palmedo H, et al. Redifferentiation therapy with retinoic acid in follicular thyroid cancer. J Nucl Med. 1998;39:1555–1558. [PubMed] [Google Scholar]
- 44.Haberkorn U, Henze M, Altmann A, Jiang S, Morr I, Mahmut M, et al. Transfer of the human NaI symporter gene enhances iodide uptake in hepatoma cells. J Nucl Med. 2001;42:317–325. [PubMed] [Google Scholar]
- 45.Nakamoto Y, Saga T, Misaki T, Kobayashi H, Sato N, Ishimori T, et al. Establishment and characterization of a breast cancer cell line expressing Na+/I-symporters for radioiodide concentrator gene therapy. J Nucl Med. 2000;41:1898–1904. [PubMed] [Google Scholar]
- 46.Boland A, Ricard M, Opolon P, Bidart JM, Yeh P, Filetti S, et al. Adenovirus-mediated transfer of the thyroid sodium/iodide symporter gene into tumors for a targeted radiotherapy. Cancer Res. 2000;60:3484–3492. [PubMed] [Google Scholar]
- 47.Spitzweg C, Dietz AB, O'Connor MK, Bergert ER, Tindall DJ, Young CY, et al. In vivo sodium iodide symporter gene therapy of prostate cancer. Gene Ther. 2001;8:1524–1531. doi: 10.1038/sj.gt.3301558. [DOI] [PubMed] [Google Scholar]
- 48.Schipper ML, Weber A, Béhé M, Göke R, Joba W, Schmidt H, et al. Radioiodide treatment after sodium iodide symporter gene transfer is a highly effective therapy in neuroendocrine tumor cells. Cancer Res. 2003;63:1333–1338. [PubMed] [Google Scholar]
- 49.Kang JH, Chung JK, Lee YJ, Shin JH, Jeong JM, Lee DS, et al. Establishment of a human hepatocellular carcinoma cell line highly expressing sodium iodide symporter for radionuclide gene therapy. J Nucl Med. 2004;45:1571–1576. [PubMed] [Google Scholar]
- 50.Scholz IV, Cengic N, Baker CH, Harrington KJ, Maletz K, Bergert ER, et al. Radioiodine therapy of colon cancer following tissue-specific sodium iodide symporter gene transfer. Gene Ther. 2005;12:272–280. doi: 10.1038/sj.gt.3302410. [DOI] [PubMed] [Google Scholar]
- 51.Petrich T, Quintanilla-Martinez L, Korkmaz Z, Samson E, Helmeke HJ, Meyer GJ, et al. Effective cancer therapy with the alpha-particle emitter [211At]astatine in a mouse model of genetically modified sodium/iodide symporter-expressing tumors. Clin Cancer Res. 2006;12:1342–1348. doi: 10.1158/1078-0432.CCR-05-1576. [DOI] [PubMed] [Google Scholar]
- 52.Dwyer RM, Bergert ER, O'Connor MK, Gendler SJ, Morris JC. Adenovirus-mediated and targeted expression of the sodium-iodide symporter permits in vivo radioiodide imaging and therapy of pancreatic tumors. Hum Gene Ther. 2006;17:661–668. doi: 10.1089/hum.2006.17.661. [DOI] [PubMed] [Google Scholar]
- 53.Kim HJ, Jeon YH, Kang JH, Lee YJ, Kim KI, Chung HK, et al. In vivo long-term imaging and radioiodine therapy by sodium iodide symporter gene expression using a lentiviral system containing ubiquitin C promoter. Cancer Biol Ther. 2007;6:1130–1135. doi: 10.4161/cbt.6.7.4342. [DOI] [PubMed] [Google Scholar]
- 54.Dingli D, Diaz RM, Bergert ER, O'Connor MK, Morris JC, Russell SJ. Genetically targeted radiotherapy for multiple myeloma. Blood. 2003;102:489–496. doi: 10.1182/blood-2002-11-3390. [DOI] [PubMed] [Google Scholar]
- 55.La Perle KM, Blomme EA, Capen CC, Jhiang SM. Effect of exogenous human sodium iodide symporter expression on growth of MATLyLu cells. Thyroid. 2003;13:133–140. doi: 10.1089/105072503321319431. [DOI] [PubMed] [Google Scholar]
