Abstract
Purpose
Bisphosphonate (BP)-related osteonecrosis of the jaw (BRONJ) is a well-known serious complication of BP treatment. This study was undertaken to evaluate the diagnostic usefulness of three-phase bone scintigraphy in patients with BRONJ.
Methods
Forty-one patients (48 lesions) with clinically proven BRONJ (2 males, 39 females, age 74.3 ± 6.7 years) underwent Tc-99 m HDP bone scintigraphy. Visual interpretation and semiquantitative analysis of uptakes using lesion-to-contralateral uptake ratios during the blood pool phase (BUR) and during the osseous phase (OUR) were performed, and relations were sought between these and various clinical parameters.
Results
Three-phase bone scintigraphy showed increased perfusion and blood pooling in 21 (63.6 %) and 27 (81.8 %) of 33 lesions, respectively. The osseous phase was positive for 45 (93.8 %) of the 48 lesions. Of the four inflammatory clinical parameters of BRONJ [pus discharge, pain, swelling, and erythrocyte sedimentation rate (ESR)], patients with three or more parameters had more positive findings in vascular and blood pool phase images (p = 0.033, p = 0.027). By semiquantitative analysis, patients with a positive ESR had statistically higher BUR and OUR (both p < 0.001). Higher stage BRONJ lesions had higher OUR than lower stage lesions (p = 0.003). In addition, bone scintigraphy revealed three clinically covert BRONJ lesions without bone exposure, and four patients were up-staged based on bone scintigraphy.
Conclusions
Bone scintigraphy provides a relatively sensitive means of detecting BRONJ, so it was helpful for accurate BRONJ staging. Furthermore, increased uptakes in vascular and blood pool phases of three-phase bone scintigraphy were related to the inflammatory activity of BRONJ.
Keywords: Three-phase bone scintigraphy, Bisphosphonate, Osteonecrosis, Jaws
Introduction
Bisphosphonates (BPs) are widely used in patients with osteoporosis, Paget’s disease, multiple myeloma, bone metastasis from solid cancers, and cancer-induced hypercalcemia [1]. Although its pathogenic mechanism has not yet been fully elucidated, osteonecrosis of the jaw, one of the most serious adverse effects of BP, is not uncommonly encountered [2–4].
To distinguish BP-related osteonecrosis of the jaw (BRONJ) from other delayed healing conditions of the jaw, the American Association of Oral and Maxillofacial Surgeons (AAOMS) has proposed a definition for BRONJ [5]. According to this, BRONJ can be diagnosed when a case meets the following three conditions: (1) current or previous treatment with a BP, (2) exposed bone in the maxillofacial region that has persisted for more than 8 weeks, and (3) no history of radiation therapy to the jaws [5].
The staging system proposed by AAOMS is based on the presence of infection and complications. Therefore, detection of these through clinical symptoms, laboratory findings, and imaging studies is crucial for the accurate staging of BRONJ. However, plain radiography is poor for visualization of BRONJ lesions; and although CT and MRI allow the BRONJ lesions and accompanying infections and complications to be visualized, no standardized imaging modality has been established for the assessment of BRONJ.
Bone scintigraphic images can demonstrate anatomy of bony structures and also enable the detection of a disease process that alters metabolic activity of the bone without any structural or anatomical alteration or detect an early disease status that has not yet produced any structural changes [6]. Three-phase bone scintigraphy is a well established nuclear medicine modality that is often used to diagnose inflammatory bone diseases, such as osteomyelitis, septic arthritis, inflammatory arthritis, prosthesis complications, diabetic foot, and complex regional pain syndrome [7]. Because high stage BRONJ is associated with inflammatory conditions with infections or complications, three-phase bone scintigraphy might be expected to be useful for the assessment of BRONJ, and recent studies have suggested that bone scintigraphy is a useful modality [8, 9]. However, the clinical role of three-phase bone scintigraphy for BRONJ has not been fully investigated [1]. Thus, this study was undertaken to evaluate the clinical implications of three-phase Tc-99 m hydroxymethylene diphosphonate (HDP) bone scintigraphy in BRONJ. In addition, relations between its findings and clinical and laboratory parameters were sought, especially regarding clinical stage.
