Abstract
Purpose
The extent and intensity of 18F-FDG uptake in prostate cancer patients are known to be variable, and the clinical significance of focal 18F-fluorodeoxyglucose (18F-FDG) uptake that is incidentally found on positron emission tomography (PET) has not been established. We investigated the clinical significance of incidental focal prostate uptake of 18F-FDG on PET/computed tomography (CT) and analyzed differential findings on PET/CT between malignant and benign uptake.
Methods
A total of 14,854 whole-body 18F-FDG PET/CT scans (4,806 that were conducted during cancer screening and 10,048 that were conducted to evaluate suspected or alleged cancer outside of the prostate) were retrospectively reviewed to determine the presence, location, multiplicity and maximum standardized uptake value (SUVmax) of focal prostate uptake and combined calcification. The final diagnosis determined by serum prostate-specific antigen (PSA) level and biopsy was compared with PET findings.
Results
Incidental focal prostate uptake was observed in 148 of 14,854 scans (1.0 %). Sixty-seven of these 148 subjects who had diagnostic confirmation were selected for further analysis. Prostate cancer was diagnosed in nine of 67 subjects (13.4%). The remaining 58 subjects had no malignancy in the prostate based on normal serum PSA level (n = 53), or elevated serum PSA level with a negative biopsy result (n = 5). While 84.6% (11/13) of malignant uptake was peripherally located in the prostate glands, 60.2% (50/83) of benign uptake was centrally located (p < 0.05). The positive predictive value of peripheral focal uptake for malignancy was 25%. The SUVmax, multiplicity and combined calcification were not significantly different between the two groups.
Conclusion
Although incidental focal 18F-FDG uptake in the prostate is not common, the incidence of cancer with focal uptake is not low. Therefore, these findings deserve further evaluation. The location of the focal prostate uptake may help with the selection of high-risk prostate cancer patients.
Keywords: 18F-fluorodeoxyglucose, PET/CT, Prostate gland, Prostate cancer
Introduction
Positron emission tomography (PET)/computed tomography (CT) using 18F-fluorodeoxyglucose (18F-FDG) is a useful whole-body imaging modality that is widely used for diagnosis, initial staging, restaging, post-therapy and follow-up for many kinds of cancers [1, 2]. Due to its advantages of whole-body coverage and exact anatomical localization, 18F-FDG PET/CT often shows focal hypermetabolic lesions in clinically unexpected sites [3]. Previous studies have suggested that these incidental lesions are associated with a second primary cancer, unexpected metastasis or other pathological lesions deserving further evaluation [3]. Detection of secondary primary cancer is one of important prognostic factors in cancer patients. For example, in patients with early-stage head and neck squamous cell carcinomas, a second primary cancer is the leading cause of treatment failure and death in patients [4–6]
However, it is controversial whether further diagnostic work-ups are necessary for all incidental focal hypermetabolic lesions. Incidental focal hypermetabolic lesions on 18F-FDG PET/CT are not rare, but the cancer risk of such lesions is not high [3, 7]. Physiological or benign incidental focal uptake is often found in the thyroid, bowel, uterus, ovary, and other organs. [3]. Further evaluation of all incidental focal hypermetabolic lesions raises problems in terms of cost and time. Therefore, it is important and necessary to recognize the differential findings that are most associated with the risk of cancer and thus deserve further diagnostic work-up. For example, one study reported that it is possible to screen focal hypermetabolic thyroid lesions incidentally found on 18F-FDG PET/CT and warranting further diagnostic work-up due to a high risk of cancer using combined PET/CT diagnostic criteria including SUVmax and CT attenuation [3].
There are only a few case reports showing incidental focal uptake lesions in the prostate on 18F-FDG PET/CT, and these were pathologically proven to be prostate cancer or granulomatous prostatitis [8–10]. As is the case with most other cancers, early detection of prostate cancer may reduce the risk of dying from prostate cancer [11]. The purpose of this study was to investigate the incidence and clinical significance of incidental focal prostate uptake on 18F-FDG PET/CT. Differential findings between malignant and benign uptake were also analyzed.
