Abstract
Leaves of the C4 plant maize have two major types of photosynthetic cells: a ring of five large bundle sheath cells (BSC) surrounds each vascular bundle and smaller mesophyll cells (MC) lie between the cylinders of bundle sheath cells. The enzyme ribulose bisphosphate carboxylase/oxygenase is encoded by nuclear rbcS and chloroplast rbcL genes. It is not present in MC but is abundant in adjacent BSC of green leaves. As reported previously, the separate regions of rbcS-m3, which are required for stimulating transcription of the gene in BSC and for suppressing expression of reporter genes in MC, were identified by an in situ expression assay; expression was not suppressed in MC until after leaves of dark-grown seedlings had been illuminated for 24 h. Now we have found that transient expression of rbcS-m3 reporter genes is stimulated in BSC via a red/far-red reversible phytochrome photoperception and signal transduction system but that blue light is required for suppressing rbcS-m3 reporter gene expression in MC. Blue light is also required for the suppression system to develop in MC. Thus, the maize gene rbcS-m3 contains certain sequences that are responsive to a phytochrome photoperception and signal transduction system and other regions that respond to a UVA/blue light photoperception and signal transduction system. Various models of "coaction" of plant photoreceptors have been advanced; these observations show the basis for one type of coaction.
Full text
PDF
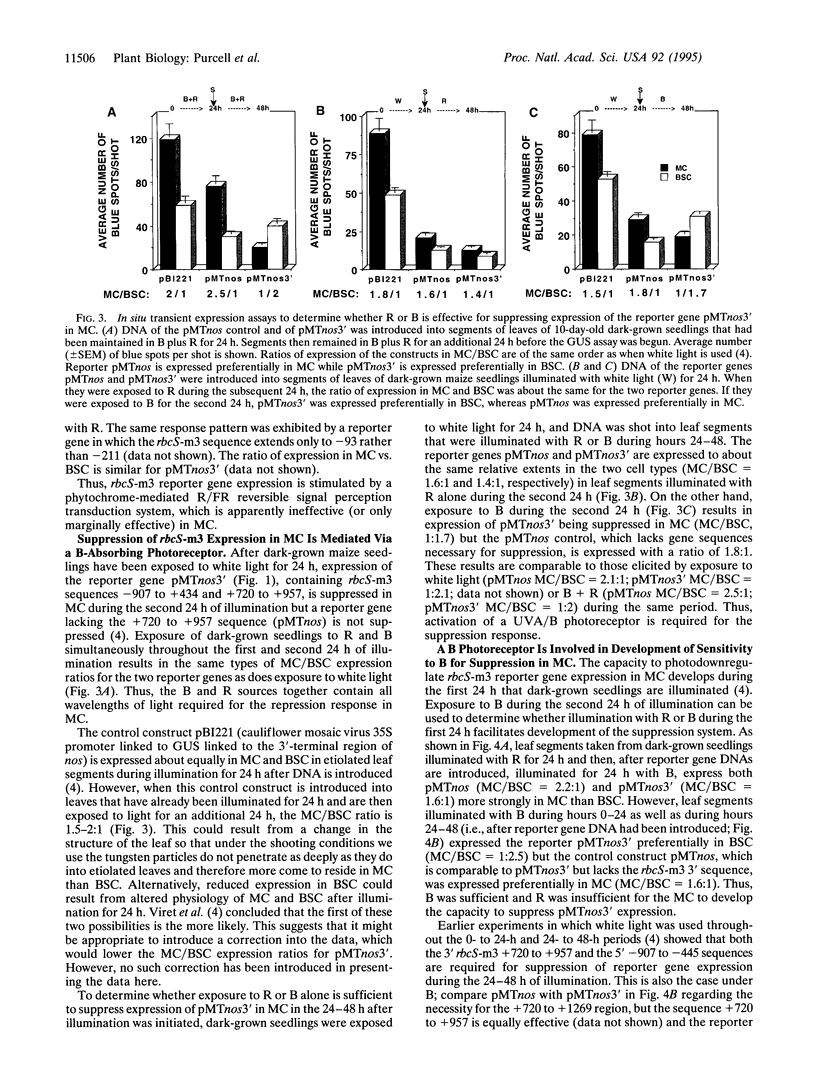
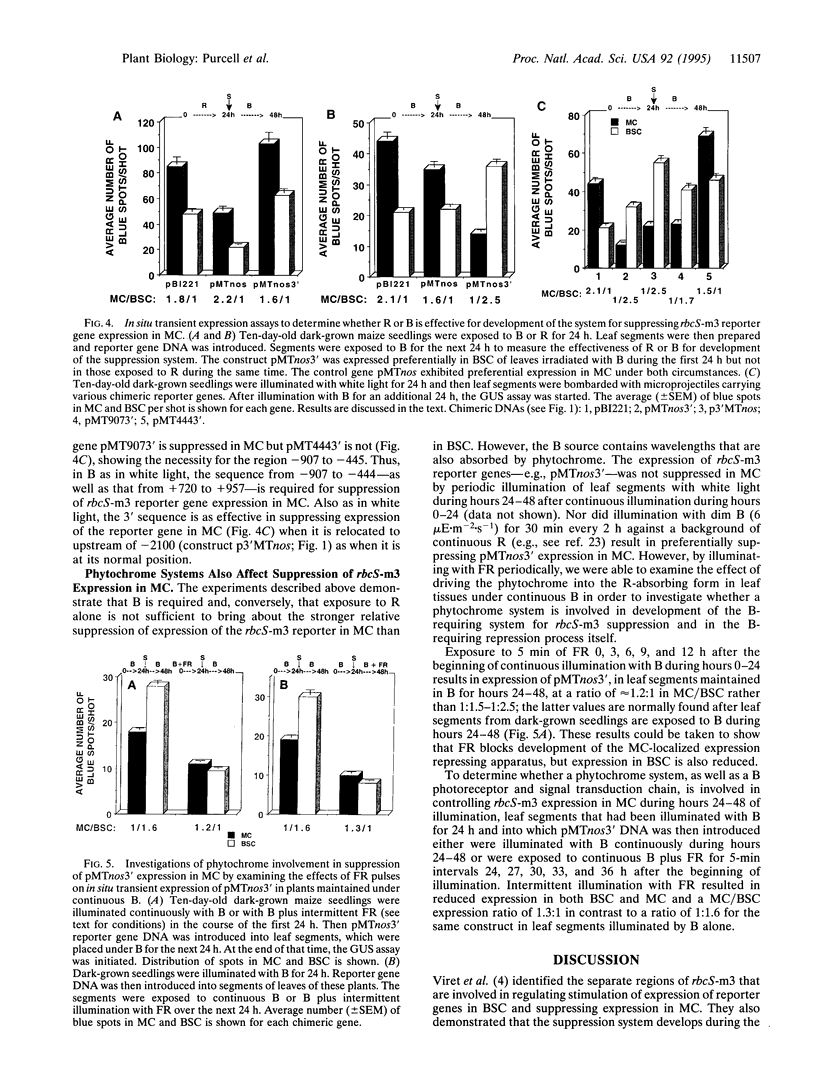

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad M., Cashmore A. R. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993 Nov 11;366(6451):162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- Bansal K. C., Bogorad L. Cell type-preferred expression of maize cab-m1: repression in bundle sheath cells and enhancement in mesophyll cells. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4057–4061. doi: 10.1073/pnas.90.9.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal K. C., Viret J. F., Haley J., Khan B. M., Schantz R., Bogorad L. Transient expression from cab-m1 and rbcS-m3 promoter sequences is different in mesophyll and bundle sheath cells in maize leaves. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3654–3658. doi: 10.1073/pnas.89.8.