Materials and Methods
This retrospective study included 41 BRONJ patients (2 male, 39 female) who were referred to our Department of Oral and Maxillofacial Surgery from February 2010 to March 2011. Mean age of the 41 enrolled patients was 74.2 ± 6.7 years (range 65–86 years), and mean duration of BP medication was 44.0 ± 30.0 months. Twenty-two patients were medicated with alendronate, four with pamidronate, four with risedronate, two with zoledronate, one with ibandronate, and eight patients took more than one type of BP. The route of BP administration was oral for 36 patients and intravenous for 5 patients. Thirty-nine patients took BPs for osteoporosis, one for bone metastasis, and one for multiple myeloma.
BRONJ was diagnosed when at least 12 months of BP administration was followed by maxillofacial bone exposure that did not heal within 8 weeks of identification in the absence of a history of local radiotherapy in accordance with the AAOMS definition [5]. A bone biopsy was performed in patients with an underlying malignancy to differentiate BRONJ from bone metastases. Age, gender, duration of BP treatment, type of BP used, and reasons for BP medication were obtained by reviewing medical records.
All 48 BRONJ lesions were staged according to the AAOMS BRONJ staging system [5]. Stage 1 includes exposed/necrotic bone in asymptomatic patients with no evidence of infection. Stage 2 includes patients with exposed/necrotic bone associated with infection as evidenced by pain and erythema in the region of exposed bone with or without purulent drainage. Stage 3 includes patients with exposed/necrotic bone associated with pain, infection, and one or more of the following; a pathologic fracture, an extra-oral fistula, or osteolysis extending into the inferior border or sinus floor.
Erythrocyte sedimentation rate (ESR) was viewed as a surrogate marker of inflammatory activity, and ESR value above the cut-off value (male, 15 mm/h; female, 20 mm/h) was interpreted as positive. Three-phase bone scintigraphy was performed immediately after a bolus injection of 740 MBq (20 mCi) of Tc-99 m HDP. First-pass radionuclide angiographic data were obtained using a 256 × 256 matrix in anterior view every 3 s for 99 s (vascular phase). Next, blood pool images were obtained for 3 min from 5 min after injection. Anterior and lateral images were obtained using a 512 × 512 matrix (blood pool phase) and a dual-headed gamma camera equipped with a high resolution collimator (Infinia, GE, Milwaukee, WI). Finally, osseous phase images were obtained 3 h after injection; anterior and posterior whole body images were obtained over 10 min and anterior and lateral static images of the skull over 3 min (osseous phase). Three-phase bone scintigraphy was performed in 29 patients with 33 clinically proven lesions, but only single osseous phase acquisition was performed in 12 patients with 15 lesions.
Visual interpretation of bone scintigraphic images was performed by two nuclear medicine physicians in consensus. Focal or diffusely increased tracer accumulation versus surrounding background or contralateral bone was regarded as a positive finding.