Materials and Methods
Subjects
From March 2005 to September 2008, 15,301 male subjects underwent 18F-FDG PET/CT scans for cancer screening, or evaluation of known or suspected malignancies. Among these patients, we excluded 447 subjects known to have prostate cancer or a previous history of prostate surgery. Therefore, 14,854 subjects were included in this study, and among these, we retrospectively reviewed the medical records of 148 with focal hypermetabolic foci in the prostate gland. When a focal hypermetabolic prostate lesion was incidentally found on PET/CT, we usually recommended a test for serum levels of prostate-specific antigen (PSA), irrespective of the degree of uptake on the initial official interpretation of PET/CT images. We investigated the clinical courses of the 148 subjects with focal hypermetabolic lesions of the prostate glands. Finally, 67 subjects who underwent further diagnostic work-up for prostate lesions were selected and included in further analyses.
Our institutional review board approved the study protocol of this retrospective study, which was exempt from the requirement for informed consent.
18F-FDG PET/CT
All patients underwent 18F-FDG PET/CT imaging after at least 6 h of fasting with liberal water intake. 18F-FDG was produced using a commercially available cyclotron (PET Trace, GE Healthcare, Milwaukee, WI, USA). The PET/CT scan was performed using a dedicated PET/CT scanner (Discovery LS, GE Healthcare). A whole-body CT from the basal skull to the mid-thigh was performed by a continuous spiral technique using an eight-slice helical CT with a gantry rotation speed of 0.8 s 45 min after an intravenous injection of ~370 MBq 18F-FDG. The CT scan data were collected with 40–120 mAs adjusted to the patient’s body weight, 140 KeV, a section width of 5 mm, and a table feed of 5 mm per rotation. No intravenous or oral contrast material was used. After the CT scan, a corresponding emission scan was obtained with 4 min per frame. Attenuation-corrected PET images using CT data were reconstructed by an ordered-subsets expectation maximization algorithm (28 subsets, 2 iterations). The standardized uptake values (SUVs) were acquired using the attenuation-corrected images, the amount of injected 18F-FDG, the body weight of each patient and the cross-calibration factors between the PET and the dose calibrator. Commercial software (Xeleris, GE Healthcare) was used to accurately coregister the separate CT and PET scan data.
Image Analysis
PET/CT image analysis focused on the focal hypermetabolic lesions of the prostate. Retrospective visual analysis was performed by the consensus of two nuclear medicine physicians. First, we reviewed the medical records for PET/CT images with focal uptake in the prostate. In this study, a focal prostate lesion was defined as focally increased 18F-FDG uptake within the prostate glands compared with the uptake of surrounding prostatic parenchyma, excluding the focal physiological uptake in the prostatic urethra which was discernible by fused PET/CT images. If focal hypermetabolic lesions were found, the maximum SUV (SUVmax) of each lesion was measured. The location of each focal hypermetabolic lesion was classified as either central or peripheral according to the distance from the central prostatic urethra. A point halfway between the central prostatic urethra and the margin of the prostate was determined to demarcate the border between the central and peripheral portions. In addition, the presence of combined calcification within a focal hypermetabolic lesion was determined. Multiplicity of hypermetabolic lesions was also evaluated.
Data Analysis
Final diagnosis was determined based on the histology of each hypermetabolic lesion or serum PSA level in cases for which the histological results were not available. Age, SUVmax, location, multiplicity and calcification were compared between benign and malignant focal hypermetabolic prostatic lesions by independent t-test or chi-square test. A p value of less than 0.05 was considered to indicate a statistically significant difference.