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Lowe S. L., Meagher R. B. Transcriptional regulation of a gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean tissue is linked to the phytochrome response. Mol Cell Biol. 1985 Aug;5(8):1910–1917. doi: 10.1128/mcb.5.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M., Barnes W. M., Chilton M. D. Structure and transcription of the nopaline synthase gene region of T-DNA. Nucleic Acids Res. 1983 Jan 25;11(2):369–385. doi: 10.1093/nar/11.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J. Out of darkness: mutants reveal pathways controlling light-regulated development in plants. Trends Genet. 1993 May;9(5):167–172. doi: 10.1016/0168-9525(93)90163-c. [DOI] [PubMed] [Google Scholar]
- Clugston C. K., Barnett L. K., Urwin N. A., Jenkins G. I. Photoreceptors controlling transcription of rbcS genes in green leaf tissue of Pisum sativum. Photochem Photobiol. 1990 Jul;52(1):23–28. doi: 10.1111/j.1751-1097.1990.tb01750.x. [DOI] [PubMed] [Google Scholar]
- Dehesh K., Franci C., Parks B. M., Seeley K. A., Short T. W., Tepperman J. M., Quail P. H. Arabidopsis HY8 locus encodes phytochrome A. Plant Cell. 1993 Sep;5(9):1081–1088. doi: 10.1105/tpc.5.9.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Chua N. H. Developmental regulation of two genes encoding ribulose-bisphosphate carboxylase small subunit in pea and transgenic petunia plants: Phytochrome response and blue-light induction. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2358–2362. doi: 10.1073/pnas.83.8.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren G., Lau A., Klein J., Golas C., Bologa-Campeanu M., Soldin S., MacLeod S. M., Prober C. Pharmacokinetics and adverse effects of amphotericin B in infants and children. J Pediatr. 1988 Sep;113(3):559–563. doi: 10.1016/s0022-3476(88)80653-x. [DOI] [PubMed] [Google Scholar]
- Lebrun M., Waksman G., Freyssinet G. Nucleotide sequence of a gene encoding corn ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit (rbcs). Nucleic Acids Res. 1987 May 26;15(10):4360–4360. doi: 10.1093/nar/15.10.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S., Bogorad L. A rice cab gene promoter contains separate cis-acting elements that regulate expression in dicot and monocot plants. Plant Cell. 1992 Aug;4(8):971–981. doi: 10.1105/tpc.4.8.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail P. H., Boylan M. T., Parks B. M., Short T. W., Xu Y., Wagner D. Phytochromes: photosensory perception and signal transduction. Science. 1995 May 5;268(5211):675–680. doi: 10.1126/science.7732376. [DOI] [PubMed] [Google Scholar]
