Schwannomas, also known as neurilemmomas, are tumors originating from nervous tissue; they have Schwann cell sheaths. According to a recent classification, about 80% of gastrointestinal mesenchymal tumors are gastrointestinal stromal tumors (GISTs) [1]. Gastrointestinal (GI) schwannomas have been reported to represent only 3% of all GI mesenchymal tumors [2]. These tumors make up only 0.2% of all gastric neoplasms [3]. Schwannomas of the GI tract are distinctive from conventional schwannomas that arise in soft tissue or the central nervous system. GI schwannomas are hypothesized to arise from the myenteric plexus within the GI tract wall. These tumors are usually benign, slow-growing, and asymptomatic, and therefore most are discovered incidentally [3].
The differentiation of schwannomas from other submucosal tumors is very difficult. The main differential diagnosis for a mass arising in the wall of the gastointestinal tract is a GIST, which is a potentially malignant mesenchymal GI tumor that arises from the interstitial cells of Cajal, which help regulate peristalsis [4]. The diagnostic determination of schwannomas requires positive histological tests for S-100 protein and vimentin, but negative histological tests for smooth muscle actin and c-KIT. In contrast, GISTs are c-KIT positive and can be S-100 positive if they are located in the small bowel [5]. Because most patients with schwannomas have excellent prognoses, surgical removal is sufficient for treatment.
Gastric schwannomas are normally benign, and malignant transformation is extremely rare [6]. However, the current case (Figs. 1 and 2) illustrates that these tumors may exhibit avid F-18 FDG uptake. It remains unclear why high F-18 FDG uptake is found in benign tumors such as schwannomas. F-18 FDG uptake in soft tissue and neural schwannomas is variable but is frequently high, possibly due to over-expression of the glucose transporter by tumor cells. In particular, glucose transporter type 3 is found in all human tissues and is the major glucose transporter on the neuronal surface [7, 8]. However, for diagnostic purposes, intense F-18 FDG uptake does not distinguish benign schwannomas from potentially malignant GISTs, which also have high F-18 FDG uptake. These data suggest that F-18 FDG PET is of limited value as a preoperative diagnostic technique for the assessment of schwannoma versus GIST.
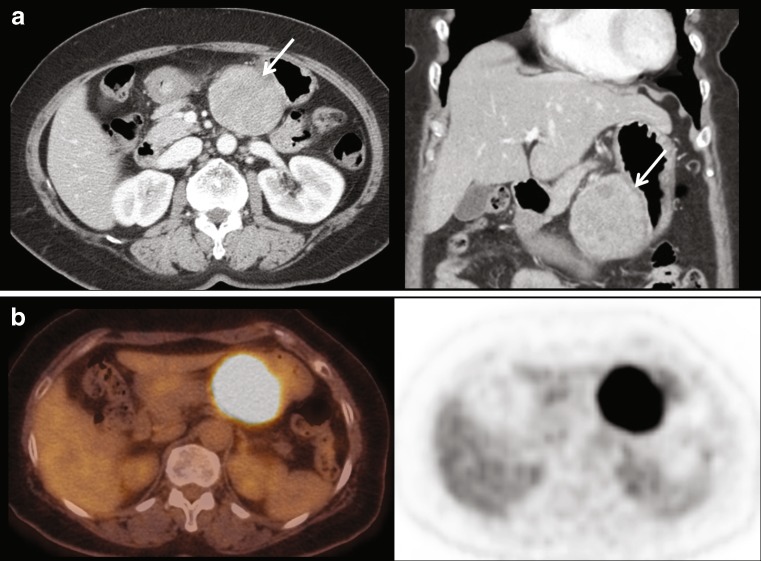
Fig. 1.
