Abstract
Purpose
L-type amino acid transporter 1 (LAT1) is essential for the transport of large neutral amino acids. However, its role in breast cancer growth remains largely unknown. The purpose of the study is to investigate whether LAT1 is a potential biomarker for the diagnosis and treatment of breast cancer.
Methods
LAT1 mRNA and protein levels in breast cancer cell lines and tissues were analyzed. In addition, the effects of targeting LAT1 for the inhibition of breast cancer cell tumorigenesis were assessed with soft agar assay. The imaging of xenograft with anti-1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid (anti-[18F]FACBC) PET was assessed for its diagnostic biomarker potential.
Results
Normal breast tissue or low malignant cell lines expressed low levels of LAT1 mRNA and protein, while highly malignant cancer cell lines and high-grade breast cancer tissue expressed high levels of LAT1. In addition, higher expression levels of LAT1 in breast cancer tissues were consistent with advanced-stage breast cancer. Furthermore, the blockade of LAT1 with its inhibitor, 2-amino-bicyclo[2.2.1]heptane-2-carboxylic acid (BCH), or the knockdown of LAT1 with siRNA, inhibited proliferation and tumorigenesis of breast cancer cells. A leucine analog, anti-[18F]FACBC, has been demonstrated to be an excellent PET tracer for the non-invasive imaging of malignant breast cancer using an orthotopic animal model.
Conclusions
The overexpression of LAT1 is required for the progression of breast cancer. LAT1 represents a potential biomarker for therapy and diagnosis of breast cancer. Anti-[18F]FACBC that correlates with LAT1 function is a potential PET tracer for malignant breast tumor imaging.
Keywords: LAT1, PET, Breast cancer
Introduction
Breast cancer is the most common malignancy and a leading cause of cancer mortality in women in the USA [1]. Worldwide, more than 1 million women are diagnosed with breast cancer every year, and approximately 14% of deaths due to cancer in women are attributed to this disease [2, 3]. Moreover, breast cancer incidence rates have been reported to be increasing by up to 5% each year in many populations in developing countries [2]. Similarly, over the past decade, China's and India’s urban cancer registries have documented increased incidence rates for breast cancer [4, 5]. These data clearly show that breast cancer, once called ‘a disease of the western world,’ has become more pervasive throughout the world. Though many molecules have been implicated in breast cancer growth and progression, the detailed mechanisms that maintain the malignant growth and progression of breast cancer cells are still not completely understood. Therefore, novel, powerful biomarkers need to be explored for insights into breast cancer diagnosis and therapy.
Amino acid transporters mediate amino acid transport across the plasma membrane. L-type amino acid transporter 1 (LAT1) is a neutral amino acid transporter and is essential for the transport of large neutral amino acids [6, 7]. It has been shown that LAT1 is overexpressed in some malignant tumor cells such as KB human oral epidermoid carcinoma cells [8], esophageal carcinoma [9], Barrett's adenocarcinoma [10], astrocytic tumors [11, 12], lung adenocarcinoma [13], oral cancer [14] and osteogenic sarcoma cells [15]. Amino acids are essential for protein synthesis, which is required for critical cell growth and proliferation [16]. Actively proliferating cancer cells require more nutrients, such as amino acids and glucose, than quiescent cells. With regard to the relationship between LAT1 and breast cancer, there have been only four publications so far reporting the role of LAT1 in two cultured breast cancer cell lines, from Shennan and his colleagues [17–20].
We have previously developed a new PET tracer, 1-amino-3-fluorocyclobutane-1-carboxylic acid (FACBC), labeled with fluorine-18 for nuclear medicine imaging. Anti-1-amino-3-[18F]-fluorocyclobutane-1-carboxylic acid (anti-[18F]FACBC) is a synthetic L-leucine analog that exhibited excellent uptake within prostate carcinoma cells and brain tumor cells [21, 22]. L-type large-neutral amino acid transport system (LAT) likely mediated the uptake of anti-[18F]FACBC [23]. Anti-[18F]FACBC has little renal excretion compared with [18F]FDG [21].
In the present study, in an effort to identify the role of LAT1 in breast cancer growth and progression and explore the mechanism of new anti-[18F]FACBC tracer action, we examined LAT1 expression in breast cancer cell lines and tissues. Furthermore, we tested whether anti-[18F]FACBC-PET, a sensitive imaging modality to detect LAT1 function, can detect tumors in an orthotopic breast cancer animal model.
Materials and Methods
Cell Lines and Cell Culture
In this study, we selected three types of cell lines, highly malignant (MDA-MB-231, MDA-MB-361, MDA-MB-435 and SKBR3), low malignant (HCC1395, and HCC1143) and non-tumorigenic (A1N4, MCF-10A). Highly malignant cell lines were derived from stage 3 or stage 4 cancers, indicating that the tumors had metastasized to local lymph nodes as well as other body organs (lung, liver, bone, etc.). Low malignant cell lines were derived from stage 1 or stage 2 cancers, indicating that the tumors were more localized and were quantitatively smaller. MDA-MB-231, MDA-MB-361, MDA-MB-435, HCC1395 and HCC1143 [24, 25] were cultured in 5% CO2 at 37°C in RPMI 1600 (Sigma, St. Louis, MO), supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 50 units/ml penicillin and 50 μg/ml streptomycin (Invitrogen, Carlsbad, CA). SKBR3 grew in DMEM (Sigma, St. Louis, MO), A1N4 in improved minimal essential medium (IMEM)[26] and MCF-10A in DMEM/F12 (Lonza, Walkersville, MD) [27, 28].
RNA Isolation and Quantitative RT-PCR Analysis
Total RNA was extracted from tumor cells by using Trizol Reagent (Invitrogen) following the previous procedure [24, 29]. The procedures for RT-PCR and the sequence of β-actin primers were described previously [24, 29]. The primers for LAT1 are 5'-AGGAGCCTTCCTTTCTCCTG and 5'- CTGCAAACCCTAAGGCAGAG (GenBank accession no. AB018009). Five hundred nanograms of total RNA was transcribed into cDNA in a 20-μl reaction volume at 42°C for 45 min. The cycle conditions for cDNA PCR were 95°C for 10 min followed by 35 cycles of 95°C for 30 s, 54°C for 20 s and 72°C for 30 s. For SYBR Green quantitative PCR amplifications, reactions were performed in a 20-μl reaction volume containing 10 μl of 2× SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). The fold-difference for LAT1 expression level was calculated. Each assay condition was performed in triplicate; the experiment was repeated once.