Results
In the study sample of 14,854 male subjects, clinically unexpected focal 18F-FDG uptake in prostate glands was identified in a total of 148 subjects (1.0%). The serum PSA level was checked in 67 of the 148 subjects. Our results were based on 96 focal hypermetabolic foci from these 67 subjects (age, 59.2 ± 10.5 years; range, 38–83 years). For 53 subjects, the focal hypermetabolic lesions in the prostate glands were not pathologically confirmed, because the serum PSA levels were normal. The remaining 14 subjects, including 12 with high serum PSA level, had histopathologic confirmation via multiple prostate biopsy (number of sites, 8.9 ± 2.7; range, 3–12), and nine subjects (13.4%) were confirmed to have prostate cancer. Due to multiple prostate biopsies, where we knew the location of each biopsy site, it was possible to perform a lesion-based correlation analysis between focal hypermetabolic lesions and pathological results. The 67 subjects were divided into two groups. The malignant group was composed of subjects who were pathologically proven to have prostate cancer. The remaining subjects, who showed normal serum PSA level or had pathologically proven benign prostate uptake, were classified as the benign group. There were nine men with an age range of 57–80 years (mean, 69.5 ± 8.2 years) in the malignant group and 58 men with an age range of 38–83 (mean, 57.6 ± 10.0 years) in the benign group. Although the mean age of the subjects in the malignant group was slightly higher than that of the subjects in the benign group, this difference was not statistically significant. On the basis of pathology and serum PSA level, the incidence of prostate cancer was 13.4% (9/67). On the basis of focal uptake number, the incidence of cancer was 13.5% (13/96) because the malignant group had a total of 13 focal hypermetabolic lesions and the benign group had 83.
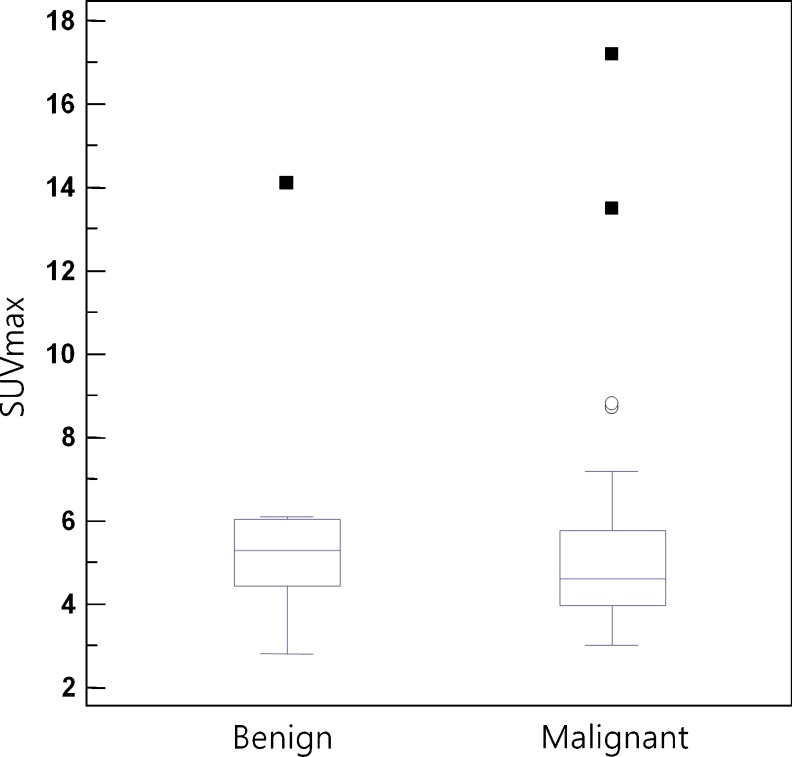
We compared the PSA levels and SUVmax of each group. The PSA levels were significantly higher in the malignant group. The SUVmax, however, was not significantly different between the two groups (Fig. 1). The cancer incidence was significantly higher when the hypermetabolic foci were peripheral (odds ratio = 8.3) (Figs. 2, 3). The positive predictive value of peripheral focal uptake for malignancy was 25.0%. The multiplicity of each hypermetabolic focal lesion and combined calcification were not correlated with cancer incidence in either group (Table 1) .
Fig. 1.
Comparison of SUVmax between patients with benign and malignant prostate lesions. There was no significant difference in SUVmax between the two groups
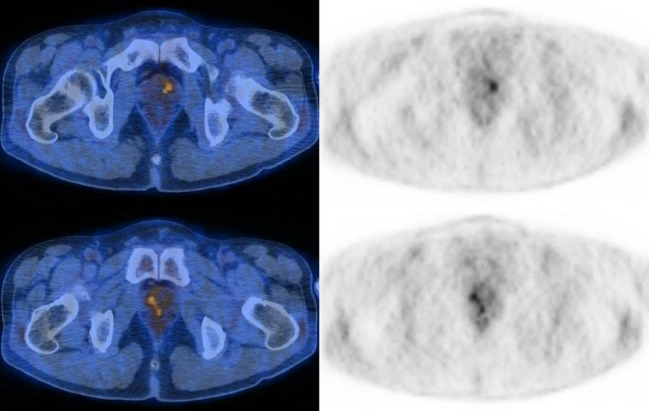
Fig. 2.