A 58-year-old woman was admitted for the evaluation and treatment of a gastric mass that had previously been detected by cancer-screening endoscopy. Endoscopy revealed a submucosal tumor that had a central ulcer on its surface. Contrast-enhanced CT showed a solitary, exophytic soft tissue mass measuring 6.1 cm in diameter at the lesser curvature of the stomach around the gastric angle. The mass exhibited central ulceration, calcification, and small necrotic portions (a, arrows). On F-18 FDG PET, this mass showed intense F-18 FDG uptake (maximum SUV = 9.7) (b). The mass was thought to be a malignant gastrointestinal stromal tumor
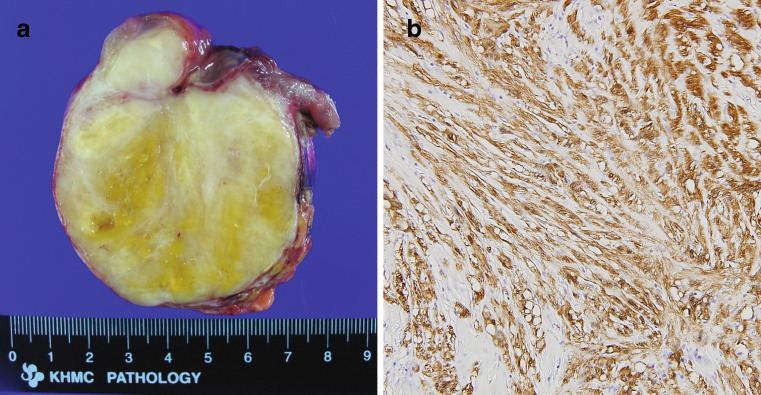
Fig. 2.
On gross inspection, the tumor was an 8 × 7 × 6 cm sized well-encapsulated ovoid, firm mass in the stomach wall. A mucosal ulceration (2 × 1.5 cm) was noted. The cut surfaces of the tumor revealed a gray and white, solid appearance with patches of yellow discoloration. Small cystic lesions were also noted, but hemorrhage or necrosis was not identified (a). Immunohistochemically, the tumor was positive for S-100 protein, while smooth muscle actin and c-KIT were negative (×200). These pathologic features are consistent with schwannoma (b)
Acknowledgments
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol. 1999;30:1213–1220. doi: 10.1016/S0046-8177(99)90040-0. [DOI] [PubMed] [Google Scholar]
- 2.Kwon MS, Lee SS, Ahn GH. Schwannomas of the gastrointestinal tract: clinicopathological features of 12 cases including a case of esophageal tumor compared with those of gastrointestinal stromal tumors and leiomyomas of the gastrointestinal tract. Pathol Res Pract. 2002;198:605–613. doi: 10.1078/0344-0338-00309. [DOI] [PubMed] [Google Scholar]
- 3.Melvin WS, Wilkinson MG. Gastric schwannoma. Clinical and pathologic considerations. Am Surg. 1993;59:293–296. [PubMed] [Google Scholar]
- 4.Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. From the Archives of the AFIP: gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003;23:283–304. doi: 10.1148/rg.232025146. [DOI] [PubMed] [Google Scholar]
- 5.Miettinen M, Shekitka KM, Sobin LH. Schwannomas in the colon and rectum: a clinicopathologic and immunohistochemical study of 20 cases. Am J Surg Pathol. 2001;25:846–855. doi: 10.1097/00000478-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Bees NR, Ng CS, Dicks-Mireaux C, Kiely EM. Gastric malignant schwannoma in a child. Br J Radiol. 1997;70:952–955. doi: 10.1259/bjr.70.837.9486074. [DOI] [PubMed] [Google Scholar]
- 7.Komatsu D, Koide N, Hiraga R, Furuya N, Akamatsu T, Uehara T, et al. Gastric schwannoma exhibiting increased fluorodeoxyglucose uptake. Gastric Cancer. 2009;12:225–228. doi: 10.1007/s10120-009-0526-7. [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu S, Rubin B, Djang D, Conrad E, Turcotte E, Eary JF. Positron emission tomography of schwannomas: emphasizing its potential in preoperative planning. AJR Am J Roentgenol. 2004;182:971–974. doi: 10.2214/ajr.182.4.1820971. [DOI] [PubMed] [Google Scholar]