Western Blotting Analyses
Twenty-five micrograms of protein was resolved in an SDS/PAGE gel and subjected to immunoblot analysis using a monoclonal antibody against LAT1 (1:1,000) (TransGenic Inc, Kumamoto, Japan) or β-actin (1:5,000) (Sigma-Aldrich, St. Louis, MO). Detection by enzyme-linked chemiluminescence was performed according to the manufacturer’s protocol (ECL; Pierce Biotechnology Inc., Rockford, IL). Quantification of protein bands was performed using ImageJ software (NIH, Bethesda, MD).
Tissue Samples
A human breast cancer tissue array was purchased (Imgenex, San Diego, CA), which contained 40 primary breast cancer and 10 normal breast tissue samples adjacent to the tumor sites. The source and characteristics of the tissue samples are summarized in Table 1.
Table 1.
Source and characteristics of tissue specimens
| Total | T stage | Sex | Age | ||||
|---|---|---|---|---|---|---|---|
| T1 and T2 | T3 and T4 | Male | Female | <50 | ≥50 | ||
| Tumor tissues | 40 | 19 | 21 | 2 | 38 | 24 | 16 |
| Normal tissues | 10 | - | - | 0 | 10 | 9 | 1 |
| Total | 50 | 19 | 21 | 2 | 48 | 33 | 17 |
Immunohistochemical Staining
For immunohistochemical staining of formalin-fixed and paraffin-embedded tissue sections for LAT1, a monoclonal antibody was used at 1:500 against LAT1 (TransGenic). The immunohistochemical staining procedure was described previously [30]. The staining intensity with brown color was scored semi-quantitatively as follows: 4+, very strong; 3+, strong; 2+, medium; 1+, weak. Additionally, another immunostaining score was calculated by multiplication of the percentage of positive tumor cells (0–100) by the staining intensity (grade 1–4), producing a total range of 0–400 [31]. Staining intensity ≥100 was considered LAT1 positive.
LAT1 Inhibition and Proliferation of Cells
MDA-MB-231 cells were seeded in 96-well plates. After the addition of 10 mM of LAT1 inhibitor, 2-amino-bicyclo[2.2.1]heptane-2-carboxylic acid (BCH) (Sigma-Aldrich, St. Louis, MO), cell proliferation was measured at 24 h and 48 h by the CellTiter 96 AQ (Promega, Madison, WI) [24].
Transfection of LAT1 siRNA and Proliferation Assay
The target sequence of small interfering RNA (siRNA) against LAT1 is 5'-AAGAAUUUCGUCACAGAGGAA-3') (Genbank accession no. AF104032) and these duplexes were purchased from Dharmacon (Lafayette, CO). The nonspecific control siRNA duplexes were used as controls from Dhamacon with the same GC content as LAT1 siRNAs (42%). The siRNAs were transfected into MDA-MB-231 cells at a final concentration of 120 nmol/l using LipofectAMINE 2000 (Invitrogen). The transfected cells were collected to measure the mRNA levels of LAT1 at 24 h post-transfection. In addition, cell proliferation was measured at 48 h post-transfection by the CellTiter 96 AQ (Promega) following a previous procedure [24]. The experiments were repeated once more.
Soft Agar Colony Formation Assay
Colony formation in soft agar was evaluated in 0.35% agar and 10% FBS in RPMI1640 medium overlaying a 0.6% agar layer. In total, there were four groups, consisting of one control group and three BCH-treated groups (0.1, 1 and 10 mM). Each well of the six-well plates containing 2500 MDA-MB-231 cells in agar was incubated at 37°C in 5% CO2 for 30 days and fed twice a week. Colonies were counted under the microscope at 30 days. Each assay treatment was performed in triplicate.
Cell Uptake Assays
The amino acid uptake assays were performed with eight cultured breast cancer cell lines as described previously [23]. In this study, approximately 2 × 106 cells were exposed to 5 μCi of anti-1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid (anti-[18F]FACBC) in amino acid free HBSS and incubated at 37°C for 60 min. Each assay condition was performed in triplicate. After incubation, cells were centrifuged twice and rinsed with ice-cold amino-acid/serum-free HBSS to remove residual activity in the supernatant. The activity in the tubes was counted in an automated γ-counter (AccuFLEXγ7001, Aloka, Co., Ltd., Japan). The data from these studies were normalized as percent uptake relative to standard per 2 × 106 cells.
Preparation of Anti-[18F]FACBC and microPET Imaging
The preparation of anti-[18F]FACBC has been previously reported [32]. One hour after tail vein injections of 150 μCi [18F]FACBC in a volume of 150 μl, the animals were anesthetized and secured on an extended scanning bed before being moved into the interior of the Inveon Preclinical CT scanner (Siemens Medical Solutions Inc, Malvern, PA). The purpose of using the microCT is to acquire a CT-based attenuation scan for later re-construction of the microPET scan data. The mouse microCT images were acquired at settings so that the number of bed positions and overlap are calculated to match the axial microPET FOV of 12.7 cm. After microCT imaging, the scanning bed is moved forward into the microPET. Mouse microPET images were acquired on an Inveon Preclinical PET/CT scanner (Siemens Medical Solutions Inc.) that has a 12-cm transaxial and 12.7-cm axial field of view. A total of 30-min imaging was acquired, first using the Inveon Preclinical CT and then using the Inveon Preclinical PET with the long axis of the mouse parallel to the long axis of the scanner. Images of the two scans were aligned using a Transformation Matrix. Quality control scans were done before scanning any animals, and the microPET scanner was calibrated by analyzing a uniform phantom of activity. The resulting images were quantitatively calibrated and had a 2-mm isotropic resolution. Data acquisition and processing, including image reconstruction, image display and analyses, were performed with the ASIPro program provided by Siemens Medical Solutions Inc.
Statistical Analysis
All significance of the difference was determined with a two-sided Student’s t-test or χ-test. P values ≤ 0.05 are considered statistically significant.
