Abstract
Aim
Due to the interesting pharmacologic properties of porphyrins, the idea of developing a possible tumor imaging agent using PET by incorporating 68Ga into a suitable porphyrin ligand was investigated.
Methods
68Ga-labeled 5,10,15,20-tetrakis(pentafluoro-13 phenyl) porphyrin (68Ga-TFPP) was prepared using freshly eluted [68Ga]GaCl3 obtained from a 68Ge/68Ga generator developed in-house and 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin (H2TFPP) for 60 min at 100°C.
Results
The complex was prepared with high radiochemical purity (>99% ITLC, >99% HPLC, specific activity: 13–14 GBq/mmol). Stability of the complex was checked in the final formulation and in human serum for 5 h. The partition coefficient was calculated for the compound (log P = 0.62). The biodistribution of the labeled compound in vital organs of Swiss mice bearing fibrosarcoma tumors was studied using scarification studies and SPECT imaging up to 1 h. The complex was mostly washed out from the circulation through kidneys and liver. The tumor-to-muscle ratio 1 h post injection was 5.13.
Conclusion
The radiolabeled porphyrin complex demonstrated potential for further imaging studies in other tumor models.
Keywords: Porphyrins, Ga-68, Fibrosarcoma, Biodistribution, Co-incidence
Introduction
Porphyrins are the focus of much attention because of their ability to mimic other chemicals in the human body, their ability to accumulate in many kinds of cancer cells, as well as their magnetic and optical properties. Photofrin has currently been approved for general use by licensing authorities for treatment of solid tumors and cancers. It makes use of photodynamic therapy (PDT), which uses a photochemical effect induced by light for treatment. Recently, meso-tetra (3-hydroxyphenyl)porphyrin was developed as one of the best tumor localizers and has also shown a favorable tissue distribution. These features make it useful in cancer medicine and photodynamic therapy [1, 2].
Various metal-porphyrin complexes have shown interesting tumor-avid activity in vitro and in vivo and have found their ways into clinical studies. Motexafin gadolinium is a novel radiation sensitizer used in the radiotherapy of brain tumors [3]. BOPP is a boronated porphyrin with 40 atoms of boron-10 per molecule against one atom alone in the BPA molecule; it is used in boron-neutron capture therapy [4]. Tumor accumulation of some gallium-porphyrin complexes has also already been reported [5]. Radiolabeled porphyrins, such as 109Pd-protoporphyrins [6], 109Pd-porphyrins [7], 109Pd-derivitized porphyrins [8], and 188Re-porphyrins [9], have been developed for therapeutic purposes.
Some diagnostic radiolabeled porphyrins have also been reported. For instance, 99mTc-porphyrin conjugate has been evaluated in rodent mammary tumors [10]. Recently 99mTc-porphine was developed for imaging despite its high hepatotoxicity [11]. At the same time, kinetic studies for 111In incorporation into m-tetraphenylporphine have been conducted, although no data have been reported for its biological evaluation [12].
The increasing trend in the production and use of PET radionuclides in nuclear medicine has offered new opportunities for researchers to focus on the production of new 6Ga-radiopharmaceuticals. Recently a 67Ga-porphyrin complex was developed with interesting biological properties for tumor imaging, but the 67Ga-complex demonstrated high liver accumulation due to low hydrophilicity [13].
A simplified scheme for the decays of 68Ge and its 68Ga daughter is shown in Fig. 1. Germanium-68 decays by pure electron capture (EC) to the ground state of 68Ga with a half-life of 270.95 days [14]. Gallium-68 in turn decays with a half-life of 67.71 min [9] by a combination of EC and positron emission primarily to the ground state of 68Zn, but also with a branch to an excited state at 1,077 keV with a probability of about 3% and a number of higher excited states with a combined probability of under 0.4 %.
Fig. 1.
