Abstract
Purpose
This study aims to compare the performance of contrast-enhanced computed tomography (CeCT) and 18 F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) in detecting small tumor implants and metastatic lymph nodes (LNs) in the abdominopelvic cavity in patients with colorectal cancer.
Methods
We enrolled 16 patients who were clinically suspected of experiencing a recurrence (6 male, 10 female; mean age 61 ± 14 years). All subjects underwent CeCT and PET/CT, and the performance of these methods was compared with regard to detecting recurrences. The final diagnosis of a recurrence was made clinically.
Results
CeCT identified 38 lesions in 12 patients, all of which were detected by PET/CT. PET/CT found 27 additional lesions in 8 patients, comprising 9 seeding nodules (2 in the right upper quadrant of the abdomen and 7 in the pelvic cavity) and 18 LNs (2 celiac, 2 paraaortic, 2 hepatic hilar, 11 common iliac, 1 external iliac). Most additional lesions were located in the pelvic cavity (approximately 78% of seeding nodules and 67% of lymph nodes). The maximum standardized uptake value (SUVmax) of the additional seeding nodules that were detected solely by PET/CT was significantly higher compared with the CeCT- and PET/CT-confirmed nodules (5.5 ± 4.2 vs. 2.9 ± 2.5, p = 0.03). The seeding nodules that were detected only by PET/CT were significantly smaller than the CeCT- and PET/CT-confirmed nodules (long axis: 1.0 ± 0.3 cm vs. 2.0 ± 1.1 cm, p = 0.001; short axis: 0.8 ± 0.3 cm vs. 1.4 ± 0.8 cm, p = 0.004; mean of both axes: 0.9 ± 0.3 cm vs. 1.7 ± 0.9 cm, p = 0.001). Similarly, PET/CT-only-detected LNs were significantly smaller than CeCT- and PET/CT-identified LNs (0.7 ± 0.1 cm vs. 2.3 ± 1.2 cm, p < 0.0001).
Conclusion
PET/CT is superior to CeCT in detecting seeding nodules and metastatic LNs in patients with recurrent colorectal cancer. Specifically, PET/CT detects subcentimeter lesions in anatomically deformed pelvic cavities.
Keywords: PET/CT, Enhanced CT, Subcentimeter, Recurrent colorectal cancer
Introduction
The pelvic cavity is a frequent site of recurrence (30–50%) in colorectal cancer patients who have been treated with surgery or radiotherapy [1, 2]. The peritoneal cavity, a potential space between the parietal and visceral peritoneum, also harbors a significant proportion (25–35%) of postoperative colorectal cancer recurrences [3].
The early identification of tumor implants in these cavities is critical for planning the proper treatment and improving the prognosis. Abdominopelvic computed tomography (CT) is widely used to monitor recurrences, but its sensitivity in identifying lesions ranges between 25% and 90%, depending on the size, site, and morphology of the lesions [4]. The principal factor that affects sensitivity is lesion size. According to De bree et al., the sensitivity of CT in detecting tumor implants varies by lesion size, ranging from 9.1% to 24.3% for tumors <1 cm and 59.3% to 66.7% for tumors >5 cm [5]. Moreover, detection of recurrence decreases significantly when the lesion lies in the pelvic cavity because of anatomical deformation in colorectal cancer patients [6]. Thus, the detection of recurrences by CT has limitations, creating demands for better modalities to identify recurrences in the abdominopelvic cavity.
18 F-fluorodeoxyglucose positron emission tomography (FDG-PET) is a useful diagnostic technique for detecting local tumor recurrences, distant metastases, and recurrence in colon cancer, and for assessing treatment responses [7–15]. In recent years, positron emission tomography/computed tomography (PET/CT) has been used to accurately localize malignant lesions [16]. However, few studies have reported the efficacy of PET/CT in evaluating small tumor implants or lymph nodes in the abdominopelvic cavity in colorectal cancer patients. Thus, we compared the performance of FDG PET/CT and contrast-enhanced CT (CeCT) in detecting abdominopelvic cavity recurrences according to tumor implant and lymph node size.
Materials and Methods
Patients
We enrolled 16 patients who were treated for colorectal cancer and clinically suspected for recurrences from March 2009 to October 2009 (6 males and 10 females; mean age, 61 ± 14 years). Of them, four patients had elevated CEA levels, two had clinical symptoms (e.g., dyspepsia, abdominal pain, bowel habit change), and ten were suspected of developing abdominopelvic cavity lesions outside of the specific organ during a routine follow-up CT scan.
