Abstract
Oncogenic osteomalacia is a rare paraneoplastic syndrome characterized by renal phosphate excretion, hypophosphatemia, and osteomalacia. This syndrome is often caused by tumors of mesenchymal origin. Patients with oncogenic osteomalacia have abnormal bone mineralization, resulting in a high frequency of fractures. Tumor resection is the treatment of choice, as it will often correct the metabolic imbalance. Although oncogenic osteomalacia is a potentially curable disease, diagnosis is difficult and often delayed because of the small size and sporadic location of the tumor. Bone scintigraphy and radiography best characterize osteomalacia; magnetic resonance imaging findings are nonspecific. Here, we report a case of oncogenic osteomalacia secondary to a phosphaturic mesenchymal tumor that was successfully detected by 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT). This case illustrates the advantages of 18F-FDG PET/CT in detecting the occult mesenchymal tumor that causes oncogenic osteomalacia.
Keywords: Oncogenic osteomalacia, 18F-fluorodeoxyglucose, Positron emission tomography
Introduction
Oncogenic osteomalacia is a rare paraneoplastic syndrome associated with benign mesenchymal tumors, with clinical features mimicking those of X-linked or autosomal-dominant hereditary hypophosphatemic rickets. Oncogenic osteomalacia is characterized by severe hypophosphatemia and hyperphosphaturia. The tumors that are frequently related to oncogenic osteomalacia are a rare class of phosphaturic mesenchymal tumors of mixed connective tissue origin [1].
It is well known that surgical removal of the mesenchymal tumors relieves the symptoms and signs of osteomalacia in oncogenic osteomalacia, and phosphate levels return to normal levels within 2–3 days after surgery [2]. Because oncogenic osteomalacia is potentially curable after tumor resection, it is of great importance to detect occult mesenchymal tumors in patients with unexplained osteomalacia. To date, there are no standard methods for detecting occult tumors causing oncogenic osteomalacia. Computed tomography (CT) and magnetic resonance imaging (MRI) are often non-contributory in detecting mesenchymal tumors [3–5]. Bone scintigraphy or radiography only reveals the osteomalacia and rarely detects the mesenchymal tumor causing the syndrome [6, 7]. Somatostatin receptor expression is elevated in mesenchymal tumors, and it has been reported that 111In-pentetreotide or octreotide scintigraphy was useful in some cases [8–10]. However, somatostatin receptor imaging was not contributory in another report [11].
To the best of our knowledge, only a few case reports have evaluated the feasibility of 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET)/CT in detecting occult mesenchymal tumors in oncogenic osteomalacia [11–15]. Despite the benign nature of mesenchymal tumors, each case report indicated that the mesenchymal tumor associated with oncogenic osteomalacia exhibited relatively high 18F-FDG uptake and was easily detected by 18F-FDG PET/CT. Here, we report a case that emphasizes the value of 18F-FDG PET/CT in identifying the occult mesenchymal tumor associated with oncogenic osteomalacia.
Case Report
A 66-year-old woman presented with severe bone pain, gait disturbance, and muscle weakness. The patient complained of pain in both hips and lumbar spine combined with thigh muscle weakness that had persisted for 2 years before admission. One year prior to admission, the pain worsened in the thighs, knees, and ankles. Progressive lower limb weakness developed, and pain extended to the thorax, elbows, and wrists. She did not have a family history of bone disease or fractures. Clinical exams were essentially normal excluding bone and muscular abnormalities. The patient had lost 8 kg since the onset of symptoms.