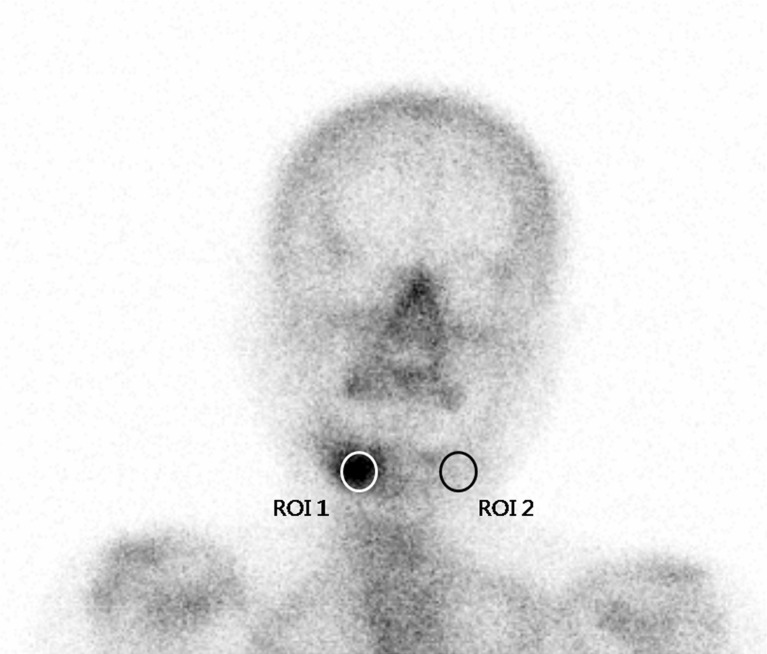
For a semiquantitative analysis, a circular region of interest (ROI) was manually placed over lesion areas showing high tracer accumulation, and an identical ROI was placed over corresponding contralateral normal regions. Uptake ratios in images of each phase were calculated by dividing mean counts of lesion ROIs by contralateral normal areas (Fig. 1). Semiquantitative uptake values, defined as lesion-to-contralateral uptake ratios in the blood pool phase (BUR) and in the osseous phase (OUR), were also calculated and correlated with several clinical parameters of BRONJ, such as duration of BP medication, clinical symptoms, and ESR. Image analysis was performed on a Xeleris workstation (GE Medical Systems, Milwaukee, WI). The semiquantitative analysis was not performed in four patients with five lesions due to bilateral abnormalities or a midline abnormality that prevented drawing contralateral ROIs.
Fig. 1.
Methods of semiquantitative analysis. A circular region of interest (ROI) was manually drawn and placed over BRONJ lesion (ROI 1), and a duplicate identical ROI was placed over the contralateral normal region for control purposes (ROI 2)
To assess lesion inflammatory activity, three clinical parameters and one laboratory parameter (pus discharge, pain, swelling, and ESR) were selected, and comparative analysis was performed. Degrees of inflammatory activity were dichotomized based on the presence of ≥3 or ≤2 positive parameters. These two groups were compared with respect to the results of visual assessments of three-phase bone scintigraphy.
Statistical analysis was performed using Fisher’s exact test for categorical variables and the independent samples t-test for continuous variables, as indicated. Pearson’s correlation coefficients were used to analyze degrees of association between OUR and the duration of BP medication. The Kruskal-Wallis test (continuous variables) and the chi-squared test (categorical variables) were used to analyze differences between bone scintigraphic findings with respect to BRONJ stage. MedCalc software (version 11.5; MedCalc, Mariakerke, Belgium) was used for all statistical calculations, and statistical significance was accepted for p values of <0.05.
Results
Forty-eight lesions were identified clinically by oromaxillary examination. Seventeen lesions were in right mandibles, 2 in the mid-anterior mandibular region, 15 in left mandibles, 6 in right maxillae, 1 in the mid-anterior maxillary region, and 7 in the left maxillae. Of seven patients with two lesions, one had bilateral maxillary involvement and six had involvements of maxilla and mandible. Clinically, bone exposure was observed in all 41 patients, gingival pain in 11, gingival swelling in 20, and discharge of pus in 33 patients. Clinical findings of the 41 subjects are summarized in Table 1, according to BRONJ stage (Table 1).
Table 1.