All subjects underwent CeCT and PET/CT to determine whether they had a recurrence; the two scans were performed 11 days apart (11 ± 11 days, range 0–36 days). All 16 patients were diagnosed clinically as developing a recurrence. Five patients were confirmed histologically; seven patients were clinically diagnosed and underwent chemotherapy immediately without histological confirmation; and four patients underwent supportive care because of their poor general health.
Of the seven patients who underwent chemotherapy without histological confirmation, five showed a partial response (PR), and two had stable disease (SD) on a follow-up CT scan based on an evaluation of response to chemotherapy. Four patients who underwent supportive care were monitored by short-term follow-up CT, all of whom experienced a progression of the lesions. Although only 5 patients were confirmed histologically, we diagnosed all 16 patients clinically as experiencing a recurrence.
Contrast-Enhanced CT Scan
CT imaging studies were performed using a 64-MDCT scanner (Brilliance; Philips Medical Systems) after 2 ml/kg injections of nonionic contrast material (iopromide, Ultravist 370; Schering, Germany) were administered at 3 ml/s. The scan was started 60 s after the aortic enhancement reached a 200-HU threshold using a bolus-tracking software. Patients were imaged in the supine position cephalocaudally, beginning from the diaphragm to the symphysis pubis; the scan was performed with a 120-kVp potential, 0.625-mm collimation, and 0.42-s gantry rotation time. To tailor the tube current to each patient, automatic modulation was performed to minimize individual variations in image noise (Dose-Right, Philips Medical Systems). From the original raw projection data, thick-section images were reconstructed with 5 mm thickness at 4-mm intervals.
PET/CT Scan
Integrated FDG PET/CT scans were performed on Discovery BGO (General Electric Medical Systems, Milwaukee, WI). Patients were asked to fast for 6 h before the scan and had their serum glucose levels measured. No patient had a glucose level that exceeded 120 mg/dl. We also recorded the patient’s weight, height, and body mass index (BMI) to calculate the standardized uptake value (SUV). Then, a radiolabeled tracer (FDG) was given intravenously at a dose of 5.18 MBq/kg (0.14 mCi/kg).
All patients took oral mebeverine 135 mg to relax the intestinal smooth muscles and to minimize image artifacts caused by bowel movements, and received a 10-mg injection of furosemide to minimize urinary retention after intravenous FDG administration. Forty minutes after the injection, noncontrast CT scanning and PET scanning were performed sequentially. The scan encompassed the skull base to the proximal thigh.
CT-based attenuation correction was applied. Then we analyzed the data with the AW 4.4 (General Electric Medical Systems) software. We selected a region of interest for each lesion and measured the maximum SUV (SUVmax), corrected for lean body mass (LBM): [tissue concentration (MBq/mL)]/[injected dose (MBq)/LBM (kg)].
Image Analysis
Two experienced radiologists read the CeCT scans, and two experienced nuclear medicine physicians evaluated the PET/CT images. On the CeCT images, seeding nodules in the abdominopelvic cavity were defined as nodular or sheet- or plaque-like implants regardless of location.
On the PET/CT images, the reviewers analyzed PET, noncontrast CT, and fusion images. Findings that were suggestive of seeding nodules in the abdominopelvic cavity were defined as suspected findings on the PET image. We defined suspected findings on the PET images as follows: focal metabolic abnormalities that show higher FDG uptake than adjacent normal structures without regard to size should have matching lesions on the concomitant noncontrast CT, and all suspected lesions should be located in the abdominopelvic cavity without regard to solid viscera. Detected lesions were confirmed as tumor implants by clinical follow-up, image analysis, or, if available, pathological examination. We measured the size and SUVmax of the seeding nodules to perform a quantitative analysis. Nodule size was measured using the long and short axes from the largest two-dimensional transverse cross-section.
We also analyzed the size and SUVmax of the detected metastatic lymph nodes in the abdominopelvic cavity. We measured the shortest dimension of the lymph nodes by implementing commonly used criteria for determining lymph node size [17]. Similarly, detected lesions were confirmed as metastases by clinical follow-up, image analysis, or, if available, pathological examination.