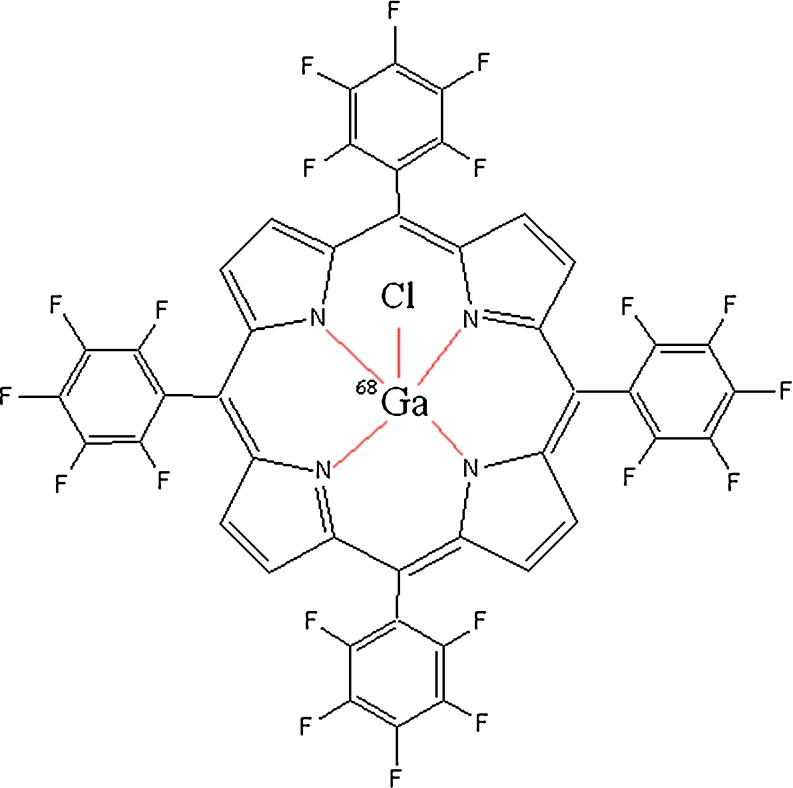
Structure of 68GaTFPP
Due to the interesting pharmacologic properties of porphyrins, such as solubility in serum, rapid wash-out, tumor avidity, and feasible complexation with various bi- and trivalent metals [15], the idea of developing a possible tumor imaging agent using PET by incorporating 68Ga into a suitable porphyrin ligand, i.e., 5,10,15,20-tetrakis(pentafluorophenyl) porphyrin (TFPP), was investigated (Fig. 1).
In this work we report synthesis, radiolabeling, quality control, stability, partition coefficient determination, and biodistribution studies (using SPECT and scarification) of 68Ga-TFPP in fibrosarcoma-bearing mice.
Materials and Methods
The 68Ge/68Ga generator (10 mCi activity) was obtained from the nuclear medicine research group at the Agricultural, Medical and Industrial Research School (AMIRS) in Karaj, Iran. Chemicals were purchased from the Aldrich Chemical (Germany) and the ion-exchange resins from Bio-Rad Laboratories (Canada). NMR spectra were recorded at room temperature on a Bruker AVANCE 300-MHz operating at 300.0, 75.0, and 23.3 MHz, and the spectra are referenced to SiMe4. Infrared spectrum was measured on a Perkin-Elmer 781 spectrometer using a KBr disc. Mass spectrum was recorded by a Finnigan Mat TSQ-70 Spectrometer. Thin layer chromatography (TLC) for cold compounds was performed on polymer-backed silica gel (F 1500/LS 254, 20 × 20 cm, TLC Ready Foil, Schleicher & Schuell®, Germany). Normal saline and sodium acetate used for labeling were of high purity and were filtered through 0.22 μm Cativex filters. Instant thin layer chromatography (ITLC) was performed by counting Whatman No. 2 papers using a thin layer chromatography scanner (Bioscan AR2000, Bioscan Europe, France). Analytical high performance liquid chromatography (HPLC) used to determine the specific activity was performed on a Shimadzu LC-10AT, armed with two detector systems, flow scintillation analyzer (Packard-150 TR) and UV-visible (Shimadzu) using Whatman Partisphere C-18 column 250 × 4.6 mm (Whatman, NJ, USA). Analytical HPLC was also used to determine the specific radioactivity of the labeled compound. A standard curve was generated to calculate the mass of the final solution. Biodistribution data were acquired by counting normal saline-washed tissues after weighting on a Canberra™ high purity germanium (HPGe) detector (model GC1020-7500SL). Radionuclidic purity was checked with the same detector. For activity measurement of the samples a CRC Capintech Radiometer (NJ, USA) was used. All calculations and tissue countings were based on the 511 keV peak. Animal studies were performed in accordance with the United Kingdom Biological Council’s Guidelines on the Use of Living Animals in Scientific Investigations, 2nd ed.