Clinical findings by stage of bisphosphonate-related osteonecrosis of the jaw (BRONJ)
| Stage | P value | |||
|---|---|---|---|---|
| 1 (n = 3) | 2 (n = 11) | 3 (n = 27) | ||
| Pain | 0 | 4 | 7 | 0.751 |
| Swelling | 0 | 7 | 13 | 0.147 |
| Pus discharge | 0 | 8 | 25 | <0.001 |
| Erythrocyte sedimentation rate (mm/h) | 18.0 ± 2.0 | 26.4 ± 18.2 | 45.4 ± 24.3 | 0.021 |
| Duration of medication (months) | 44.0 ± 30.2 | 55.4 ± 37.2 | 39.3 ± 26.7 | 0.375 |
Three-phase bone scintigraphy showed increased tracer uptake during the vascular and blood pool phases in 21 (63.6 %) and 27 (81.8 %) of 33 lesions, respectively. The osseous phase revealed increased tracer uptake in 45 (93.8 %) of 48 lesions. BRONJ lesions in patients with a positive ESR had a higher incidence of positive findings than lesions in patients with a negative ESR based on visual interpretations of all three image phases (vascular, blood pool, osseous phases) (p = 0.047, p < 0.001, p = 0.017 respectively; Table 2). We compared the results of visual interpretations and the four parameters of active inflammation (pus discharge, pain, swelling, and ESR), and these results are summarized in Table 3. The patients with ≥3 positive parameters had a significantly higher incidence of positive findings based on visual interpretations of vascular and blood pool phase images (p = 0.033, p = 0.027, respectively). However, the patients with ≥3 positive parameters did not have statistically higher incidence of positive findings based on visual interpretations of osseous phase images (p = 0.239).
Table 2.
Results of visual interpretations in erythrocyte sedimentation rate (ESR) positive and negative cases
| Vascular phase | Blood pool phase | Osseous phase | ||||
|---|---|---|---|---|---|---|
| (n = 33) | (n = 33) | (n = 48) | ||||
| Positive | Negative | Positive | Negative | Positive | Negative | |
| ESR (+) | 20 | 6 | 26 | 0 | 35 | 0 |
| ESR (−) | 1 | 6 | 1 | 6 | 10 | 3 |
| P valuea | 0.047 | <0.001 | 0.017 | |||
aFisher’s exact test
Table 3.
Result of visual interpretations according to number of inflammatory parametersa
| Vascular phase | Blood pool phase | Osseous phase | ||||
|---|---|---|---|---|---|---|
| (n = 34) | (n = 34) | (n = 48) | ||||
| Numbers of (+) parameters | Positive | Negative | Positive | Negative | Positive | Negative |
| ≤2 | 9 | 10 | 13 | 6 | 23 | 3 |
| ≥3 | 12 | 2 | 14 | 0 | 22 | 0 |
| P valueb | 0.033 | 0.027 | 0.239 | |||
aInflammatory parameters: pus discharge, pain, swelling, and an elevated erythrocyte sedimentation rate
bFisher’s exact test
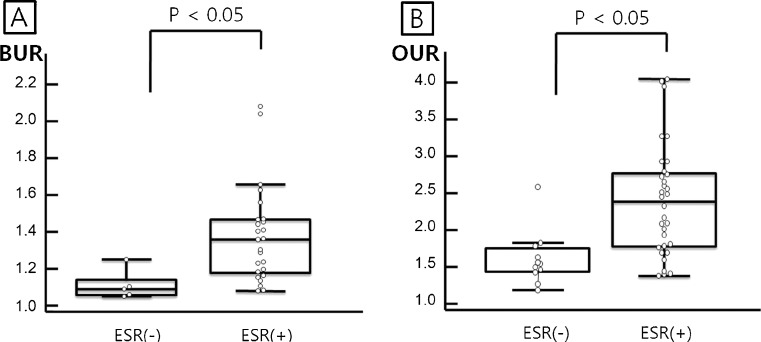
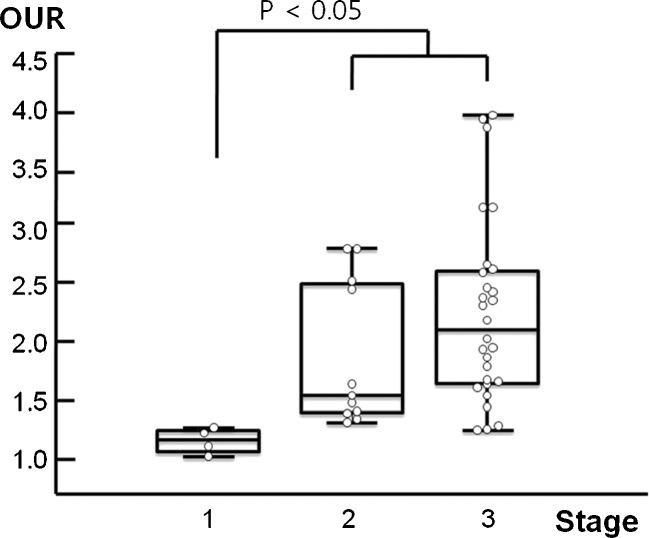
By semiquantitative analysis, BRONJ lesions in patients with a positive ESR had a statistically higher BUR (Fig. 2a, p < 0.001) and OUR (Fig. 2b, p < 0.001) than those of patients with a negative ESR. BUR seemed to be higher in BRONJ lesions of stage 2 or 3 as compared with stage 1, but this was not significant (p = 0.099; Table 4). OURs were significantly affected by BRONJ stage (p = 0.003, Table 4). Post-hoc analysis revealed OURs of stages 2 plus 3 were higher than stage 1 (Fig. 3). OURs were not found to differ significantly according to BP type. No significant correlation was found between BUR or OUR and duration of BP medication (r = 0.041, p = 0.828; r = 0.030, p = 0.849).