Results
Overexpression of LAT1 in Highly Malignant Tumor Cell Lines
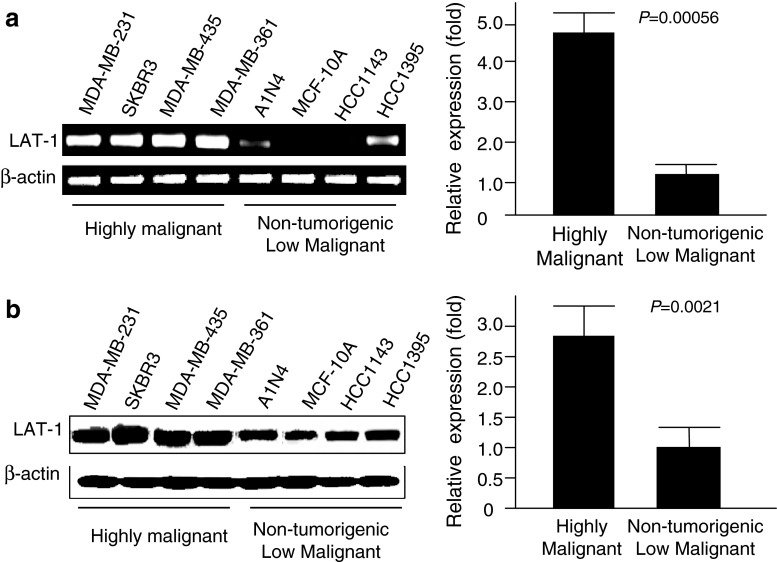
LAT1 has been shown to be highly expressed in human fetal liver, bone marrow, placenta and testis, but not in normal breast tissues [33]. To assess whether LAT1 is overexpressed in highly malignant breast cancer cell lines and further elucidate the role of LAT1 in breast cancer progression, we analyzed and compared expression levels of LAT1 mRNA and protein in four highly malignant and four low malignant and non-tumorigenic breast tumor cell lines. After qRT-PCR analysis, overexpression of LAT1 mRNA was observed in all four highly malignant breast cancer cell lines: MDA-MB-231, SKBR3, MDA-MB-435 and MDA-MB-361. Lower levels of LAT1 were expressed in all four non-tumorigenic and low malignant tumor cell lines: A1N4, MCF-10A, HCC1143 and HCC1395 (Fig. 1a). Expression levels of LAT1 mRNA in highly malignant cell lines were 4.8-fold higher than in low malignant cell lines (Fig. 1a) with qRT-PCR analysis. Similarly, Western blotting analysis (Fig. 1b) showed that highly malignant tumor cells expressed high levels of LAT1 protein, whereas non-tumorigenic and low malignant tumor cells expressed significantly lower levels of LAT1 protein. Expression levels of the LAT1 protein in highly malignant breast cancer cells were 2.8-fold higher than in non-tumorigenic and low malignant tumor cells (Fig. 1b).
Fig. 1.
Expression of LAT1 in breast cancer cell lines. (a) Expression of LAT1 mRNA. The left panel shows expression levels of LAT1 mRNA determined by RT-PCR in highly malignant breast cancer cell lines, MDA-MB-231, SK-BR-3, MDA-MB-435 and MDA-MB-361, and non-tumorigenic and low malignant breast cancer cell lines, A1N4, MCF-10A, HCC1143 and HCC1395. The right panel is a comparison of LAT1 mRNA levels in highly and low malignant breast cancer cell lines determined by quantitative RT-PCR analysis. β-Actin was used to normalize. (b) Expression of LAT1 protein. The left panel shows the protein expression of LAT1 in highly, and non-tumorigenic and low malignant breast cancer cell lines determined by Western blotting analysis. The right panel is a comparison of average LAT1 protein levels in highly and low malignant breast cancer cell lines with Western blotting analysis. Protein bands were measured quantitatively using the ImageJ software, and β-actin was used as a loading control
Overexpression of LAT1 in Malignant Tumor Tissues Compared to Normal Tissues
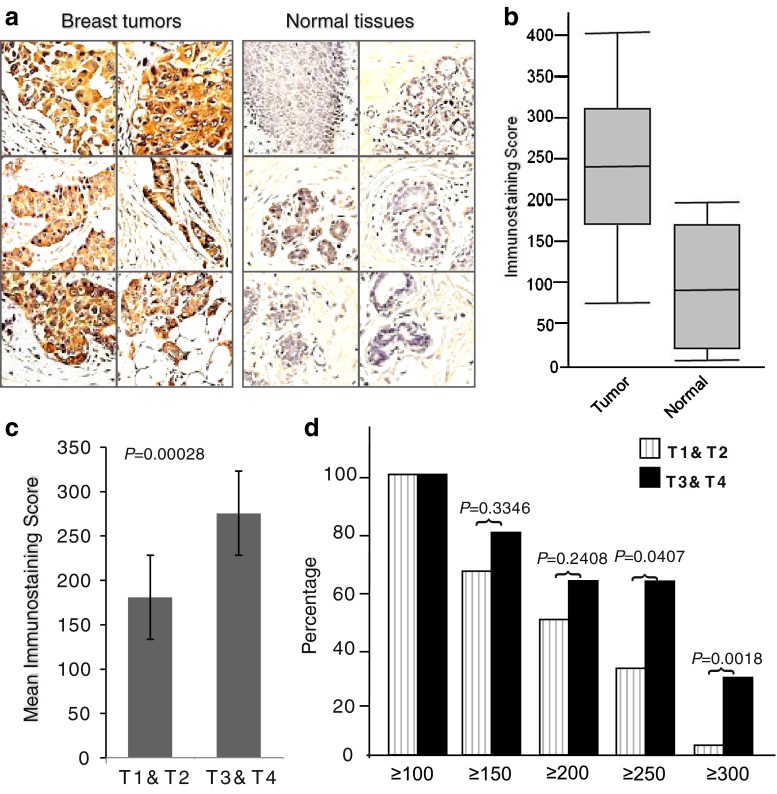
To examine whether LAT1 is overexpressed in malignant breast cancer tissues, we analyzed 40 primary and 10 normal breast tissue samples with immunohistochemistry. We identified the majority of breast cancer samples as overexpressing LAT1, whereas normal breast tissues expressed little to no LAT1 (Fig. 2a). Ninety-five percent of breast carcinoma samples were positive for immunostaining with LAT1 (≥100). Conversely, only three out of ten normal breast tissue samples from the adjacent area of the tumor demonstrated positive staining (Table 2). Although these tissues were LAT1-positive after staining, their intensities were generally lower than those in tumor tissues (all three ≤200). Furthermore, the average score (241) of staining intensity of the tumor samples with LAT1 was significantly greater than the score (90) of normal tissues (P = 0.000064) (Fig. 2b).
Fig. 2.