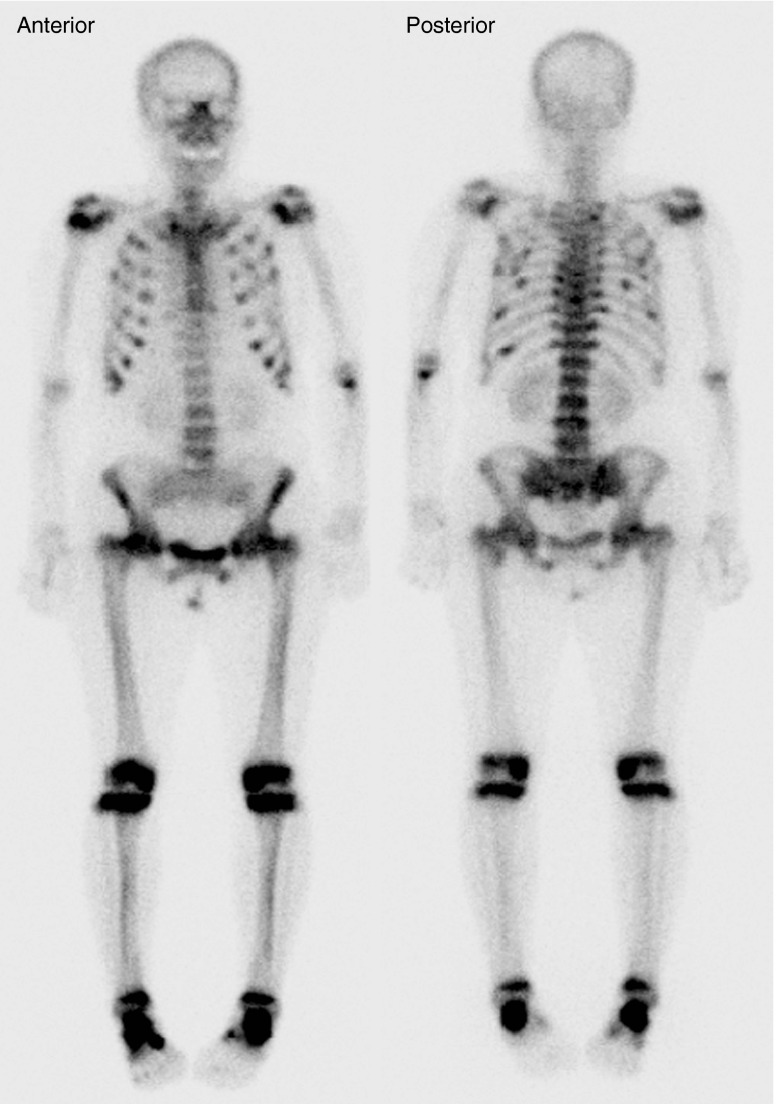
Radiographs of the skeleton revealed bilateral femoral neck fractures and osteopenia, which are suggestive of osteomalacia. Bone scintigraphy using 99mTc-hydroxymethylene diphosphonate (HDP) demonstrated focally increased uptake in both rib cages and diffuse increased uptake in the thoracic and lumbar spines, pelvis, knees, ankles, shoulders, and left elbow (Fig. 1). Electromyography revealed no definite evidence of denervation potential or myopathy. Bone densitometry revealed decreased bone mass in the lumbar vertebrae (T-score = −3.1), whole hip (T-score = −3.6), and femoral neck (T-score = −1.6).
Fig. 1.
99mTc-HDP whole-body bone scan findings on admission. Multifocal increased uptake in both rib cages due to pseudofracture and fracture was noted. Symmetric uptake in two costochondral junctions (rosary pattern) is shown. Increased uptake was observed on both shoulders, left elbow, femoral necks, and knees. These findings represent osteomalacia. There was no focal area of significantly increased activity in the left ischium
Laboratory tests revealed marked hypophosphatemia (1.4 mg/dL; normal range: 2.5–4.5 mg/dL), normal serum calcium levels (9.3 mg/dL), and increased total alkaline phophatase levels (394 IU/L; normal range: 38–115 U/L). The serum level of osteocalcin was 33.12 ng/mL (normal range: 7.6 ± 0.5 ng/mL), and the plasma concentration of 25-OH D was normal (18.13 ng/mL). Serum parathyroid hormone, protein electrophoresis, creatinine, and muscle enzyme levels, liver and thyroid function tests, erythrocyte sedimentation rate, and blood cell count were within normal ranges on laboratory findings.