Fig. 2.
Blood pool and osseous uptake ratios according to erythrocyte sedimentation rate (ESR) positivity. Bisphosphonate-related osteonecrosis of the jaw (BRONJ) lesions in ESR-positive patients had significantly higher blood pool phase uptake ratio (BUR) and osseous phase uptake ratio (OUR) values than the lesions in ESR-negative patients
Table 4.
Lesion-to-contralateral uptake ratios during the blood pool and osseous phases according to different stages of bisphosphonate-related osteonecrosis of the jaw (BRONJ)
| Stage | P valuea | |||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| BUR | 1.11 ± 0.07 | 1.37 ± 0.44 | 1.35 ± 0.17 | 0.099 |
| OUR | 1.30 ± 0.11 | 2.02 ± 0.62 | 2.39 ± 0.78 | 0.003 |
BUR Blood pool phase uptake ratio, OUR osseous phase uptake ratio
aKruskal-Wallis test
Fig. 3.
Osseous phase uptake ratio (OUR) according to different stages of bisphosphonate-related osteonecrosis of the jaw (BRONJ). The OURs of stage 1 and stage 2 plus 3 were significantly different
In addition, osseous phase imaging uncovered three clinically covert BRONJ lesions and up-staged four BRONJ lesions from stage 2 to stage 3 by demonstrating maxillary sinus involvement. Whole body bone scintigraphy also demonstrated additional extra-maxillofacial bone lesions in 14 patients including an insufficiency fracture (spines, ribs, or sternum) in 13 and disseminated cancer metastasis in 1.
Discussion
BRONJ is associated with significant morbidity, and this has encouraged re-evaluations of the indications and duration of BP therapy for osteopenia, osteoporosis, and osseous metastasis from malignant tumors [4]. BRONJ is usually diagnosed using clinical manifestations and the presence of a positive BP medical history. Plain radiography, CT, and MRI can be used both to diagnose BRONJ and to evaluate disease extent. These radiologic modalities are useful to visualize bony change and evaluate the involvement of adjacent structures such as inferior alveolar nerve or sinuses. However, these findings are not specific enough to be diagnostically useful [10, 11]. These modalities also only visualize the lesions after structure changes and do not represent early change in BRONJ and inflammation activities. Dental prostheses are widely used in the elderly patients who are the usual candidates for bisphosphonate medications, and these artifacts add to the difficulty of interpreting these radiologic images. Although bone scintigraphy is also known to be useful for the early detection of BRONJ [8, 12], the role of three-phase bone scintigraphy for the assessment of clinical status and the correlation between uptake value and clinical status has not been well determined.