Overexpression of LAT1 in breast cancer tissues. (a) Representative micrographs of immunohistochemical staining of LAT1 protein on paraffin-embedded breast cancer and normal breast tissues. LAT1 protein is shown in brown color (DAB), whereas blue color represents nuclei counterstaining. (b) A box-and-whisker plot diagram showing the expression levels (immunostaining scores) of LAT1 from breast cancer and normal breast tissues by immunostaining. The scores were calculated by multiplying the percentage of positive tumor cells (1–100) by staining intensity (1–4), producing a score range of 0–400. Horizontal lines in the boxes represent the median value of LAT1 immunostaining scores of each group. Top and bottom edges of the boxes indicate the score values of the 75th and the 25th percentile, respectively. Whiskers represent the highest and lowest values. The range is shown as a vertical line. (c) Comparison of the expression levels (immunostaining score mean) of LAT1 between T1 and T2 and T3 and T4. (d) Correlation of LAT1 protein expression levels with tumor T stage. Stage 3 and stage 4 tumors expressed high levels of LAT1, whereas stage 1 and 2 tumors showed low levels of LAT1. This difference was more profound when ≥250 or ≥300 was used as a threshold
Table 2.
Sensitivity and specificity for tumor progression with LAT1
| Sensitivity | Specificity | |
|---|---|---|
| Tumor prediction (≥100) | 95% (38/40) | 70% (7/10) |
| Prediction of lymph node positive (≥150) | 92% (23/25) | 60% (9/15) |
Correlation of LAT1 Expression Levels to Progression of Breast Cancer
Further analysis showed that stage 3 or 4 breast tumors expressed higher levels (mean score: 276) of LAT1, whereas stage 1 or 2 breast tumors exhibited significantly lower levels (mean score: 181) of LAT1 (Fig. 2c). When ≥100, ≥150 or even ≥200 was used as the threshold, there was no observable difference between T1&2 and T3&4, whereas drastic differences were observed between these two groups with LAT1 staining when ≥250 or ≥300 was used as the threshold level (Fig. 2d). Twenty-nine percent of the stage 3 or 4 tumors exhibited ≥300 IHC score with LAT1, whereas only 4% of stage 1 or 2 tumors resulted in ≥300 staining grade (Fig. 2d). In addition, we found that 92% of the tumor samples from node-positive patients were ≥150, whereas only 60% of the tumor samples from node-negative patients were ≥150 for LAT1 staining (Table 2). These results demonstrate that LAT1 expression levels are correlated to breast cancer progression.
Blockade and Downregulation of LAT1 Inhibits the Proliferation and the Tumorigenesis of Breast Cancer Cells
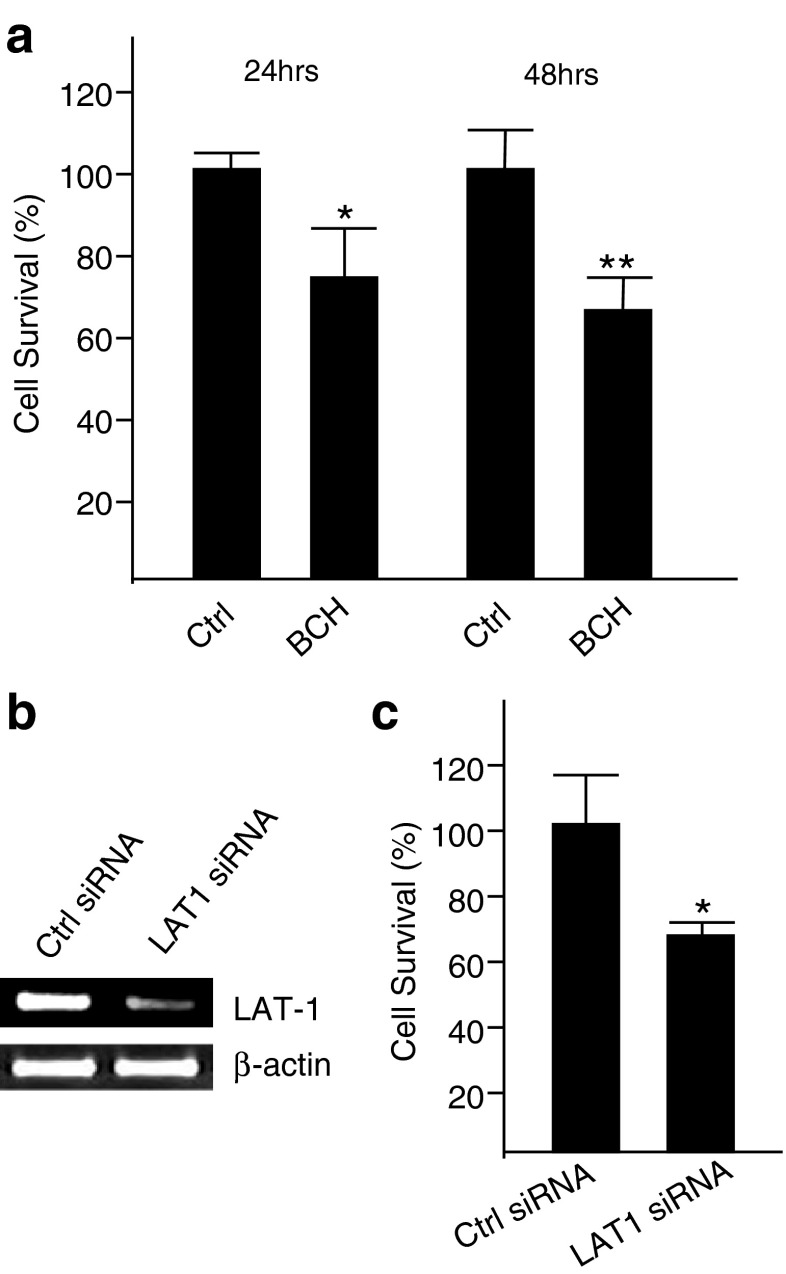
LAT1 is a major nutrient transporter responsible for the transport of large neutral essential amino acids. It has been shown that LAT1 is overexpressed in some tumor tissues [34, 35] and is actively involved in the proliferation of vascular smooth muscle cells [36]. In order to investigate whether or not LAT1 is involved in the proliferation of breast cancer cells, we employed BCH, a selective inhibitor of LAT1 [37, 38], to inhibit the proliferation of malignant MDA-MB-231 cells. In the presence of 10 mM BCH, the proliferation of MDA-MB-231 cells was significantly reduced in comparison with control cells grown in the absence of BCH at either 24 h or 48 h post-cell treatment (Fig. 3a). Moreover, LAT1 expression was downregulated efficiently with a LAT1 siRNA (Fig. 3b) to determine the effects of the knockdown of LAT1 on the proliferation of MDA-MB-231 cells. Figure 3c shows that the knockdown of LAT1 significantly reduced the proliferation of MDA-MB-231 cells at 72 h post-transfection.