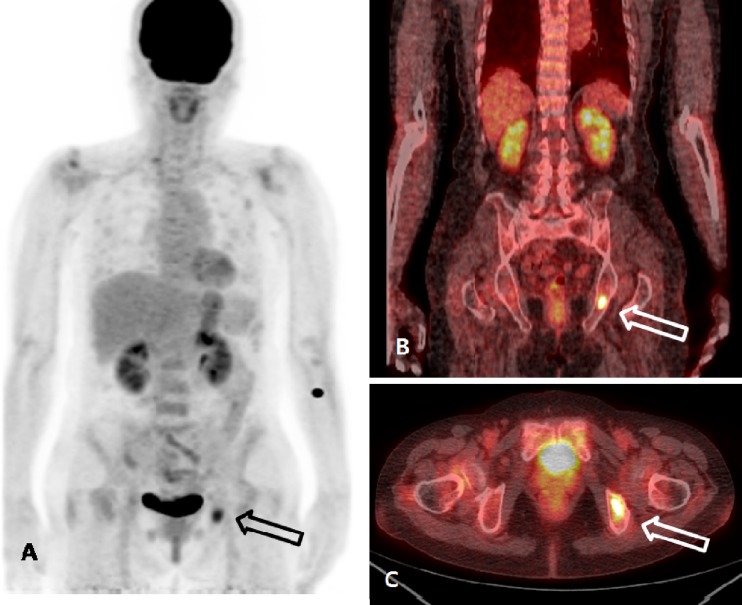
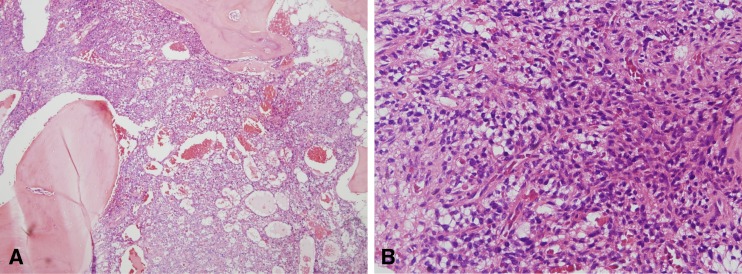
With the clinical diagnosis of hypophosphatemic osteomalacia, 18F-FDG PET/CT was performed to detect the occult tumor causing osteomalacia. An 18F-FDG PET/CT image was acquired according to routine protocols after the injection of 370 MBq of 18F-FDG. 18F-FDG PET/CT revealed an isolated focus of hypermetabolism in the left ischium (Fig. 2). The standardized uptake value (SUV) of the lesion was calculated to be 4.7. No other abnormal finding was found in the rest of the imaged body. Additional MRI confirmed the presence of a well-defined ovoid tumor in the ischium with gadolinium enhancement that measured 2.1 × 1.2 cm in diameter (Fig. 3). A mesenchymal tumor of mixed connective tissue origin was confirmed by histopathologic examination (Fig. 4). In the histologic examination, a large number of small spindle cells with vascular networks, which is suggestive of a typical mesenchymal tumor of mixed connective tissue origin, were noted.
Fig. 2.
a–c 18F-FDG PET/CT findings. 18F-FDG PET/CT revealed the increased 18F-FDG uptake in the left ischium (arrow), and the maximum SUV was calculated to be 4.7, which is suggestive of bone tumor. There was no abnormal 18F-FDG uptake, suggestive of malignant processes, in the rest of the imaged body (a MIP image, b coronal view, c axial view)
Fig. 3.
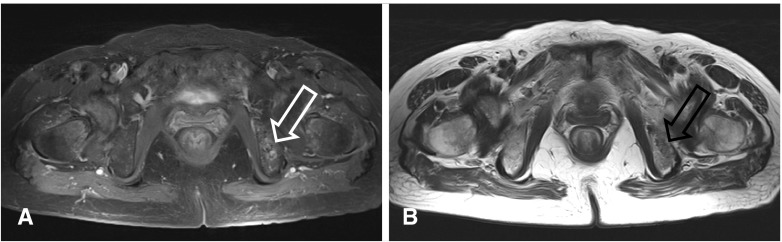
a, b Pelvic MRI findings. a Axial gadolinium-enhanced T1-weighted fat-suppressed MR image, b axial T2-weighted MR image. A 2.1-cm ovoid lesion was identified on the left ischium. T1-weighted fat-suppressed MRI identified a chondroid matrix tumor with contrast enhancement (a; white arrow). T2-weighted MR image revealed a low-to-intermediate-signal-intensity tumor (b; black arrow)
Fig. 4.