Statistics
Statistical analysis was performed using SPSS 12.0. Descriptive analyses were used for all quantitative variables. We counted all lesions that were detected by CeCT or PET/CT, and analyzed the data on a per-lesion basis. As lesion parameters, we measured the size and SUVmax of all lesions that were detected by CeCT or PET/CT. The results for the CeCT and PET/CT groups were compared by the Mann-Whitney U-test with 95% CIs.
Results
CeCT detected 38 lesions in 12 patients, all of which were identified by PET/CT. The lesions comprised 24 seeding nodules (8 in the pelvic cavity, 2 in the left paracolic gutter, 1 in the parietal peritoneum of the right upper quadrant of the abdomen, and 13 in the mesentery) and 14 lymph nodes (5 paraaortic, 1 perigastric, 1 celiac, 5 external iliac, and 2 internal iliac). PET/CT found 27 additional lesions in 8 patients that were not detected by CeCT-9 seeding nodules (1 in the subdiaphragmatic area of the right upper quadrant of the abdomen, 1 in the parietal peritoneum of the right upper quadrant of the abdomen, and 7 in the pelvic cavity) and 18 lymph nodes (2 celiac artery, 2 paraaortic, 2 hepatic hilar, 11 common iliac, and 1 external iliac). Representative images are shown in Figs. 1 and 2. All of these lesions are listed in Table 1.
Fig. 1.
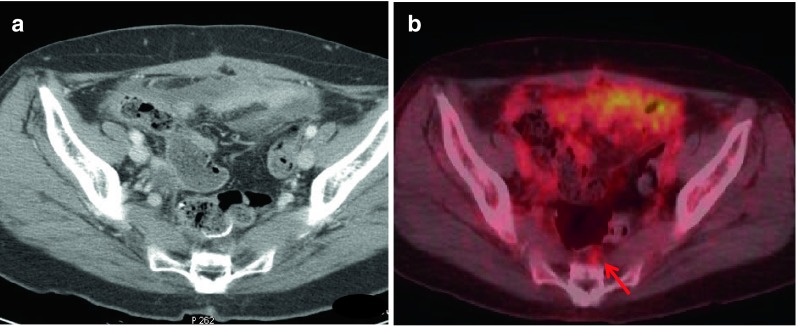
By CeCT (a), abnormal fluid collection was observed in the pelvic cavity, and no definite lesion was detected. However, PET/CT (b) showed an additional small presacral nodule with FDG uptake (red arrow, SUVmax: 1.9, size: 0.6 cm) (patient no. 16)
Fig. 2.
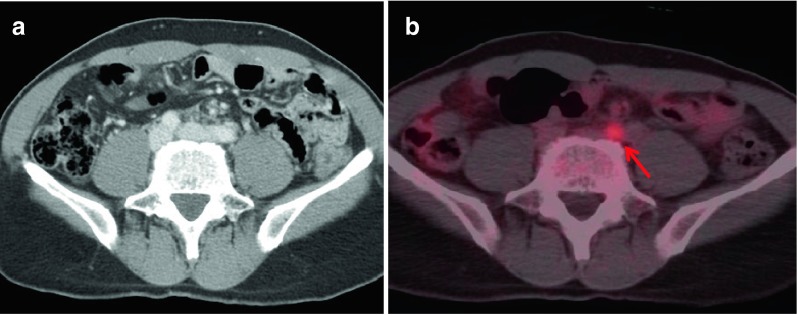
By CeCT (a), there was no definite abnormal finding. However, the PET/CT image (b) showed an additional hypermetabolic lymph node in the left common iliac area (red arrow, SUVmax: 2.2, size: 0.7 cm) (patient no. 6)
Table 1.