68Ge/68Ga Generator
A prototype 5 mCi 68Ge/68Ga generator developed at AMIRS was used in this study. Briefly, metallic gallium powder was melted and transferred to niobium capsules, which were then sealed. The target was irradiated at 29 MeV energy in a 30 MeV IBA cyclotron (effective 22 MeV protons on the target material). After irradiation the target material was dissolved in 12 M H2SO4 while being gently heated and stirred. To increase the solubility, 30% hydrogen peroxide was added to the mixture followed by the addition of 2 M HCl (30 ml). After completion of the dissolution, carbon tetrachloride (150 ml) was added to the mixture, and the organic layer containing 68Ge was extracted from 68Ga-containing aqueous solution. 68Ge was then back-extracted into aqueous solution using 0.05 M HCl. The final solution was then used in the manufacture of a SnO2-based generator.
Quality Control of the Product
Control of Radionuclide Purity
Gamma spectroscopy of the final sample was carried out by counting with an HPGe detector coupled to a Canberra™ multi-channel analyzer for 1,000 s.
Chemical Purity Control
This step was carried out to ensure that the amounts of Sn, Fe, and Cu ions resulting from the target material and backing in the final product were acceptable according to internationally accepted limits. Chemical purity was checked by ICP method differential. The detection limit of our system was 0.1 ppm for both zinc and copper ions.
Preparation of Tetraphenyl Porphyrin (H2TFPP)
This compound was prepared according to the reported method using freshly distilled pentafluoro benzaldehyde, pyrrole, and propionic acid followed by oxidation [16]. UV (CH2Cl2) λmax (ε) = 412,506,584 nm. 1HNMR : −2.91 (NH), 9.40 (βH) .19FNMR: −136.9 (d), 151.7 (t), −161.8 (m),
Preparation of 68Ga-TFPP
The acidic solution (2 ml) of [68Ga]GaCl3 (111 MBq, 3 mCi) was transferred to a 3 ml borosilicate vial and heated to dryness using a flow of N2 gas at 50–60°C. Fifty microliters of TFPP in absolute ethanol (5 mg/ml ≈409 nmoles) was added to the gallium-containing vial followed by the addition of acetate buffer pH 5.5 (450 μl). The mixture refluxed at 100°C for 60 min. The active solution was checked for radiochemical purity by ITLC and HPLC. The final solution was then passed through a 0.22 μm filter and pH was adjusted to 5.5–7.
Quality Control of 68Ga-TFPP
Radio Thin Layer Chromatography
A 5 μl sample of the final fraction was spotted on a chromatography Whatman No. 2 paper and developed in mobile phase mixture, 10% NH4OAc and methanol 1:1.
High Performance Liquid Chromatography
HPLC was performed with a flow rate of 1 ml/min and pressure of 130 kgF/cm2 for 20 min. HPLC was performed on the final preparation using a mixture of water:acetonitrile 3:2(v/v) as the eluent by means of reversed phase column Whatman Partisphere C18 (4.6 × 250 mm).