- Reed J. W., Nagatani A., Elich T. D., Fagan M., Chory J. Phytochrome A and Phytochrome B Have Overlapping but Distinct Functions in Arabidopsis Development. Plant Physiol. 1994 Apr;104(4):1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Sakihama T., Kamikubo T., Shinozaki K. Phytochrome-mediated regulation of two mRNAs, encoded by nuclei and chloroplasts of ribulose-1,5-bisphosphate carboxylase/oxygenase. Eur J Biochem. 1983 Jul 1;133(3):617–620. doi: 10.1111/j.1432-1033.1983.tb07507.x. [DOI] [PubMed] [Google Scholar]
- Sheen J. Y., Bogorad L. Differential expression of the ribulose bisphosphate carboxylase large subunit gene in bundle sheath and mesophyll cells of developing maize leaves is influenced by light. Plant Physiol. 1985 Dec;79(4):1072–1076. doi: 10.1104/pp.79.4.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Y., Bogorad L. Expression of the ribulose-1,5-bisphosphate carboxylase large subunit gene and three small subunit genes in two cell types of maize leaves. EMBO J. 1986 Dec 20;5(13):3417–3422. doi: 10.1002/j.1460-2075.1986.tb04663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short T. W., Briggs W. R. Characterization of a Rapid, Blue Light-Mediated Change in Detectable Phosphorylation of a Plasma Membrane Protein from Etiolated Pea (Pisum sativum L.) Seedlings. Plant Physiol. 1990 Jan;92(1):179–185. doi: 10.1104/pp.92.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viret J. F., Mabrouk Y., Bogorad L. Transcriptional photoregulation of cell-type-preferred expression of maize rbcS-m3: 3' and 5' sequences are involved. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8577–8581. doi: 10.1073/pnas.91.18.8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warpeha K. M., Kaufman L. S. Blue-Light Regulation of Epicotyl Elongation in Pisum sativum. Plant Physiol. 1989 Feb;89(2):544–548. doi: 10.1104/pp.89.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. S., Kung S. D., Bogorad L. Phytochrome control of levels of mRNA complementary to plastid and nuclear genes of maize. Plant Physiol. 1985 Oct;79(2):371–376. doi: 10.1104/pp.79.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]