List of lesions detected on CeCTa and PET/CT
| Patient no. | Seeding nodules | Lymph nodes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Both CeCT and PET/CT found | PET/CT additionally found | Both CeCT and PET/CT found | PET/CT additionally found | |||||||||
| Site (no.) | SUVmax | Sizeb (cm) | Site (no.) | SUVmaax | Sizeb (cm) | Site (no.) | SUVmaax | Size (cm) | Site (no.) | SUVmaax | Size (cm) | |
| 1 | No | No | - | Subdiaphragmatic | 2.0 | 1.2 | Paraaortic (1) | 2.0 | 1.2 | Paraaortic | 1.9 | 0.7 |
| 1.7 | 0.7 | |||||||||||
| (1) | (2) | |||||||||||
| 2 | No | No | - | No | - | - | Perigastic (1) | 3.4 | 1.0 | No | - | - |
| - | - | |||||||||||
| 3 | Paracolic gutter (2) | 2.0 (PG) | 1.6 | No | - | - | - | No | - | No | - | - |
| Pelvic cavity (1) | 1.1 (PG) | 1.4 | ||||||||||
| 3.1 (PC) | 2.6 | |||||||||||
| 4 | Pelvic cavity (1) | 6.2 | 2.2 | No | - | - | No | - | No | - | - | |
| 5 | No | - | - | No | - | - | Celiac artery (1) | 1.3 | 1.2 | No | - | - |
| 6 | No | - | - | No | - | - | No | - | - | Common | 4.0 | 0.8 |
| iliac (3) | 2.4 | 0.6 | ||||||||||
| 2.2 | 0.7 | |||||||||||
| 7 | Parietal peritoneum (1) | 5.8 | 1.9 | No | - | - | No | - | - | No | - | - |
| - | - | - | - | - | - | - | - | |||||
| 8 | No | - | - | Parietal | 10.0 (PP) | 1.1 | No | - | - | Hepatic | 6.6 | 0.9 |
| peritoneum (1) | 14.1 (PC) | 1.0 | hilar (2) | 5.1 | 0.6 | |||||||
| Pelvic cavity (1) | Celiac | 1.1 (CA) | 0.6 | |||||||||
| artery (2) | 1.2 (CA) | 0.5 | ||||||||||
| 9 | Mesentery | 2.3 | 1.5 | No | - | - | No | - | - | No | - | - |
| (5) | 2.4 | 1.6 | ||||||||||
| 2.9 | 1.5 | |||||||||||
| 2.4 | 1.8 | |||||||||||
| 2.6 | 1.6 | |||||||||||
| 10 | No | - | - | Pelvic cavity (1) | 6.8 | 1.0 | No | - | - | No | - | - |
| 11 | No | - | - | No | - | - | No | - | - | Common | 3.3 (CI) | 0.6 |
| iliac (2) | 4.5 (CI) | 0.7 | ||||||||||
| Internal | 4.4 (II) | 1.0 | ||||||||||
| iliac (1) | ||||||||||||
| 12 | Mesentery | 2.4 (M) | 3.4 | No | - | - | No | - | - | No | - | - |
| (3) | 1.0 (M) | 1.0 | ||||||||||
| Pelvic | 1.1 (M) | 0.9 | ||||||||||
| cavity (3) | 1.0 (PC) | 0.9 | ||||||||||
| 1.2 (PC) | 1.6 | |||||||||||
| 1.4 (PC) | 1.1 | |||||||||||
| 13 | Pelvic | 11.4 | 5.4 | Pelvic cavity (2) | 3.6 | 1.5 | External iliac | 4.1 (EI) | 1.7 | Common | 4.1 | 0.7 |
| cavity (1) | 2.3 | 0.8 | (3) | 4.8 (EI) | 4.5 | iliac (6) | 4.0 | 0.8 | ||||
| Internal iliac | 3.0 (EI) | 4.9 | 4.0 | 0.7 | ||||||||
| (2) | 3.4 (II) | 3.3 | 4.2 | 0.7 | ||||||||
| 2.7 (II) | 1.9 | 4.0 | 0.6 | |||||||||
| 3.9 | 0.8 | |||||||||||
| 14 | Pelvic cavity (1) | 5.3 | 4.1 | Pelvic cavity (2) | 3.8 | 1.5 | Paraaortic (4) | 6.4 (PA) | 2.2 | No | ||
| 5.2 | 0.9 | External iliac | 5.8 (PA) | 2.1 | ||||||||
| (2) | 6.1 (PA) | 2.3 | ||||||||||
| 5.2 (PA) | 1.5 | |||||||||||
| 6.5 (EI) | 2.5 | |||||||||||
| 4.6 (EI) | 1.5 | |||||||||||
| 15 | Pelvic cavity (1) | 5.8 | 4.0 | No | - | - | No | - | - | No | ||
| 16 | Mesentery (5) | 1.4 | 1.2 | Pelvic cavity (1) | 1.9 | 0.7 | No | - | - | No | ||
| 1.8 | 1.3 | |||||||||||
| 1.2 | 1.4 | |||||||||||
| 1.5 | 1.8 | |||||||||||
| 1.1 | 2.0 | |||||||||||
| Total | 24 | 9 | 14 | 18 | ||||||||
a: Contrast-enhanced CT
b: Size of long axis
CA: Celiac artery, CI: common iliac, EI: external iliac, II: internal iliac, M: mesentery, PA: paraaortic, PC: pelvic cavity, PG: paracolic gutter, PP: parietal peritoneum
The SUVmax of the additional seeding nodules that were detected by PET/CT was significantly higher than that of nodules that were identified by both CeCT and PET/CT (5.5 ± 4.2 vs. 2.9 ± 2.5, p = 0.03). We analyzed the size of the seeding nodules by short axis, long axis, and the mean of both values. All three measurements in PET/CT-only-detected nodules differed significantly from those of the CeCT- and PET/CT-confirmed nodules (long axis: 1.0 ± 0.3 cm vs. 2.0 ± 1.1 cm, p = 0.001; short axis: 0.8 ± 0.3 cm vs. 1.4 ± 0.8 cm, p = 0.004; mean of both axes: 0.9 ± 0.3 cm vs. 1.7 ± 0.9 cm, p = 0.001). Notably, the PET/CT-only-detected lesions were significantly smaller than CeCT- and PET/CT-confirmed lesions, regardless of the measurement method used.