Fig. 3.
Impact of blockade or knockdown of LAT1 on proliferation of MDA-MB-231 cells. (a) Impact of blockade of LAT1 with an inhibitor on proliferation of breast cancer cells. MDA-MB-231 cells were treated with/without 10 mM of a LAT1 inhibitor, BCH. Proliferation of these cells was measured at either 24 h or 48 h post-treatment. *P < 0.05, **P < 0.01. (b) Knockdown of LAT1 mRNA at 24 h post-siRNA transfection in MDA-MB-231 cells. β-Actin was used as a loading control. (c) Impact of LAT1 knockdown via siRNA on proliferation of MDA-MB-231 cells. Relative growth at 72 h post-transfection of LAT1 siRNA on MDA-MB-231 cells compared to the control. *P < 0.05
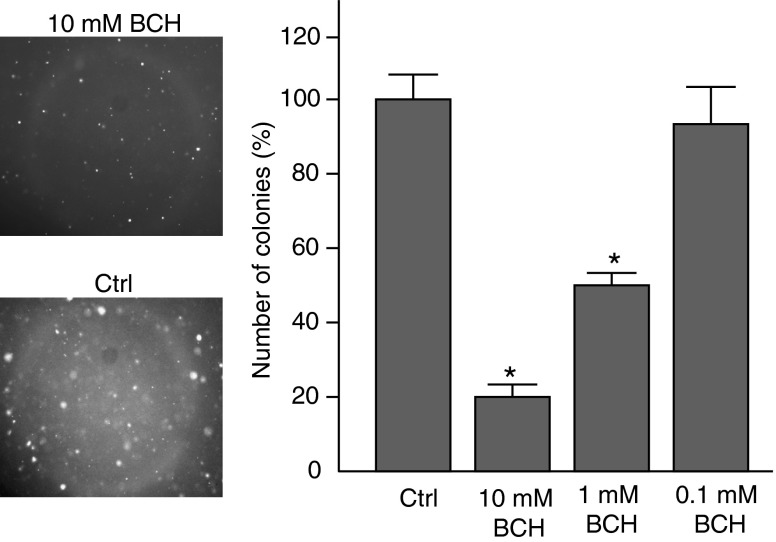
To determine whether the blockade of LAT1 affects the tumorigenesis of breast tumor cells, we performed a soft agar colony-formation assay. MDA-MB-231 cells were implanted in semisolid medium and treated with increasing concentrations of BCH. After 30 days, results showed that the blockade of LAT1 with BCH efficiently inhibited colony formation of MDA-MB-231 cells in a dose-dependent manner (Fig. 4).
Fig. 4.
Effect of LAT1 blockade on tumorigenesis of MDA-MB-231 cells determined by soft agar colony formation assay. MDA-MB-231 cells were treated with increasing concentrations of BCH (0.1 mM–10 mM) for 24 h and plated onto semisolid medium. The representatives of colonies from control and 10 mM BCH treatment groups are shown in the left panel. The colonies were quantified by light microscopy (right) after 1 month. Five fields were quantified and compared per condition (right). *Versus control P < 0.01
LAT1 Function Correlates with Breast Cancer Malignancy in Vitro
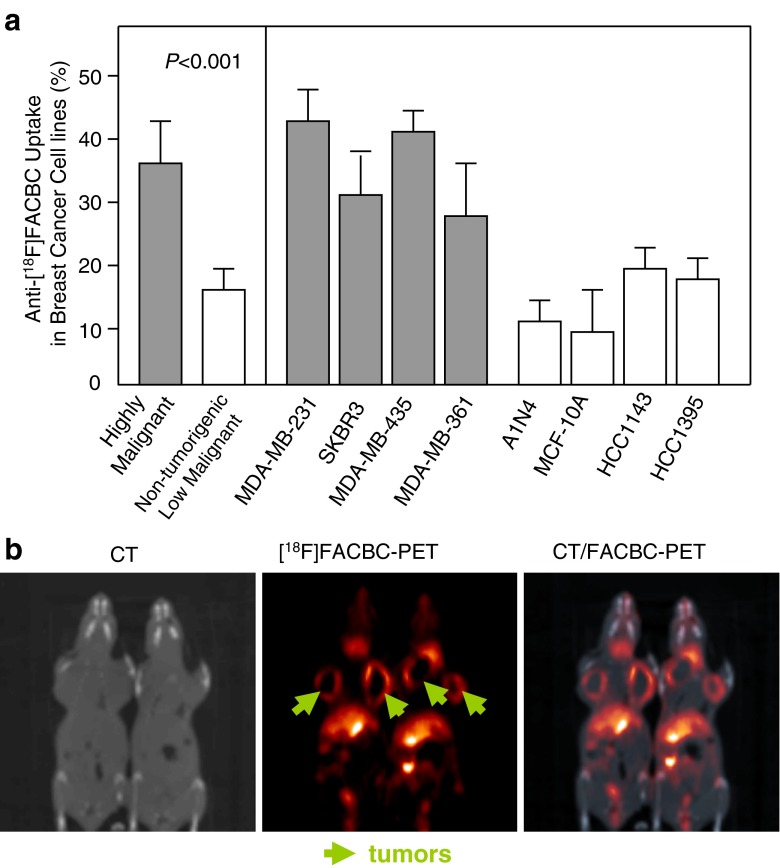
We performed in vitro uptake studies in all eight breast cancer cell lines described above to evaluate LAT1 function. Anti-[18F]FACBC revealed high levels of intracellular accumulation in all four highly malignant cell lines. In contrast, lower uptake levels were detected with anti-[18F]FACBC in all four non-tumorigenic and low malignant cell lines (Fig. 5a).
Fig. 5.