Determination of Partition Coefficient
Partition coefficient (log P) of 6Ga-TFPP was calculated followed by the determination of P (P = the ratio of specific activities of the organic and aqueous phases). A mixture of 1 ml of 1-octanol and 1 ml of isotonic acetate-buffered saline (pH = 7) containing approximately 3.7 MBq of the radiolabeled gallium complex at 37°C was vortexed for 1 min and left 5 min. Following centrifugation at >1,200 g for 5 min, the octanol and aqueous phases were sampled and counted in an automatic well-type counter. A 500 μl sample of the octanol phase from this experiment was shaken again two to three times with fresh buffer samples. The reported log P values are the average of the second and third extractions from three to four independent measurements.
Stability Tests
The stability of the complex was checked according to the conventional ITLC method [17]. A sample of 68Ga-TFPP (37 MBq) was kept at room temperature for 2 days while being checked by ITLC at time intervals in order to check stability in the final product using the chromatography system described above. For serum stability studies, to 36.1 MBq (976 μCi) of 6Ga-TFPP was added 500 μl of freshly collected human serum, and the resulting mixture was incubated at 37°C for 5 h. Aliquots (5 μl) were analyzed by ITLC.
Induction of Fibrosarcoma Tumors in Mice
Tumor induction was performed with the use of polyaromatic hydrocarbon injection in rodents as reported previously [18]. For tumor model preparation, 10 μl of 3-methyl cholanthrene solution in extra-virgin olive oil (4 mg/ml) was injected subcutaneously into the dorsal area of the mice. After 14–16 weeks, the tumors weighed 0.2–0.4 g and were not grossly necrotic. Tumor tissues of some random animals were sent for pathological tests and were diagnosed as fibrosarcoma.
Biodistribution in Fibrosarcoma-bearing Mice
The distribution of the radiolabelled complex among tissues was determined for Swiss mice bearing fibrosarcoma tumors immediately after imaging. The total amount of radioactivity injected into each animal was determined by measuring the contents of a 1-ml syringe before and after injection in a dose calibrator with fixed geometry. The animals were sacrificed using the animal care protocols at selected times after injection (30, 60, and 120 min). The tissues (blood, heart, lung, brain, intestine, feces, skin, stomach, kidneys, liver, muscle, and bone) were weighed and rinsed with normal saline, and their specific activities were determined with an HPGe detector equipped with a sample holder device as percentage of injected dose per gram of tissues. Blood samples were rapidly taken from rodent aorta after scarification.
Imaging of 68Ga-TFPP in Swiss Mice Bearing Fibrosarcoma Tumors
Images were taken up to 1 h after administration of the radiopharmaceutical by a dual-head SPECT system at co-incidence mode. The mouse-to-high energy septa distance was 12 cm. Images were taken from both normal and tumor-bearing mice. The useful field of view (UFOV) was 540 × 400 mm.
Results
Development and Quality Control of 68Ga Generator
Radiochemical separation was performed by a two-step extraction in an organic solution followed by back extraction with 96% yield. The presence and absence of 68Ga and 68Ge were checked at each step using an HPGe detector.
Radionuclidic control showed the presence of 511 and 1,077 keV all originating from 68Ga and showed a radionuclidic purity higher than 99% (E.O.S.). The concentrations of tin (from generator material), iron (from the sealing parts), and copper were determined using inductively coupled plasma (ICP) method. The data are summarized in Table 1.
Table 1.