Also, PET/CT-only-detected lymph nodes were significantly smaller than CeCT- and PET/CT-confirmed lymph nodes (0.7 ± 0.1 cm vs. 2.3 ± 1.2 cm, p < 0.0001). However, with regard to SUVmax, there was no statistically significant difference (3.5 ± 1.5 vs. 4.2 ± 1.7, p = 0.17).
Most of these additional lesions were located in pelvic cavities that had been operated on. Approximately 78% (7/9) of seeding nodules and 67% (12/18) of lymph nodes were located in the pelvic cavity.
Discussion
In our study, PET/CT was superior to CeCT in detecting seeding nodules and metastatic lymph nodes in the abdominopelvic cavity in recurrent colorectal cancer patients. Specifically, PET/CT identified more subcentimeter lesions than CeCT. All lesions that were detected by CeCT were detected by PET/CT as well; yet PET/CT found additional lesions, which were significantly smaller than CeCT-detected lesions. Moreoever, most of these additional lesions were located in pelvic cavities that had been previously operated on (78% of seeding nodules and 67% of lymph nodes).
Anatomical imaging methods, such as CT, are nonetheless important tools for detecting the recurrence of cancer. However, anatomical imaging relies primarily on size to discriminate malignant from benign lesions [18]. Metastasis to the lymph node is defined generally as a lesion that is larger than 1 cm [17]. Metastatic abdominal lymph nodes in colorectal cancer are frequently small (less than 1 cm in diameter). Thus, the sensitivity of CT for metastatic lymph nodes is not satisfactory in colorectal cancer [19, 20]. Jacquet et al. reported varying sensitivities of CT, depending on tumor size (90% for >5 cm and 28% for <0.5 cm) [21]. A similar study by De bree et al. also reported diverse sensitivities of CT according to lesion size (9.1% to 24.3% for tumors <1 cm, 59.3% to 66.7% for tumors >5 cm) [5]. Thus, size-dependent discrimination of lesions is not ideal.
The anatomical region also affects detection by CT, and according to several studies, sensitivities are lower in the pelvic cavity [6, 21]. Most recurrent colorectal cancer patients have been treated with surgery and/or radiotherapy and, therefore, usually have distorted pelvic anatomies. Scar formation after surgery and fibrotic changes after radiation therapy are frequent events. Thus, radiologists face challenges in discriminating malignant from benign lesions in the pelvic cavity in patients with recurrent colorectal cancer by CT imaging [2].
With the advent of PET/CT, it has become possible to use metabolic information as well as anatomical information. It is reasonable to believe that size-dependent discrimination will be aided by such data. Recent studies have determined that of PET/CT, CT alone, and PET alone, PET/CT is the most sensitive method for detecting tumor implants. Turlakow et al. studied 88 patients, and 23 were confirmed as having peritoneal seeding lesions. They reported sensitivities of 57% (13/23) for PET, 42% (10/23) for CT, and 78% (18/23) for their combination in detecting peritoneal seeding lesions [22]. Similarly, we observed a significantly higher detection rate with PET/CT versus CeCT.