Uptake of anti-[18F]FACBC, a PET tracer and substrate of LAT1, in vitro and in vivo. (a) Uptake of anti-[18F]FACBC into highly, and non-tumorgenic and low malignant breast cancer cell lines. “Highly malignant” represents the average of anti-[18F]FACBC uptake in four highly malignant breast cancer cell lines. “Non-tumorigenic and low malignant” is the average of anti-[18F]FACBC uptake in four non-tumorigenic and low malignant breast cancer cell lines. (b) Representatives of anti-[18F]FACBC-PET images of a mouse orthotopic xenograft model of MDA-MB-231 after intravenous administration of 150 μCi [18F]FACBC (1 h after injection). CT imaging is used as a control
Efficient Detection of Breast Tumors by anti-[18F]FACBC-PET
We determined whether LAT1 overexpression could be a diagnostic marker for breast tumor detection by using non-invasive anti-[18F]FACBC-PET in an orthotopic breast cancer animal model. MDA-MB-231 breast cancer cells were injected into the mammary fat pads of the nude mice. When the average tumor size reached 700 mm3, animals were injected with anti-[18F]FACBC intravenously and scanned in microPET/CT at 1 h post-injection. CT scan showed anatomical information (Fig. 5b left), and anti-[18F]FACBC-PET images showed that tumors are bright (Fig. 5b middle), especially the rings. The corresponding PET/CT fused images are shown in Fig. 5b right. Our study provides the mechanism that allows anti-[18F]FACBC-PET, which correlates with LAT1 function, to be a potential non-invasive diagnostic tool for malignant breast tumor detection.
Discussion
The present study investigated the potential of LAT1 as a biomarker for immunohistochemistry and non-invasive PET imaging. Our study revealed, for the first time, that LAT1 is overexpressed in highly malignant breast cancer cell lines and tissues compared to low malignant breast cancer cell lines and tissues. Previous studies have shown that LAT1 is expressed at different levels in primary brain tumors vs. normal brain tissues [39], in human normal osteoblast cells vs. osteogenic sarcoma cells [15], in normal lung vs. adenocarcinoma of the lung [13] and in human normal oral keratinocytes vs. human oral cancer cells [14]. Furthermore, we demonstrated for the first time that breast cancer tissues overexpressed LAT1 compared to normal breast tissues. Our study found that higher staining scores were exhibited in highly malignant tumor (T3 and T4) tissues compared to those in low malignant tumor (T1 and T2) tissues. In addition, we have shown that the blockade or knockdown of LAT1 inhibited the proliferation and colony formation of breast cancer cells. Our findings, coupled with previous studies, suggest that overexpression of LAT1 may be required for the growth and the progression of breast cancer.
Our results suggest that LAT1 may be a potential biomarker for the diagnosis of breast cancer. [18F]FDG-PET has been widely accepted as a clinical imaging tool in the evaluation of patients with various carcinomas [40–42]. [18F]FDG-PET relies on increased glucose metabolism to determine the location of malignant lesions [43]. However, the major limitation of [18F]FDG-PET is that many active inflammatory diseases and some benign tumors have high [18F]FDG uptake [44–46]. Our previous studies have shown that anti-[18F]FACBC, a substrate of LAT1, has an excellent uptake rate within primary and metastatic prostate carcinoma, brain and renal tumors with little renal excretion compared to [18F]FDG [47–49]. In this study, we showed for the first time that uptake of anti-[18F]FACBC is correlated with breast tumor cell malignancy. Furthermore, anti-[18F]FACBC has an excellent uptake rate in a breast cancer xenograft. Therefore, our data suggest that using anti-[18F]FACBC-PET may be a potential strategy for non-invasively assessing LAT1 function, which correlates well with breast cancer malignancy.
LAT1 has great potential to be an efficient target for the treatment of metastatic breast cancer. Metastasis is the leading cause of breast cancer death. Traditional surgery and radiotherapy are not efficient in decreasing morbidity and mortality associated with metastatic breast cancer, whereas chemotherapy and targeted therapy are critical to treat this class of breast cancer. Currently, the HER2-targeting antibody trastuzumab and anti-estrogen drugs such as tamoxifen are the only molecularly targeted drugs for advanced breast cancer treatment. However, the utility of these treatments is limited. Approximately 25% of patients with breast cancer have tumors that overexpress HER2/neu[50]. Administration of trastuzumab as a single agent resulted in a response rate of only 21%. [51]. There is about a 55% estrogen receptor-positive ratio in breast cancer tumors [52], but normal and benign breast tissues possess a high positive ratio of estrogen receptors as well. In our study, we observed that 94% of breast tumor tissues are LAT1 positive. Previous investigations have shown that LAT1 is not detectable in normal breast tissues [17]. Our results demonstrated that the normal breast tissues adjacent to the tumor site expressed a low intensity of LAT1.
In conclusion, we demonstrated that LAT1 promotes the proliferation, tumorigenesis and progression of breast cancer cells. Taken together, these combined data suggest that LAT1 may be a potential biomarker for treatment and diagnosis of breast cancer, and in particular, for treatment of advanced and metastatic breast cancer.
Acknowledgements
This research was supported by grants from NIH R01 5R01CA121320 (M. Goodman) and P50CA128301 (H. Shim). We thank Mr. Vernon Camp for his assistance with [18F]FACBC cell uptake study. We thank Jessica Paulishen and Carol J. So for proof-reading. We acknowledge Dr. James Provenzale for stimulating discussions.