PET/CT images of a 52-year-old male. There are two focal hypermetabolic lesions in the central portion of the prostate gland (SUVmax = 3.5, 3.3) that were proven to be benign based on normal serum PSA level
Fig. 3.
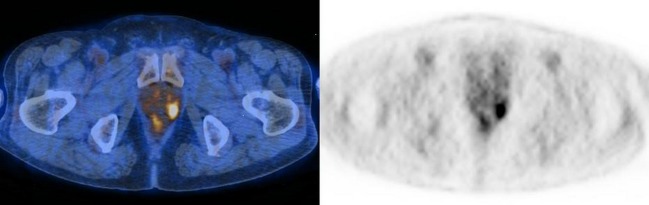
PET/CT images of a 77-year-old male. There is a focal hypermetabolic lesion in the peripheral portion of the left prostate gland (SUVmax = 6.1). The serum PSA level was elevated (7.06 ng/ml) and the lesion was pathologically proven to be prostate cancer
Table 1.
Comparisons between the malignant group and the benign group
| Malignant group | Benign group | p value | |
|---|---|---|---|
| Age (years)a | 69.5 ± 8.2 | 57.6 ± 10.0 | NS |
| PSA (ng/ml) | 33.4 ± 37.9 | 1.5 ± 2.7 | <0.001 |
| SUVmax | 5.5 ± 3.1 | 5.5 ± 2.6 | NS |
| Location (peripheral/central) | 11/2 | 33/50 | <0.001 |
| Multiplicity | 3/9 | 2/58 | NS |
| Calcification | 3/13 | 25/83 | NS |
NS not significant
a Patient-based. The remaining results are lesion-based
Discussion
Our results suggest that although incidental focal hypermetabolic lesions on the prostate glands are not common (1.0%), the cancer incidence of such lesions (13.4%) may suggest the need for further diagnostic evaluation, such as serum PSA level or prostate biopsy. There was a significant difference in 18F-FDG PET/CT between benign and malignant lesions according to the location of the hypermetabolic foci. In other words, peripherally located focal hypermetabolic foci in the prostate glands were more likely to be malignant than centrally located foci (odds ratio = 8.3). This finding may assist in the screening of focal hypermetabolic lesions, and justify further diagnostic work-up due to high risk of malignancy. These results are in agreement with the results of previous studies that demonstrated that the incidence of prostate cancer differs according to anatomical zone [12]. Most prostate cancers originate from the peripheral zone, while only 15% of cancers originate from the transitional zone, and very few cases originate from the central zone.
The SUVmax of the hypermetabolic lesions was not helpful for differentiating benign from malignant prostate lesions. This result may be explained by several facts as follows. First, prostate cancer cells are usually well differentiated, having lower glucose utilization than tumor cells of other types [10, 13–15]. Second, the intense urine radioactivity of the bladder and prostatic urethra, which are close to the prostate gland, may have affected difficult differential diagnoses [16]. Finally, benign prostate disease, such as benign prostatic hypertrophy (BPH) and prostatitis, is relatively common, and can show focal 18F-FDG uptake that mimics malignancy [17].
Calcification is considered to be a diagnostic criterion suggesting benign lesions in several clinical situations, such as single pulmonary nodule or mediastinal lymph node in lung cancer [18]. In this study, however, the presence of calcification on the focal prostate lesion was not helpful for differential diagnosis. First, prostatic calcification is one of the common findings in the aging process [19]. Second, prostatic calcification is known to be generated by the stasis of prostatic fluid due to obstruction within the prostatic ducts. Although prostatitis is the leading cause of calcification, this finding cannot guarantee the absence of malignancy [20]. Multiplicity of focal hypermetabolic lesions is not a significant differential diagnostic criterion. Both prostate cancer and benign disease, such as BPH or prostatitis, may show multifocal uptake.