a, b Histopathologic examination with hematoxylin and eosin staining. a Band-like short spindle cell proliferation infiltrated into marrow space, and abundant small capillaries were present. A partial shell of woven bone was also present (×40). b Cell proliferation with a vascular network was prominent in the high-magnification image (×200)
Surgical excision was performed to remove the mesenchymal tumor. Final histopathology revealed a mesenchymal tumor of mixed connective tissue origin. Special staining resulted in partial positive SMA, positive vimentin, and negative ER, PR, TTF-1, inhibin, and calretinin, consistent with oncogenic osteomalacia. FGF-23 staining was not performed in this study.
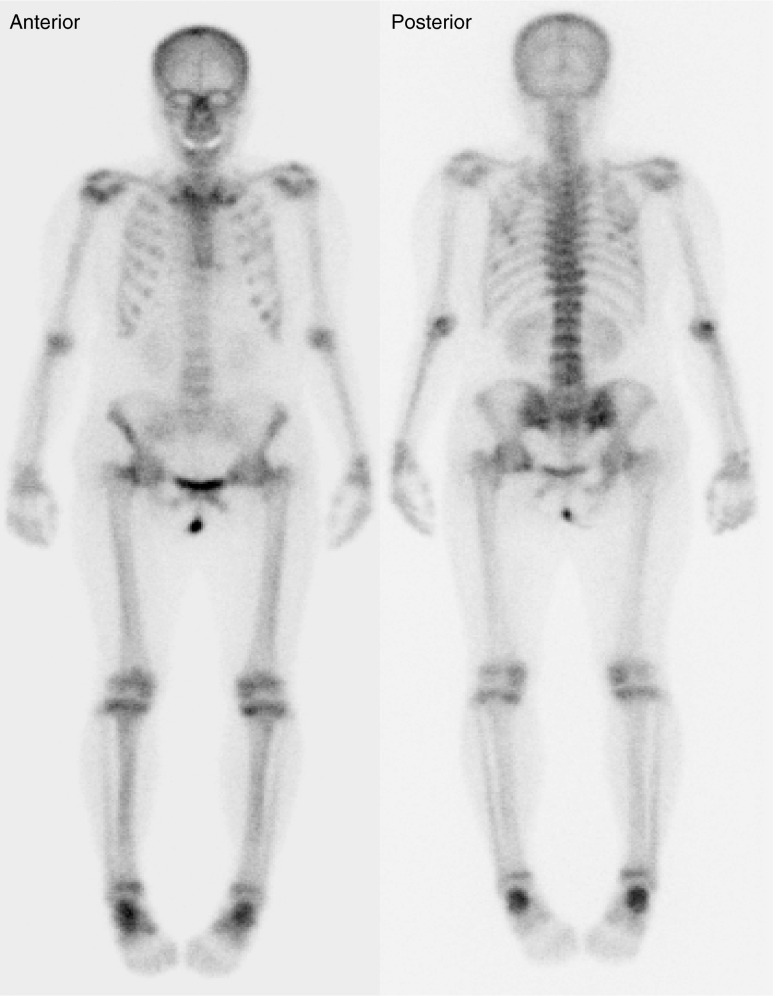
Two days after tumor removal, serum phosphorus levels normalized to 2.5 mg/dL, and a normal value was maintained in follow-up studies. Bone pain and muscle weakness were ameliorated 13 days after the operation. Six months later, follow-up bone scintigraphy was performed to evaluate the recovery of osteomalacia. Compared with preoperative bone scintigraphy, the increased uptake in both shoulders, knees, and ankles was markedly decreased, but mild residual uptake in both ribs, femoral necks, and axial spine was still noted (Fig. 5). The gait function was improved, and the serum level of alkaline phosphate decreased to 188 IU/L.
Fig. 5.