The analytical data for chemical purity of five prototype generators developed in this work at the Agricultural, Medical and Industrial Research School (AMIRS)
| Generator | Cu2+(ppm) | Sn2+ (ppm) | Fe2+(ppm) |
|---|---|---|---|
| AMIRS-1 | 0.33 | 2.47 | 1.07 |
| AMIRS-2 | 0.15 | 0.12 | 0.49 |
| AMIRS-3 | 0.15 | 8.82 | 0.74 |
| AMIRS-4 | 0.17 | 0.46 | 0.68 |
| AMIRS-5 | 0.06 | 0.29 | 0.04 |
Radiolabeling
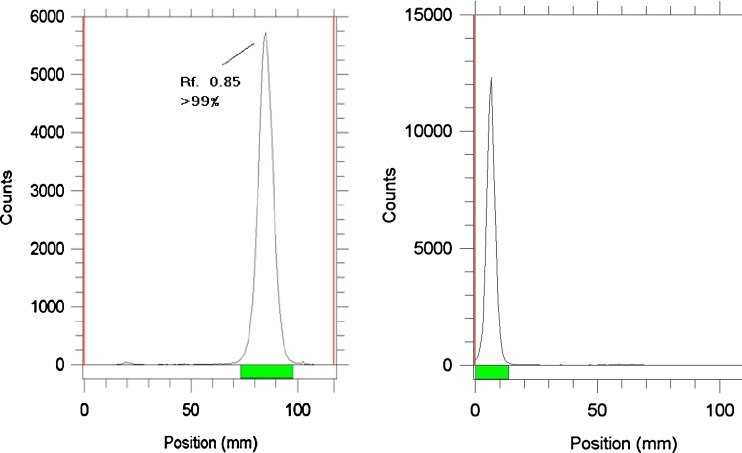
Because of the engagement of NH polar functional groups in its structure, labeling of H2TFPP with gallium cation affects its chromatographic properties, and the final complex is more lipophilic. A chromatographic system was used for the detection of the radiolabeled compound from the free gallium cation. Using 10% NH4OAc and methanol 1:1 mixture, free gallium remains at the origin of the paper as a single peak, while the radiolabeled compound migrates to higher Rf (0.85) (Fig. 2).
Fig. 2.
ITLC of 68Ga-TFPP (left) and [6Ga]GaCl3 (right)
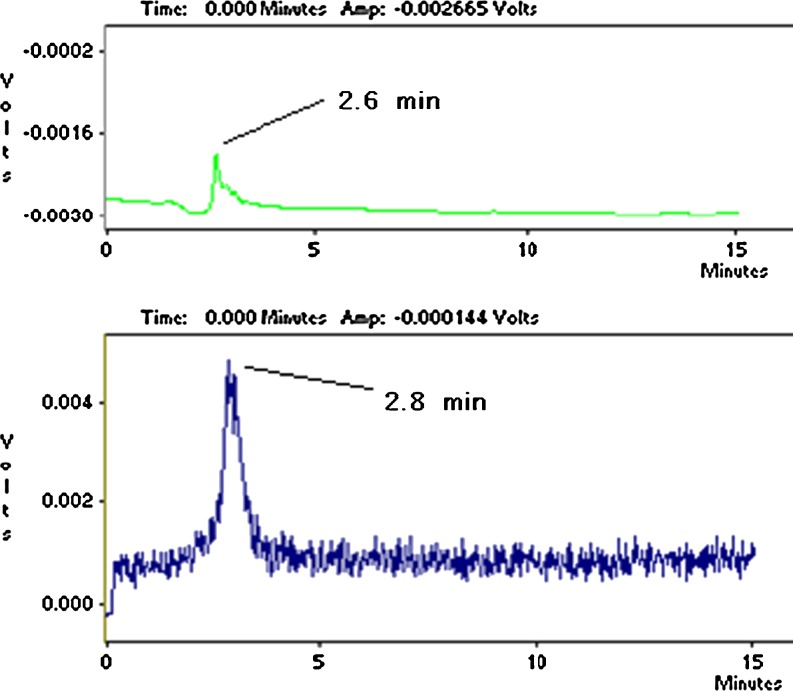
ITLC studies indicated the presence of the radiolabeled compound, a finding that was supported by HPLC, which demonstrated the existence of the radiolabeled species using both techniques (Fig. 3).
Fig. 3.