Conflict of Interest
One of the authors is entitled to royalties derived from Nihon Medi-Physics Ltd., Japan, for the sale of products related to the research described in the paper. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
Abbreviations
- LAT1
L-type amino acid transporter 1
- BCH
2-amino-bicyclo[2.2.1]heptane-2-carboxylic acid
- Anti-[18F]FACBC
anti-1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid
- [18F]FDG-PET
the 2-F-fluoro-2-deoxygluose-positron emission tomography
References
- 1.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coughlin SS, Ekwueme DU. Breast cancer as a global health concern. Cancer Epidemiol. 2009;33:315–318. doi: 10.1016/j.canep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Anderson BO, Yip CH, Smith RA, Shyyan R, Sener SF, Eniu A, et al. Guideline implementation for breast healthcare in low-income and middle-income countries: overview of the Breast Health Global Initiative Global Summit 2007. Cancer. 2008;113:2221–2243. doi: 10.1002/cncr.23844. [DOI] [PubMed] [Google Scholar]
- 5.Porter P. “Westernizing” women's risks? Breast cancer in lower-income countries. N Engl J Med. 2008;358:213–216. doi: 10.1056/NEJMp0708307. [DOI] [PubMed] [Google Scholar]
- 6.Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 7.Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB, et al. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- 8.Yoon JH, Kim YB, Kim MS, Park JC, Kook JK, Jung HM, et al. Expression and functional characterization of the system L amino acid transporter in KB human oral epidermoid carcinoma cells. Cancer Lett. 2004;205:215–226. doi: 10.1016/j.canlet.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi H, Ishii Y, Takayama T. Expression of L-type amino acid transporter 1 (LAT1) in esophageal carcinoma. J Surg Oncol. 2005;90:233–238. doi: 10.1002/jso.20257. [DOI] [PubMed] [Google Scholar]
- 10.Lin J, Raoof DA, Thomas DG, Greenson JK, Giordano TJ, Robinson GS, et al. L-type amino acid transporter-1 overexpression and melphalan sensitivity in Barrett's adenocarcinoma. Neoplasia. 2004;6:74–84. doi: 10.1593/neo.04205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nawashiro H, Otani N, Shinomiya N, Fukui S, Ooigawa H, Shima K, et al. L-type amino acid transporter 1 as a potential molecular target in human astrocytic tumors. Int J Cancer. 2006;119:484–492. doi: 10.1002/ijc.21866. [DOI] [PubMed] [Google Scholar]
- 12.Nawashiro H, Otani N, Shinomiya N, Fukui S, Nomura N, Yano A, et al. The role of CD98 in astrocytic neoplasms. Hum Cell. 2002;15:25–31. doi: 10.1111/j.1749-0774.2002.tb00096.x. [DOI] [PubMed] [Google Scholar]
- 13.Nakanishi K, Matsuo H, Kanai Y, Endou H, Hiroi S, Tominaga S, et al. LAT1 expression in normal lung and in atypical adenomatous hyperplasia and adenocarcinoma of the lung. Virchows Arch. 2006;448:142–150. doi: 10.1007/s00428-005-0063-7. [DOI] [PubMed] [Google Scholar]
- 14.Yoon JH, Kim IJ, Kim H, Kim HJ, Jeong MJ, Ahn SG, et al. Amino acid transport system L is differently expressed in human normal oral keratinocytes and human oral cancer cells. Cancer Lett. 2005;222:237–245. doi: 10.1016/j.canlet.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 15.Kim SG, Kim HH, Kim HK, Kim CH, Chun HS, Kanai Y, et al. Differential expression and functional characterization of system L amino acid transporters in human normal osteoblast cells and osteogenic sarcoma cells. Anticancer Res. 2006;26:1989–1996. [PubMed] [Google Scholar]
- 16.Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990;70:43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- 17.Shennan DB, Thomson J, Barber MC, Travers MT. Functional and molecular characteristics of system L in human breast cancer cells. Biochim Biophys Acta. 2003;1611:81–90. doi: 10.1016/S0005-2736(03)00028-2. [DOI] [PubMed] [Google Scholar]
- 18.Travers MT, Gow IF, Barber MC, Thomson J, Shennan DB. Indoleamine 2, 3-dioxygenase activity and L-tryptophan transport in human breast cancer cells. Biochim Biophys Acta. 2004;1661:106–112. doi: 10.1016/j.bbamem.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Shennan DB, Thomson J, Gow IF, Travers MT, Barber MC. L-leucine transport in human breast cancer cells (MCF-7 and MDA-MB-231): kinetics, regulation by estrogen and molecular identity of the transporter. Biochim Biophys Acta. 2004;1664:206–216. doi: 10.1016/j.bbamem.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Shennan DB, Thomson J. Inhibition of system L (LAT1/CD98hc) reduces the growth of cultured human breast cancer cells. Oncol Rep. 2008;20:885–889. [PubMed] [Google Scholar]
- 21.Oka S, Hattori R, Kurosaki F, Toyama M, Williams LA, Yu W, et al. A preliminary study of anti-1-amino-3-18F-fluorocyclobutyl-1-carboxylic acid for the detection of prostate cancer. J Nucl Med. 2007;48:46–55. [PubMed] [Google Scholar]
- 22.Shoup TM, Olson J, Hoffman JM, Votaw J, Eshima D, Eshima L, et al. Synthesis and evaluation of [18F]1-amino-3-fluorocyclobutane-1-carboxylic acid to image brain tumors. J Nucl Med. 1999;40:331–338. [PubMed] [Google Scholar]
- 23.Martarello L, McConathy J, Camp VM, Malveaux EJ, Simpson NE, Simpson CP, et al. Synthesis of syn- and anti-1-amino-3-[18F]fluoromethyl-cyclobutane-1-carboxylic acid (FMACBC), potential PET ligands for tumor detection. J Med Chem. 2002;45:2250–2259. doi: 10.1021/jm010242p. [DOI] [PubMed] [Google Scholar]
- 24.Liang Z, Wu T, Lou H, Yu X, Taichman RS, Lau SK, et al. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res. 2004;64:4302–4308. doi: 10.1158/0008-5472.CAN-03-3958. [DOI] [PubMed] [Google Scholar]
- 25.Fu OY, Hou MF, Yang SF, Huang SC, Lee WY. Cobalt chloride-induced hypoxia modulates the invasive potential and matrix metalloproteinases of primary and metastatic breast cancer cells. Anticancer Res. 2009;29:3131–3138. [PubMed] [Google Scholar]