Follow-up 99mTc-HDP bone scintigraphy 6 months after surgical removal of the tumor. Compared to the preoperative bone scintigraphy, a decrease in intensity is observed in the entire skeleton. Mild uptake is observed in both rib cages, axial spine, femoral necks, knees, and ankles. These findings indicate a favorable treatment response after tumor resection
Discussion
Oncogenic osteomalacia is almost exclusively associated with mesenchymal benign tumors, which are frequently located in the bone. Oncogenic osteomalacia is difficult to diagnose because the tumor is frequently small; additionally, it can be located in any soft tissue or skeleton, and it generally causes no pain symptoms. According to the reported cases, difficulty in tumor detection results in a considerable delay in diagnosis and a delay between the onset of symptoms and surgical resection of 5 years on average [16]. In many cases, CT and MRI are not contributory in diagnosing oncogenic osteomalacia [3] because this tumor is often small, grows slowly, and develops in unpredictable locations such as feet [17], mandibular ramus [18], and frontal bone [19]. Previous case reports suggested that 18F-FDG PET/CT is a useful modality in detection of oncogenic tumors causing oncogenic osteomalacia [11–15]. In the present report, the mesenchymal tumor causing oncogenic osteomalacia was easily detected by 18F-FDG PET/CT.
In our report, the patient had typical symptoms and signs of oncogenic osteomalacia. However, initial diagnostic studies including physical examination and radiography failed to localize the tumor. During the follow-up period, radiography revealed only osteopenia and pseudofracture. The constellation of bone scan findings was consistent with osteomalacia, but the tumor in the left ischium was not localized, probably due to low-level bone turnover and limited localization within bone marrow. The present study demonstrated the limitations of conventional imaging modalities in detecting occult mesenchymal tumors. 18F-FDG PET/CT is advantageous in that it has a high lesion-to-background ratio for mesenchymal tumors, can evaluate the entire body in contrast to regional scanning by MRI, and can visualize the tumor itself rather than reactive bone formation as detected in bone scintigraphy.
Although mesenchymal tumors causing oncogenic osteomalacia have benign characteristics, it has been reported that 18F-FDG uptake was relatively high, which allows 18F-FDG PET/CT to be easily located. Our case presented with an SUV of 4.7, which is slightly higher than that in the literature. It may be because the mesenchymal tumor in this case had high cellularity and a large vascular supply, which were observed in the histologic examination. However, the mechanism of high glucose uptake should be further elucidated.
We report a case of oncogenic osteomalacia that was easily localized by 18F-FDG PET/CT. We suggest that 18F-FDG PET should be performed in patients with hypophosphatemic osteomalacia to localize occult tumors and to allow for proper surgical resection.
Acknowledgments
Conflict of Interest
We declare that we have no conflict of interest.
Reference
- 1.Folpe AL, Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, Cho JY, et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28:1–30. doi: 10.1097/00000478-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Weber TJ, Liu S, Indridason OS, Quarles LD. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18:1227–1234. doi: 10.1359/jbmr.2003.18.7.1227. [DOI] [PubMed] [Google Scholar]
- 3.Casari S, Rossi V, Varenna M, Gasparini M, Parafioriti A, Failoni S, et al. A case of oncogenic osteomalacia detected by 111In-pentetreotide total body scan. Clin Exp Rheumatol. 2003;21:493–496. [PubMed] [Google Scholar]