HPLC chromatograms of 68Ga-TPFP on a reversed phase column using acetonitrile:water 40:60. Top UV chromatogram, bottom scintillation chromatogram
Partition Coefficient of 68Ga-TFPP
As expected from the chemical formula in Fig. 1, the lipophilicity of the 68Ga-TFPP compound is not high due to the ionic nature of the radiocomplex. The measured octanol/water partition coefficient, P, for the complex was found to depend on the pH of the solution. At pH 7, the log P was 0.62.
Stability
The chemical stability of 68Ga-TFPP was high enough to perform further studies. Incubation of 68Ga-TFPP in freshly prepared human serum for 2 days at 37°C showed no loss of 68Ga from the complex. The radiochemical purity of the complex remained at 99% for 5 h under physiologic conditions.
Biodistribution Studies of 68Ga-TFPP in Swiss Mice Bearing Fibrosarcoma Tumors
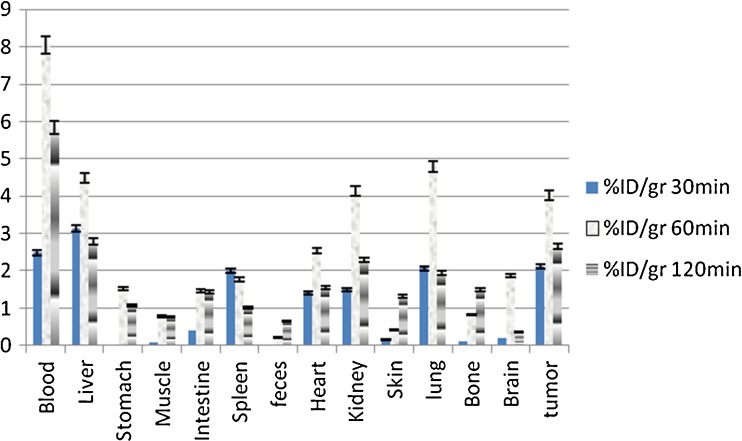
The uptake of 68Ga-TFPP complex in different organs or tissues of tumor-bearing mice was calculated as a percentage of the injected dose per gram of organs or tissues of rats (% ID/g). The results of the biodistribution studies revealed significant tumor uptake within 1 h post injection. The radiocomplex circulates in blood for at least 2 h affording high bioavailabity of the tracer. Kidney is a major route of excretion for the complex especially beyond 60 min post administration. As an imaging agent, the target-to-nontarget (tumor-to-muscle) ratio was 5.13 at 60 min post injection, however the blood content was high leading to low tumor-to-blood ratios at all time intervals (Fig. 4).
Fig. 4.
Percentage of injected dose per gram (% ID/g) of 68Ga-TFPP in Swiss mice bearing fibrosarcoma tumor tissue at 30, 60, and 120 min post injection
Imaging of 68Ga-TFPP in Swiss Mice Bearing Fibrosarcoma Tumors
As shown in Fig. 5, the Swiss mice bearing fibrosarcoma tumors showed accumulation of the radiotracer in the tumor after injection. Most of the activity was washed out from the body after 1 h and the rest remained in the tumor.
Fig. 5.
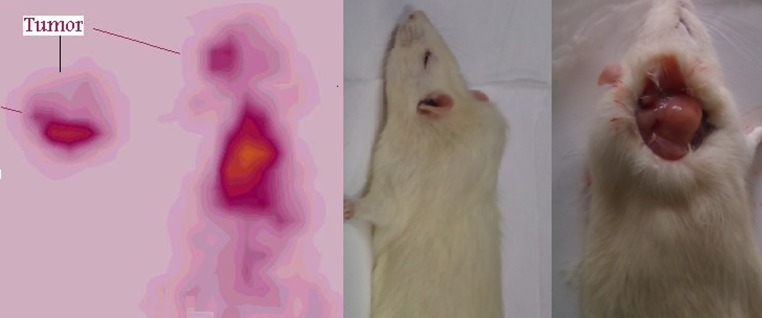
SPECT images of 68Ga-TFPP (90 MBq, 22 μCi) in Swiss mice bearing fibrosarcoma tumors 1 h post injection
Discussion
Development and Quality Control of 68Ga Generator
In this study, metallic gallium was irradiated in a sealed niobium chamber followed by laser welding. The leak control was performed in a hot water container by direct bubble visualization as well as spectrophotometric assay for the presence of the Ga cation in the solution.