In terms of the diagnosis of BRONJ lesions, this study shows that the sensitivities of the vascular and blood pool phases were lower than that of the osseous phase (63.6 and 79.4 vs. 93.9 %). All BRONJ lesions that exhibited positivity on vascular or blood pool phase images also exhibited positivity on osseous phase images. Only 3 of the 48 BRONJ lesions were not visualized on osseous phase images. However, although these three lesions were not distinguished from surrounding normal-looking bone, their OURs were higher than those of the contralateral sides. We believe that the poor resolution of bone scintigraphy images may have been responsible for these negative results [1].
Osseous phase imaging revealed three additional BRONJ lesions that were overlooked clinically at initial evaluation. After bone scintigraphy, careful examinations including other radiological studies were performed, and BRONJ was finally diagnosed. Figure 4 demonstrates a case in which bone scintigraphy uncovered an additional BRONJ lesion. In a recent study, O’Ryan et al. suggested a potential role of bone scintigraphy in assessing early markers of BRONJ [9]. They found that 65.7 % of patients with regions of abnormal bone tracer subsequently developed BRONJ. Our results also suggest that careful physical examination and close follow-up are warranted for additional jaw lesions found on bone scintigraphy in patients with BRONJ.
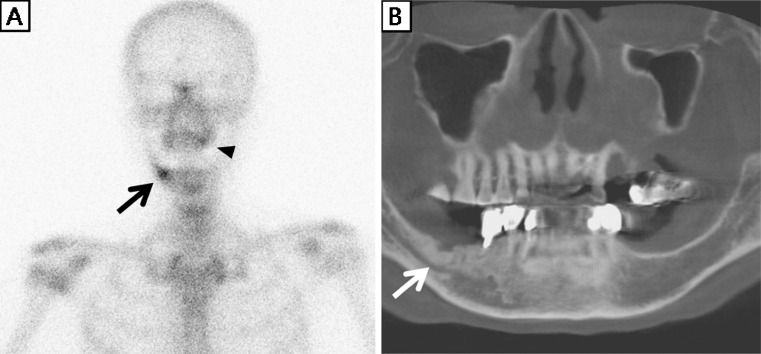
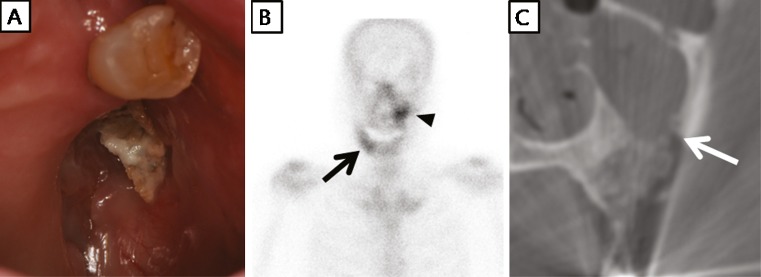
Fig. 4.
A 68-year-old woman had taken alendronate for 72 months due to idiopathic osteoporosis. After extraction of a left upper tooth, she suffered maxillary alveolar bone exposure for 2 months without pain. Thus, she was classified as having stage 1 bisphosphonate-related osteonecrosis of the jaw (BRONJ). a Osseous phase bone scintigraphy showed mild tracer uptake at the left maxillary alveolar bone (black arrowhead), and focal tracer uptake in the right side mandible (black arrow). b Cone-beam CT showed sclerotic change by a hidden BRONJ lesion in the right side mandible, which corresponded with bone scintigraphy findings (white arrow)
Concomitant infection is common, and its recognition is clinically important for the staging of BRONJ [3, 5]. Thus, serologic markers of acute inflammation can be important in terms of suggesting the presence of BRONJ lesion infection. Vascular and blood pool phases of three-phase bone scintigraphy also can visualize inflammatory activity. In the present study, patients with BRONJ lesions visualized on vascular and blood pool phases had three or more inflammatory parameters representing active inflammation, and all patients with BRONJ lesions not visualized during the osseous phase had two or fewer inflammatory parameters.