- 4.Von Falck C, Rodt T, Rosenthal H, Langer F, Goesling T, Knapp WH, et al. 68 Ga-DOTANOC PET/CT for the detection of a mesenchymal tumor causing oncogenic osteomalacia. Eur J Nucl Med Mol Imaging. 2008;35:1034. doi: 10.1007/s00259-008-0755-8. [DOI] [PubMed] [Google Scholar]
- 5.Westerberg PA, Olauson H, Toss G, Wikstrom B, Morales O, Linde T, et al. Preoperative tumor localization by means of venous sampling for fibroblast growth factor-23 in a patient with tumor-induced osteomalacia. Endocr Pract. 2008;14:362–367. doi: 10.4158/EP.14.3.362. [DOI] [PubMed] [Google Scholar]
- 6.Dowman JK, Khattak FH. Oncogenic hypophosphataemic osteomalacia mimicking bone metastases on isotope bone scan. Ann Rheum Dis. 2006;65:1664. doi: 10.1136/ard.2006.057943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia CA, Spencer RP. Bone and In-111 octreotide imaging in oncogenic osteomalacia: a case report. Clin Nucl Med. 2002;27:582–583. doi: 10.1097/00003072-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen BD, Wang EA. Indium-111 pentetreotide scintigraphy of mesenchymal tumor with oncogenic osteomalacia. Clin Nucl Med. 1999;24:130–131. doi: 10.1097/00003072-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Sandhu FA, Martuza RL. Craniofacial hemangiopericytoma associated with oncogenic osteomalacia: case report. J Neurooncol. 2000;46:241–247. doi: 10.1023/A:1006352106762. [DOI] [PubMed] [Google Scholar]
- 10.Cheung FM, Ma L, Wu WC, Siu TH, Choi PT, Tai YP. Oncogenic osteomalacia associated with an occult phosphaturic mesenchymal tumour: clinico-radiologico-pathological correlation and ultrastructural studies. Hong Kong Med J. 2006;12:319–321. [PubMed] [Google Scholar]
- 11.Dupond JL, Mahammedi H, Prie D, Collin F, Gil H, Blagosklonov O, et al. Oncogenic osteomalacia: diagnostic importance of fibroblast growth factor 23 and F-18 fluorodeoxyglucose PET/CT scan for the diagnosis and follow-up in one case. Bone. 2005;36:375–378. doi: 10.1016/j.bone.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Khadgawat R, Singh Y, Kansara S, Tandon N, Bal C, Seith A, Kotwal P. PET/CT localisation of a scapular haemangiopericytoma with tumour-induced osteomalacia. Singapore Med J. 2009;50:e55–e57. [PubMed] [Google Scholar]
- 13.Chua SC, O'Connor SR, Wong WL, Ganatra RH. Case report: solitary plasmacytoma of bone with oncogenic osteomalacia: recurrence of tumour confirmed by PET/CT. A case report with a review of the radiological literature. Br J Radiol. 2008;81:e110–e114. doi: 10.1259/bjr/58168443. [DOI] [PubMed] [Google Scholar]
- 14.Jagtap VS, Sarathi V, Lila AR, Malhotra G, Sankhe SS, Bandgar T, Menon P, Shah NS. Tumor-induced osteomalacia: a single center experience. Endocr Pract. 2011;17:177–184. doi: 10.4158/EP10151.OR. [DOI] [PubMed] [Google Scholar]
- 15.Roarke MC, Nguyen BD. PET/CT localization of phosphaturic mesenchymal neoplasm causing tumor-induced osteomalacia. Clin Nucl Med. 2007;32:300–301. doi: 10.1097/01.rlu.0000257180.03964.51. [DOI] [PubMed] [Google Scholar]
- 16.Furuya K, Isobe Y, Morita M. Hypertrophic osteoarthropathy and hypophosphatemic osteomalacia associated with tumor. Gan To Kagaku Ryoho. 1986;13:2056–2064. [PubMed] [Google Scholar]
- 17.Crouzet J, Mimoune H, Beraneck L, Juan LH. Hypophosphatemic osteomalacia with plantar neurilemoma. A review of the literature (100 cases). Rev Rhum Engl Ed 1995;62:463–6. [PubMed]
- 18.Reyes-Mugica M, Arnsmeier SL, Backeljauw PF, Persing J, Ellis B, Carpenter TO. Phosphaturic mesenchymal tumor-induced rickets. Pediatr Dev Pathol. 2000;3:61–69. doi: 10.1007/s100240050008. [DOI] [PubMed] [Google Scholar]
- 19.David K, Revesz T, Kratimenos G, Krausz T, Crockard HA. Oncogenic osteomalacia associated with a meningeal phosphaturic mesenchymal tumor. Case report. J Neurosurg. 1996;84:288–292. doi: 10.3171/jns.1996.84.2.0288. [DOI] [PubMed] [Google Scholar]