Increased glucose metabolism of lesions might facilitate the identification of lesions in anatomically distorted pelvic cavities [23]. Selzner et al. reported a sensitivity of 93% for PET/CT in detecting recurrences around the primary resection site compared with 53% for CeCT [6]. All of our patients that had undergone operations for colorectal cancer had various degrees of postoperative pelvic adhesion and anatomical deformation. Thus, PET/CT remains useful in detecting the recurrence of colorectal cancer, especially in anatomically deformed regions.
In our study, additional seeding nodules that were detected only by PET/CT showed significantly higher SUVmax values than lesions detected by both CeCT and PET/CT, despite the significantly smaller size. This is somewhat natural because FDG-avid lesions are more likely to be detected on PET/CT scans. For lesions large enough to overcome the partial volume effect, FDG-less-avid lesions can also be detected. On the contrary, small lesions with low SUV values may be missed. Therefore, FDG additionally found lesions had larger SUV values in our study.
Our results indicate that PET/CT has an additional value in detecting and distinguishing small tumor implants and metastatic lymph nodes, particularly in anatomically distorted pelvic cavities. However, our study has some limitations. The number of patients in our study was small. Although we obtained significant results, a more reliable study will require a larger sample size. Also, pathological confirmation was unavailable for most lesions because of the difficulties in the surgical approach and poor overall health of the enrollees. Thus, we could not determine the sensitivity, specificity, or accuracy of PET/CT.
Conclusion
PET/CT is superior to CeCT in detecting tumor implants and metastatic lymph nodes in the abdominopelvic cavity in patients with recurrent colorectal cancer. PET/CT was particularly successful in detecting subcentimeter lesions in anatomically deformed pelvic cavities compared to CeCT. Thus, we believe that PET/CT is an effective supplementary method for detecting abdominopelvic cavity recurrences in patients with colorectal cancer.
Acknowledgement
This research was supported by grant no. 11-2010-022 from the SNUBH Research Fund.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Kelvin FM, Korobkin M, Heaston DK, Grant JP, Akwari O. The pelvis after surgery for rectal carcinoma: serial CT observations with emphasis on nonneoplastic features. AJR Am J Roentgenol. 1983;141:959–64. doi: 10.2214/ajr.141.5.959. [DOI] [PubMed] [Google Scholar]
- 2.Even-Sapir E, Parag Y, Lerman H, Gutman M, Levine C, Rabau M, et al. Detection of recurrence in patients with rectal cancer: PET/CT after abdominoperineal or anterior resection. Radiology. 2004;232:815–22. doi: 10.1148/radiol.2323031065. [DOI] [PubMed] [Google Scholar]
- 3.Brodsky JT, Cohen AM. Peritoneal seeding following potentially curative resection of colonic carcinoma: implications for adjuvant therapy. Dis Colon Rectum. 1991;34:723–7. doi: 10.1007/BF02050360. [DOI] [PubMed] [Google Scholar]
- 4.Pfannenberg C, Konigsrainer I, Aschoff P, Oksuz M, Zieker D, Beckert S, et al. 18 F-FDG-PET/CT to select patients with peritoneal carcinomatosis for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2009;16:1295–303. doi: 10.1245/s10434-009-0387-7. [DOI] [PubMed] [Google Scholar]
- 5.de Bree E, Koops W, Kroger R, van Ruth S, Witkamp A, Zoetmulder F. Peritoneal carcinomatosis from colorectal or appendiceal origin: correlation of preoperative CT with intraoperative findings and evaluation of interobserver agreement. J Surg Oncol. 2004;86:64–73. doi: 10.1002/jso.20049. [DOI] [PubMed] [Google Scholar]