Stage 1 lesions had a lower OUR than stage 2 or 3 lesions, a finding that suggests that lesions with concomitant infection show greater tracer uptake during the osseous phase. It is difficult to detect concomitant inflammation of BRONJ lesions with physical examination in certain cases and ESR is a systemic inflammatory marker, therefore bone scintigraphy might have a role in the assessment of inflammation severity at specific sites.
A substantial percentage of patients with severe maxillary BRONJ suffer from associated maxillary sinusitis that is frequently resistant to conventional therapies [13]. Furthermore, maxillary sinusitis is one of the most serious complications of maxillary BRONJ, and up-stages any stage of BRONJ to stage 3. Bone scintigraphy is known to be highly sensitive for the detection of paranasal sinusitis, and in the present study, maxillary sinusitis was further diagnosed by scintigraphy in four patients, including one with bilateral involvement. Accordingly, these lesions were up-staged to stage 3 and their therapeutic strategies were changed (Fig. 5).
Fig. 5.
A 72-year-old woman took pamidronate for 41 months due to idiopathic osteoporosis and experienced spontaneous bone exposure, pus discharge, and swelling at the left maxilla and right mandible. a Left maxillary alveolar bone was exposed, and the bisphosphonate-related osteonecrosis of the jaw (BRONJ) lesion of the left maxilla was of stage 2. b Osseous phase bone scintigraphy showed focal tracer uptakes at the left maxilla (black arrowhead) and right mandible (black arrow). In addition, tracer uptake in the left maxilla extended to the maxillary sinus area, suggesting maxillary sinusitis. Bone scintigraphic findings suggested a stage 3 BRONJ lesion of the left maxilla. c CT scan confirmed left maxillary sinusitis by a BRONJ lesion of the left maxilla (white arrow). The BRONJ lesion in the left maxilla was up-staged to stage 3 based on bone scintigraphy
In addition to jaw images, whole body images were also obtained during the osseous phase and provided bone metabolic information for the entire skeletal system. Because BPs are usually administered for osteoporosis or bone metastases, insufficiency fractures or bone metastases can often be detected on whole body scintigraphic images. In the present study, 14 patients (34.1 %) showed extra-maxillofacial bone lesions. Furthermore, the additional information was obtained without any additional radiation exposure, which assisted with general management planning; therefore our experience suggests that an additional whole body image should be acquired during bone scintigraphy for BRONJ.
Mild and focal tracer uptakes at maxillae and mandibles are usually overlooked during bone scintigraphy performed for the detection and evaluation of bone metastasis because of frequent alveolar uptake by reactive changes associated with dental prostheses or previous dental procedures [14]. The proportion of older patients taking BPs is gradually increasing [15], and BRONJ lesions exhibit variable and noncharacteristic uptake on bone scintigraphy. According to the definition of BRONJ [5], bone exposure is needed to diagnose BRONJ, and previous dental procedures or a dental prosthesis can be predisposing factors. Therefore visual inspections of the uptake lesions at clinics and careful medical history regarding BP medication may be required for the interpretation of bone scintigraphy showing maxillary or mandibular uptake, and BRONJ should be considered as a potential cause.
Recently, other nuclear medicine methods have been used to diagnose and evaluate BRONJ lesions. F-18 FDG and F-18 fluoride PET can be used to detect and analyze the extent of these lesions [16], and hybrid SPECT/CT, PET/CT, or PET/MRI with appropriate radiopharmaceuticals may further improve diagnostic and severity assessments of BRONJ by combining metabolic, functional and morphologic imaging [16–18].
This study has some limitations that require consideration. First, it was a retrospective study and clinical data were obtained by chart review. Second, the total number of subjects was relatively small (n = 41) and only three patients had stage 1 BRONJ. In fact, no stage 0 BRONJ lesion was included. Therefore, a prospective study with a large number of subjects is warranted to verify the usefulness of three-phase bone scintigraphy for evaluation of BRONJ.