In this study, the only significant difference between benign and malignant prostate lesions was the location of the hypermetabolic lesions within the prostate gland. In other words, peripheral location of prostate uptake was significantly associated with malignancy. Recently, Hans et al. [21] reported that all three cases of pathologically confirmed prostate cancer in their sample had focal 18F-FDG uptake in the peripheral portion of the prostate gland. However, they could not find a statistical difference between benign and malignant hypermetabolic foci, because of the small number of cases. Minamimoto et al. [22] reported that 18F-FDG PET/CT is appropriate for detecting peripheral zone prostate cancer in patients with more than an intermediate risk. In their study, they enrolled 50 subjects who had serum PSA levels that were already elevated prior to the 18F-FDG PET/CT scan. However, in contrast to the present study, the hypermetabolic lesions on the prostate glands in their study were not incidental [22]. Another different finding was that the positive predictive value of elevated PSA in our study was 75.0%, which was higher than the 42.9% of a previous study [22]. This may result from higher PSA levels of our study population than that of the previous study (33.4 ± 37.9 ng/ml vs 15.9 ± 14.9 ng/ml).
This study had several limitations. Firstly, this was a retrospective study in design. Not all subjects with focal hypermetabolic lesions in the prostate gland underwent further diagnostic work-ups due to underlying advanced primary cancers, poor general condition or follow-up loss. The use of random biopsies was another potential limitation. It is possible that the tissue specimens happened to lack cells showing focal hypermetabolism by chance. There may have been false-negative results for prostate biopsies. In addition, as we used CT images for the localization within the prostate glands by the distance from central prostatic urethra, the terms, central/peripheral uptake, cannot completely reflect the anatomical zones of prostate.
In conclusion, although incidental focal hypermetabolic lesions in the prostate glands are not common, the cancer incidence of such lesions is not low. Therefore, further diagnostic work-ups for those lesions are warranted, especially when the hypermetabolic lesions are located peripherally. The SUVmax, combined calcification, and multiplicity of incidental focal prostate uptake are not associated with the risk of malignancy.
Acknowledgments
Conflicts of Interest
None.
References
- 1.Agress H, Jr, Cooper BZ. Detection of clinically unexpected malignant and premalignant tumors with whole-body FDG PET: histopathologic comparison. Radiology. 2004;230:417–22. doi: 10.1148/radiol.2302021685. [DOI] [PubMed] [Google Scholar]
- 2.Ozkol V, Alper E, Aydin N, Ozkol HF, Topal NB, Akpinar AT. The clinical value of incidental 18F-fluorodeoxyglucose-avid foci detected on positron emission tomography/computed tomography. Nucl Med Commun. 2010;31:128–36. doi: 10.1097/MNM.0b013e328332b30e. [DOI] [PubMed] [Google Scholar]
- 3.Choi JY, Lee KS, Kim HJ, Shim YM, Kwon OJ, Park K, et al. Focal thyroid lesions incidentally identified by integrated 18F-FDG PET/CT: clinical significance and improved characterization. J Nucl Med. 2006;47:609–15. [PubMed] [Google Scholar]
- 4.León X, Ferlito A, Myer CM, 3rd, Saffiotti U, Shaha AR, Bradley PJ, et al. Second primary tumors in head and neck cancer patients. Acta Otolaryngol. 2002;122:765–78. doi: 10.1080/003655402/000028048. [DOI] [PubMed] [Google Scholar]
- 5.Lippman SM, Hong WK. Second malignant tumors in head and neck squamous cell carcinoma: the overshadowing threat for patients with early-stage disease. Int J Radiat Oncol Biol Phys. 1989;17:691–4. doi: 10.1016/0360-3016(89)90126-0. [DOI] [PubMed] [Google Scholar]