- 26.Bouker KB, Skaar TC, Harburger DS, Riggins RB, Fernandez DR, Zwart A, et al. The A4396G polymorphism in interferon regulatory factor 1 is frequently expressed in breast cancer cell lines. Cancer Genet Cytogenet. 2007;175:61–64. doi: 10.1016/j.cancergencyto.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Park SA, Na HK, Kim EH, Cha YN, Surh YJ. 4-hydroxyestradiol induces anchorage-independent growth of human mammary epithelial cells via activation of IkappaB kinase: potential role of reactive oxygen species. Cancer Res. 2009;69:2416–2424. doi: 10.1158/0008-5472.CAN-08-2177. [DOI] [PubMed] [Google Scholar]
- 28.Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr, Brenz R, McGrath CM, et al. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- 29.Liang Z, Yoon Y, Votaw J, Goodman M, William L, Shim H. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res. 2005;65:967–971. [PMC free article] [PubMed] [Google Scholar]
- 30.Shim H, Lau SK, Devi S, Yoon Y, Cho HT, Liang Z. Lower expression of CXCR4 in lymph node metastases than in primary breast cancers: potential regulation by ligand-dependent degradation and HIF-1alpha. Biochem Biophys Res Commun. 2006;346:252–258. doi: 10.1016/j.bbrc.2006.05.110. [DOI] [PubMed] [Google Scholar]
- 31.Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97:643–655. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 32.McConathy J, Voll RJ, Yu W, Crowe RJ, Goodman MM. Improved synthesis of anti-[18F]FACBC: improved preparation of labeling precursor and automated radiosynthesis. Appl Radiat Isot. 2003;58:657–666. doi: 10.1016/S0969-8043(03)00029-0. [DOI] [PubMed] [Google Scholar]
- 33.Yanagida O, Kanai Y, Chairoungdua A, Kim DK, Segawa H, Nii T, et al. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta. 2001;1514:291–302. doi: 10.1016/S0005-2736(01)00384-4. [DOI] [PubMed] [Google Scholar]
- 34.Tamai S, Masuda H, Ishii Y, Suzuki S, Kanai Y, Endou H. Expression of L-type amino acid transporter 1 in a rat model of liver metastasis: positive correlation with tumor size. Cancer Detect Prev. 2001;25:439–445. [PubMed] [Google Scholar]
- 35.Kim DK, Ahn SG, Park JC, Kanai Y, Endou H, Yoon JH. Expression of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (4F2hc) in oral squamous cell carcinoma and its precusor lesions. Anticancer Res. 2004;24:1671–1675. [PubMed] [Google Scholar]
- 36.Liu XM, Reyna SV, Ensenat D, Peyton KJ, Wang H, Schafer AI, et al. Platelet-derived growth factor stimulates LAT1 gene expression in vascular smooth muscle: role in cell growth. FASEB J. 2004;18:768–770. doi: 10.1096/fj.03-0948com. [DOI] [PubMed] [Google Scholar]
- 37.Kim DK, Kanai Y, Choi HW, Tangtrongsup S, Chairoungdua A, Babu E, et al. Characterization of the system L amino acid transporter in T24 human bladder carcinoma cells. Biochim Biophys Acta. 2002;1565:112–121. doi: 10.1016/S0005-2736(02)00516-3. [DOI] [PubMed] [Google Scholar]
- 38.Christensen HN, Handlogten ME, Lam I, Tager HS, Zand R. A bicyclic amino acid to improve discriminations among transport systems. J Biol Chem. 1969;244:1510–1520. [PubMed] [Google Scholar]
- 39.Nawashiro H, Otani N, Uozumi Y, Ooigawa H, Toyooka T, Suzuki T, et al. High expression of L-type amino acid transporter 1 in infiltrating glioma cells. Brain Tumor Pathol. 2005;22:89–91. doi: 10.1007/s10014-005-0188-z. [DOI] [PubMed] [Google Scholar]
- 40.Vansteenkiste JF, Stroobants SG, De Leyn PR, Dupont PJ, Bogaert J, Maes A, et al. Lymph node staging in non-small-cell lung cancer with FDG-PET scan: a prospective study on 690 lymph node stations from 68 patients. J Clin Oncol. 1998;16:2142–2149. doi: 10.1200/JCO.1998.16.6.2142. [DOI] [PubMed] [Google Scholar]
- 41.Pieterman RM, van Putten JW, Meuzelaar JJ, Mooyaart EL, Vaalburg W, Koeter GH, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343:254–261. doi: 10.1056/NEJM200007273430404. [DOI] [PubMed] [Google Scholar]
- 42.Ueda S, Tsuda H, Asakawa H, Omata J, Fukatsu K, Kondo N, et al. Utility of 18F-fluoro-deoxyglucose emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in combination with ultrasonography for axillary staging in primary breast cancer. BMC Cancer. 2008;8:165. doi: 10.1186/1471-2407-8-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brock CS, Meikle SR, Price P. Does fluorine-18 fluorodeoxyglucose metabolic imaging of tumours benefit oncology? Eur J Nucl Med. 1997;24:691–705. doi: 10.1007/BF00841411. [DOI] [PubMed] [Google Scholar]
- 44.Stober B, Tanase U, Herz M, Seidl C, Schwaiger M, Senekowitsch-Schmidtke R. Differentiation of tumour and inflammation: characterisation of [methyl-3H]methionine (MET) and O-(2-[18F]fluoroethyl)-L-tyrosine (FET) uptake in human tumour and inflammatory cells. Eur J Nucl Med Mol Imaging. 2006;33:932–939. doi: 10.1007/s00259-005-0047-5. [DOI] [PubMed] [Google Scholar]
- 45.Larson SM. Cancer or inflammation? A Holy Grail for nuclear medicine. J Nucl Med. 1994;35:1653–1655. [PubMed] [Google Scholar]
- 46.Bakheet SM, Powe J. Benign causes of [18F]FDG uptake on whole body imaging. Semin Nucl Med. 1998;28:352–358. doi: 10.1016/S0001-2998(98)80038-X. [DOI] [PubMed] [Google Scholar]
- 47.Schuster DM, Votaw JR, Nieh PT, Yu W, Nye JA, Master V, et al. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med. 2007;48:56–63. [PubMed] [Google Scholar]
- 48.Schuster DM, Nye JA, Nieh PT, Votaw JR, Halkar RK, Issa MM, et al. Initial experience with the radiotracer anti-1-amino-3-[18F]Fluorocyclobutane-1-carboxylic acid (anti-[ 18F]FACBC) with PET in renal carcinoma. Mol Imaging Biol. 2009;11:434–438. doi: 10.1007/s11307-009-0220-5. [DOI] [PubMed] [Google Scholar]
- 49.Yu W, Williams L, Camp VM, Malveaux E, Olson JJ, Goodman MM. Stereoselective synthesis and biological evaluation of syn-1-amino-3-[18F]fluorocyclobutyl-1-carboxylic acid as a potential positron emission tomography brain tumor imaging agent. Bioorg Med Chem. 2009;17:1982–1990. doi: 10.1016/j.bmc.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 50.Pegram MD, Pauletti G, Slamon DJ. HER-2/neu as a predictive marker of response to breast cancer therapy. Breast Cancer Res Treat. 1998;52:65–77. doi: 10.1023/A:1006111117877. [DOI] [PubMed] [Google Scholar]
- 51.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 52.Yamasaki S (1978) Estrogen Receptor in Malignant and Benign Breast Tumors and Normal Breast Tissues of Japanese Patients Japanese Journal of Clinical Oncology 8:37–47