Radiochemical Purity Determination
ITLC demonstrated a high radiochemical purity using common chemical solvent, and the data were found to be consistent and reproducible. However for a fast quality assurance technique, HPLC was performed not only due to concerns about time but also to afford a parallel chemical control as well.
In HPLC studies, a fast-eluting compound at 2.6 min (UV detector) related to the 2.8 min peak (scintillation detector) demonstrated a pure single peak for the labeled compound. Free Ga cation eluted at 1.02 min (not shown).
Partition Coefficient of 68Ga-TFPP
The water solubility of the radiocomplex reduces unnecessary uptake in tissues including liver and fat and faster kidney wash-out.
Stability
The stability of the complex in the final formulation as well as in human serum at normal body conditions proved to be enough to allow for any medical procedure.
Biodistribution Studies
Many porphyrin complexes show significant water solubility, leading to the fast removal of unwanted radioactivity after the target tissue has been affected. This was also observed for 68Ga-TFPP as expected. However biodistribution studies in other tumor models and various animals are also interesting. Tumor uptake is mostly observed near the physical half life, suggesting the importance of the proper choice of the ligand and the radionuclide.
Imaging
Accurate imaging using the expected time table for 68Ga-TFPP was performed based on the biodistribution results giving significant tumor uptake at 1 h post injection.
Conclusion
Total labeling and formulation of 68Ga-TFPP took about 30–60 min (radiochemical purity: >97% ITLC, >98% HPLC, specific activity: 13–14 GBq/mmol). The complex was stable in final formulation and in human serum at least for 5 h. At pH 7, the logP was 0.69. The biodistribution of the labeled compound in vital organs of wild-type rats was studied using scarification studies and SPECT imaging up to 1 h.
A detailed comparative pharmacokinetic study was performed for 67Ga cation and [67Ga]-TFPP. The complex is mostly washed out from the circulation through the kidneys and liver. The tumor-to-blood ratios 2 and 24 h post injection were 8.22 and 18.03, respectively. The tumor-to-muscle ratios 2 and 24 h post injection were 15.37 and 38.15, respectively. Higher water solubility of the complex due to its ionic nature is an advantage for rapid wash-out of the complex from the system leading to an enhanced target-to-nontarget ratio.
Acknowledgments
Authors wish to thank Mr. S. Daneshvari for conducting the animal studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sanderson DR, Fontana RS, Lipson RL, Baldes EJ. Hematoporphyrin as a diagnostic tool. A preliminary report of new techniques. Tetrahedron. 1998;54:4151–4202. doi: 10.1016/S0040-4020(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 2.Fawwaz R, Bohdiewicz P, Lavallee D, Wang T, Oluwole S, Newhouse J, et al. Use of metalloporphyrins in diagnostic imaging. Int J Rad Appl Instrum B. 1990;17:65–72. doi: 10.1016/0883-2897(90)90009-p. [DOI] [PubMed] [Google Scholar]