- 56.Groot-Wassink T, Aboagye EO, Glaser M, Lemoine NR, Vassaux G. Adenovirus biodistribution and noninvasive imaging of gene expression in vivo by positron emission tomography using human sodium/iodide symporter as reporter gene. Hum Gene Ther. 2002;13:1723–1735. doi: 10.1089/104303402760293565. [DOI] [PubMed] [Google Scholar]
- 57.Lee YJ, Chung JK, Shin JH, Kang JH, Jeong JM, Lee DS, et al. In vitro and in vivo properties of a human anaplastic thyroid carcinoma cell line transfected with the sodium iodide symporter gene. Thyroid. 2004;14:889–895. doi: 10.1089/thy.2004.14.889. [DOI] [PubMed] [Google Scholar]
- 58.Snade JV, Massart C, Beauwens R, Schoutens A, Costagliola S, Dumont JE, et al. Anion selectivity by the sodium iodide symporter. Endocrinology. 2003;144:247–252. doi: 10.1210/en.2002-220744. [DOI] [PubMed] [Google Scholar]
- 59.Boland A, Magnon C, Filetti S, Bidart JM, Schlumberger M, Yeh P, et al. Transposition of the thyroid iodide uptake and organification system in nonthyroid tumor cells by adenoviral vector-mediated gene transfers. Thyroid. 2002;12:19–26. doi: 10.1089/105072502753451922. [DOI] [PubMed] [Google Scholar]
- 60.Furuya F, Shimura H, Miyazaki A, Taki K, Ohta K, Haraguchi K, et al. Adenovirus-mediated transfer of thyroid transcription factor-1 induces radioiodide organification and retention in thyroid cancer cells. Endocrinology. 2004;145:5397–5405. doi: 10.1210/en.2004-0631. [DOI] [PubMed] [Google Scholar]
- 61.Dadachova E, Carrasco N. The Na/I symporter (NIS): imaging and therapeutic applications. Semin Nucl Med. 2004;34:23–31. doi: 10.1053/j.semnuclmed.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 62.McDevitt MR, Sgouros G, Finn RD, Humm JL, Jurcic JG, Larson SM, et al. Radioimmunotherapy with alpha-emitting nuclides. Eur J Nucl Med. 1998;25:1341–1351. doi: 10.1007/s002590050306. [DOI] [PubMed] [Google Scholar]
- 63.Willhauck MJ, Samani BR, Gildehaus FJ, Wolf I, Senekowitsch-Schmidtke R, Stark HJ, et al. Application of rhenium-188 as an alternative radionuclide for treatment of prostate cancer after tumor-specific sodium iodide symporter gene expression. J Clin Endocrinol Metab. 2007;92:4451–4458. doi: 10.1210/jc.2007-0402. [DOI] [PubMed] [Google Scholar]
- 64.Willhauck MJ, Samani BR, Wolf I, Senekowitsch-Schmidtke R, Stark HJ, Meyer GJ, et al. The potential of 211astatine for NIS-mediated radionuclide therapy in prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35:1272–1281. doi: 10.1007/s00259-008-0775-4. [DOI] [PubMed] [Google Scholar]
- 65.Spitzweg C, O'Connor MK, Bergert ER, Tindall DJ, Young CY, Morris JC. Treatment of prostate cancer by radioiodine therapy after tissue-specific expression of the sodium iodide symporter. Cancer Res. 2000;60:6526–6530. [PubMed] [Google Scholar]
- 66.Jin YN, Chung HK, Kang JH, Lee YJ, Kimm KI, Kim YJ, et al. Radioiodine gene therapy of hepatocellular carcinoma targeted human alpha fetoprotein. Cancer Biother Radiopharm. 2008;23:551–560. doi: 10.1089/cbr.2008.0467. [DOI] [PubMed] [Google Scholar]
- 67.Willhauck MJ, Sharif Samani BR, Klutz K, Cengic N, Wolf I, Mohr L, et al. Alpha-fetoprotein promoter-targeted sodium iodide symporter gene therapy of hepatocellular carcinoma. Gene Ther. 2008;15:214–223. doi: 10.1038/sj.gt.3303057. [DOI] [PubMed] [Google Scholar]
- 68.Kim SH, Chung HK, Kang JH, Kim KI, Jeon YH, Jin YN, et al. Tumor-targeted radionuclide imaging and therapy based on human sodium iodide symporter gene driven by a modified telomerase reverse transcriptase promoter. Hum Gene Ther. 2008;19:951–957. doi: 10.1089/hum.2008.030. [DOI] [PubMed] [Google Scholar]