- 6.Selzner M, Hany T, Wildbrett P, McCormack L, Kadry Z, Clavien P. Does the novel PET/CT imaging modality impact on the treatment of patients with metastatic colorectal cancer of the liver? Ann Surg. 2004;240:1027. doi: 10.1097/01.sla.0000146145.69835.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flanagan FL, Dehdashti F, Ogunbiyi OA, Kodner IJ, Siegel BA. Utility of FDG-PET for investigating unexplained plasma CEA elevation in patients with colorectal cancer. Ann Surg. 1998;227:319–23. doi: 10.1097/00000658-199803000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delbeke D, Vitola JV, Sandler MP, Arildsen RC, Powers TA, Wright JK, Jr, et al. Staging recurrent metastatic colorectal carcinoma with PET. J Nucl Med. 1997;38:1196–201. [PubMed] [Google Scholar]
- 9.Langenhoff BS, Oyen WJ, Jager GJ, Strijk SP, Wobbes T, Corstens FH, et al. Efficacy of fluorine-18-deoxyglucose positron emission tomography in detecting tumor recurrence after local ablative therapy for liver metastases: a prospective study. J Clin Oncol. 2002;20:4453–8. doi: 10.1200/JCO.2002.12.134. [DOI] [PubMed] [Google Scholar]
- 10.Ruers TJ, Langenhoff BS, Neeleman N, Jager GJ, Strijk S, Wobbes T, et al. Value of positron emission tomography with [F-18]fluorodeoxyglucose in patients with colorectal liver metastases: a prospective study. J Clin Oncol. 2002;20:388–95. doi: 10.1200/JCO.20.2.388. [DOI] [PubMed] [Google Scholar]
- 11.Pijl ME, Chaoui AS, Wahl RL, van Oostayen JA. Radiology of colorectal cancer. Eur J Cancer. 2002;38:887–98. doi: 10.1016/S0959-8049(02)00052-7. [DOI] [PubMed] [Google Scholar]
- 12.Lonneux M, Reffad AM, Detry R, Kartheuser A, Gigot JF, Pauwels S. FDG-PET improves the staging and selection of patients with recurrent colorectal cancer. Eur J Nucl Med Mol Imaging. 2002;29:915–21. doi: 10.1007/s00259-002-0802-9. [DOI] [PubMed] [Google Scholar]
- 13.Victor K, Rodney JH, Robert EW, Annette H, et al. The clinical impact of (18)F-FDG PET in patients with suspected or confirmed recurrence of colorectal cancer: a prospective study. J Nucl Med. 2002;43:492. [PubMed] [Google Scholar]
- 14.Valk PE, Abella-Columna E, Haseman MK, Pounds TR, Tesar RD, Myers RW, et al. Whole-body PET imaging with [18 F]fluorodeoxyglucose in management of recurrent colorectal cancer. Arch Surg. 1999;134:503–11. doi: 10.1001/archsurg.134.5.503. [DOI] [PubMed] [Google Scholar]
- 15.Park EK, Kang WJ, Eo JS, Lee DS, Chung J-K, Lee MC. Diagnostic accuracy of PET and MR for detecting liver metastasis from colorectal cancer. Nucl Med Mol Imaging. 2006;40:249–56. [Google Scholar]
- 16.Kang S, Song BI, Lee HJ, Seo JH, Lee SW, Yoo J, et al. Diagnostic role of F-18 FDG PET/CT in the follow-up of patients with colorectal cancer: comparison with serum CEA, CA 19–9 levels and computed tomography. Nucl Med Mol Imaging. 2009;43:120–8. [Google Scholar]
- 17.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka T, Kawai Y, Kanai M, Taki Y, Nakamoto Y, Takabayashi A. Usefulness of FDG-positron emission tomography in diagnosing peritoneal recurrence of colorectal cancer. Am J Surg. 2002;184:433–6. doi: 10.1016/S0002-9610(02)01004-8. [DOI] [PubMed] [Google Scholar]
- 19.Vogel WV, Wiering B, Corstens FH, Ruers TJ, Oyen WJ (2005) Colorectal cancer: the role of PET/CT in recurrence. Cancer Imaging 5 Spec No A:S143-149 [DOI] [PMC free article] [PubMed]
- 20.Monig SP, Baldus SE, Zirbes TK, Schroder W, Lindemann DG, Dienes HP, et al. Lymph node size and metastatic infiltration in colon cancer. Ann Surg Oncol. 1999;6:579–81. doi: 10.1007/s10434-999-0579-1. [DOI] [PubMed] [Google Scholar]
- 21.Jacquet P, Jelinek JS, Steves MA, Sugarbaker PH. Evaluation of computed tomography in patients with peritoneal carcinomatosis. Cancer. 1993;72:1631–6. doi: 10.1002/1097-0142(19930901)72:5<1631::AID-CNCR2820720523>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 22.Turlakow A, Yeung HW, Salmon AS, Macapinlac HA, Larson SM. Peritoneal carcinomatosis: role of (18)F-FDG PET. J Nucl Med. 2003;44:1407–12. [PubMed] [Google Scholar]
- 23.Vikram R, Iyer RB (2008) PET/CT imaging in the diagnosis, staging, and follow-up of colorectal cancer. Cancer Imaging 8 Spec No A:S46-51 [DOI] [PMC free article] [PubMed]