- 3.Thomas SR, Khuntia D. Motexafin gadolinium injection for the treatment of brain metastases in patients with non-small cell lung cancer. Int J Nanomed. 2007;2:79–87. doi: 10.2147/nano.2007.2.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahl SB, Koo MS. Synthesis and properties of tetrakiscarborane-carboxylate esters of 2, 4-bis (−dihydroxyethyl) deuteroporphyrin IX. In: Allen BJ, Moore DE, Harrington BV, editors. Progress in neutron capture therapy for cancer. New York: Plenum Press; 1992. pp. 223–226. [Google Scholar]
- 5.Sasaki K, Yumita N, Nishigaki R, Sakata I, Nakajima S, Umemura S-I. Pharmacokinetic study of a gallium-porphyrin photo- and sono-sensitizer, ATX-70, in tumor-bearing mice. Jpn J Cancer Res. 2001;92:989–995. doi: 10.1111/j.1349-7006.2001.tb01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fawwaz RA, Frye F, Loughman WD, Hemphill W. Survival of skin homografts in dogs injected with 109Pd-protoporphyrin. J Nucl Med. 1974;15:997–1002. [PubMed] [Google Scholar]
- 7.Fawwaz RA, Hemphill W, Winchell HS. Potential use of 109Pd-porphyrin complexes for selective lymphatic ablation. J Nucl Med. 1971;12:231–236. [PubMed] [Google Scholar]
- 8.Chakraborty S, Das T, Banerjee S, Sarma HD, Venkatesh M. Preparation and preliminary biological evaluation of a novel 109Pd labeled porphyrin derivative for possible use in targeted tumor therapy. Q J Nucl Med Mol Imaging. 2007;15:16–23. [PubMed] [Google Scholar]
- 9.Sarma HD, Das T, Banerjee S, Venkatesh M, Vidyasagar PB, Mishra KP. Biologic evaluation of a novel 188Re-labeled porphyrin in mice tumor model. Cancer Biother Radiopharm. 2010;25:47–54. doi: 10.1089/cbr.2009.0675. [DOI] [PubMed] [Google Scholar]
- 10.Murugesan S, Shettyc SJ, Srivastava TS, Noronha OPD, Samuel AM. A technetium-99 m-labelled cyclam acid porphyrin (CAP) for tumour imaging. Appl Radiat Isot. 2001;55:641–646. doi: 10.1016/S0969-8043(01)00113-0. [DOI] [PubMed] [Google Scholar]
- 11.Wang A-Y, Lin J-L, Lin W-C. Studies on the porphine labeled with 99mTc–pertechnetate. J Radioanal Nucl Chem. 2010;284:121–128. [Google Scholar]
- 12.Nunn AD. Medical radionuclides and the quality of radiopharmaceuticals. The kinetics of incorporation of 111In into m-tetraphenylporphine. J Radioanal Nucl Chem. 1978;53:291–298. [Google Scholar]
- 13.Fazaeli Y, Jalilian AR, Amini MM, Rahiminejad-kisomi A, Saeed Rajabifar S, Bolourinovin F, et al. Preparation and preliminary evaluation of [67 Ga]-tetra phenyl porphyrin complexes as possible imaging agents. J Radioanal Nucl Chem. 2011;288:17–24. doi: 10.1007/s10967-010-0962-1. [DOI] [Google Scholar]
- 14.Laboratoire National Henri Becquerel. Decay Data Evaluation Project Data (DDEP). http://www.nucleide.org/DDEP_WG/DDEPdata.htm (2008).
- 15.Falk JE. Porohyrins and metalloporphyrins. Elsevier Science: New York; 1975. [Google Scholar]
- 16.Adler AD, Longo FR, Finarelli JD, Goldmacher J, Assour J, Korsakoff L. A simplified synthesis for meso-tetraphenylporphine. J Org Chem. 1967;32:476. doi: 10.1021/jo01288a053. [DOI] [Google Scholar]
- 17.Jalilian AR, Rowshanfarzad P, Sabet M, Novinrooz A, Raisali G. Preparation of [66Ga]bleomycin complex as a possible PET radiopharmaceutical. J Radioanal Nucl Chem. 2005;264:617.
- 18.DiGiovanni J, Rymer J, Slaga TJ, Boutwell RK. Anticarcinogenic and co-carcinogenic effects of benzo[e]pyrene and dibenz[a, c]anthracene on skin tumor initiation by polycyclic hydrocarbons. Carcinogenesis. 1982;3(4):371–375. doi: 10.1093/carcin/3.4.371. [DOI] [PubMed] [Google Scholar]