- 6.Rheingold SR, Neugut AI, Meadows AT, et al. Secondary cancers: incidence, risk factors, and management. In: Kufe DW, Pollock RE, Weichselbaum RR, Bast RC Jr, Gansler TS, Holland JF, et al., editors. Holland-Frei Cancer Medicine. 6. Hamilton: BC Decker; 2003. pp. 2623–31. [Google Scholar]
- 7.Zhai G, Zhang M, Xu H, Zhu C, Li B. The role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography whole body imaging in the evaluation of focal thyroid incidentaloma. J Endocrinol Invest. 2010;33:151–5. doi: 10.1007/BF03346574. [DOI] [PubMed] [Google Scholar]
- 8.Hung GU, Hsiung CY, Huang ML, Lin ST. Synchronous prostate cancer incidentally detected by FDG-PET/CT in staging a patient with newly diagnosed nasopharyngeal cancer. Clin Nucl Med. 2009;34:962–3. doi: 10.1097/RLU.0b013e3181bed0ae. [DOI] [PubMed] [Google Scholar]
- 9.Ilgan S, Koca G, Gundogdu S. Incidental detection of granulomatous prostatitis by F-18 FDG PET/CT in a patient with bladder cancer: a rare complication of BCG instillation therapy. Clin Nucl Med. 2009;34:613–4. doi: 10.1097/RLU.0b013e3181b06c89. [DOI] [PubMed] [Google Scholar]
- 10.Monet A, Merino B, Lupo R. Interesting image. Incidental diagnosis of prostate cancer by F-18 FDG PET/CT. Clin Nucl Med. 2010;35:34–5. doi: 10.1097/RLU.0b013e3181c361d6. [DOI] [PubMed] [Google Scholar]
- 11.Wolf AM, Wender RC, Etzioni RB, Thompson IM, D’Amico AV, Volk RJ, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60:70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 12.Scher HI, Leibel SA, Fuks Z, Cordon-cardo C, Scardino PT. Cancer of the prostate : Anatomy of the prostate. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 7. Philadelphia: Lippincott Williams & Wilkins; 2005. p. 1194. [Google Scholar]
- 13.Takahashi N, Inoue T, Lee J, Yamaguchi T, Shizukuishi K. The roles of PET and PET/CT in the diagnosis and management of prostate cancer. Oncology. 2007;72:226–33. doi: 10.1159/000112946. [DOI] [PubMed] [Google Scholar]
- 14.Jadvar H. FDG PET in prostate cancer. PET Clin. 2009;1(4):155–161. doi: 10.1016/j.cpet.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang BJ OJH, Baik JH, Jung SL, Park YH, Chung SK. Incidental thyroid uptake on F-18 FDG PET/CT: correlation with ultrasonography and pathology. Ann Nucl Med. 2009;23:729–37. doi: 10.1007/s12149-009-0299-4. [DOI] [PubMed] [Google Scholar]
- 16.Seltzer MA, Barbaric Z, Belldegrun A, Naitoh J, Dorey F, Phelps ME, et al. Comparison of helical computerized tomography, positron emission tomography and monoclonal antibody scans for evaluation of lymph node metastases in patients with prostate specific antigen relapse after treatment for localized prostate cancer. J Urol. 1999;162:1322–8. doi: 10.1016/S0022-5347(05)68277-8. [DOI] [PubMed] [Google Scholar]
- 17.Mathews D, Oz OK. Positron emission tomography in prostate and renal cell carcinoma. Curr Opin Urol. 2002;12:381–5. doi: 10.1097/00042307-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Lee EJ, Choi JY, Lee KS, Chung HW, Lee SJ, Cho YS, et al. Improving diagnostic accuracy for malignant nodes and N staging in non-small cell lung cancer using CT-corrected FDG-PET. Korean J Nucl Med. 2005;39:231–8. [Google Scholar]
- 19.Klimas R, Bennett B, Gardner WA., Jr Prostatic calculi: a review. Prostate. 1985;7:91–6. doi: 10.1002/pros.2990070110. [DOI] [PubMed] [Google Scholar]
- 20.Taylor JS. Gross calcification within the prostate gland. Br J Urol. 1998;81:645–6. doi: 10.1046/j.1464-410x.1998.00404.x. [DOI] [PubMed] [Google Scholar]
- 21.Han EJ HOJ, Choi WH, Yoo IR, Chung SK. Significance of incidental focal uptake in prostate on 18-fluoro-2-deoxyglucose positron emission tomography CT images. Br J Radiol. 2010;83:915–20. doi: 10.1259/bjr/19887771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minamimoto R, Uemura H, Sano F, Terao H, Nagashima Y, Yamanaka S, et al. The potential of FDG-PET/CT for detecting prostate cancer in patients with an elevated serum PSA level. Ann Nucl Med. 2011;25:21–7. doi: 10.1007/s12149-010-0424-4. [DOI] [PubMed] [Google Scholar]