Conclusion
Three-phase bone scintigraphy was found to provide a sensitive means of detecting BRONJ lesions and to be useful for the accurate staging of BRONJ. In addition, the study shows that increased tracer uptake by a BRONJ lesion during the vascular or blood pool phases of three-phase bone scintigraphy is related to the presence of an active inflammatory status suggesting concomitant infection.
Acknowledgments
This research was financially supported by the Ministry of Knowledge Economy (MKE), Korea Institute for Advancement of Technology (KIAT) and Daegyeong Leading Industry Office through the Leading Industry Development for Economic Region, a grant from the Nuclear Research & Development Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science & Technology (MEST), and the Brain Korea 21 Project in 2012.
References
- 1.Yoneda T, Hagino H, Sugimoto T, Ohta H, Takahashi S, Soen S, et al. Bisphosphonate-related osteonecrosis of the jaw: position paper from the Allied Task Force Committee of Japanese Society for Bone and Mineral Research, Japan Osteoporosis Society, Japanese Society of Periodontology, Japanese Society for Oral and Maxillofacial Radiology, and Japanese Society of Oral and Maxillofacial Surgeons. J Bone Miner Metab. 2010;28:365–383. doi: 10.1007/s00774-010-0162-7. [DOI] [PubMed] [Google Scholar]
- 2.Migliorati CA, Siegel MA, Elting LS. Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatment. Lancet Oncol. 2006;7:508–514. doi: 10.1016/S1470-2045(06)70726-4. [DOI] [PubMed] [Google Scholar]
- 3.Allen MR, Burr DB. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. J Oral Maxillofac Surg. 2009;67:61–70. doi: 10.1016/j.joms.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Ruggiero SL. Bisphosphonate-related osteonecrosis of the jaw: an overview. Ann N Y Acad Sci. 2011;1218:38–46. doi: 10.1111/j.1749-6632.2010.05768.x. [DOI] [PubMed] [Google Scholar]
- 5.Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaw—2009 update. J Oral Maxillofac Surg. 2009;67:2–12. doi: 10.1016/j.joms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Ahn BC, Kim HJ, Lee SW, Yoo J, Choi JK, Lee J. New quantitative method for bone tracer uptake of temporomandibular joint using Tc-99 m MDP skull SPECT. Ann Nucl Med. 2009;23:651–656. doi: 10.1007/s12149-009-0287-8. [DOI] [PubMed] [Google Scholar]
- 7.Davenport MS, Brown RK, Frey KA. Utility of delayed whole-body bone scintigraphy after directed three-phase scintigraphy. AJR Am J Roentgenol. 2009;193:338–342. doi: 10.2214/AJR.08.2142. [DOI] [PubMed] [Google Scholar]
- 8.Zanglis A, Andreopoulos D, Dima M, Baltas G, Baziotis N. Jaw uptake of technetium-99 methylene diphosphonate in patients on biphosphonates: a word of caution. Hell J Nucl Med. 2007;10:177–180. [PubMed] [Google Scholar]
- 9.O'Ryan FS, Khoury S, Liao W, Han MM, Hui RL, Baer D, et al. Intravenous bisphosphonate-related osteonecrosis of the jaw: bone scintigraphy as an early indicator. J Oral Maxillofac Surg. 2009;67:1363–1372. doi: 10.1016/j.joms.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Bisdas S, Chambron Pinho N, Smolarz A, Sader R, Vogl TJ, Mack MG. Biphosphonate-induced osteonecrosis of the jaws: CT and MRI spectrum of findings in 32 patients. Clin Radiol. 2008;63:71–77. doi: 10.1016/j.crad.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 11.Hutchinson M, O'Ryan F, Chavez V, Lathon PV, Sanchez G, Hatcher DC, et al. Radiographic findings in bisphosphonate-treated patients with stage 0 disease in the absence of bone exposure. J Oral Maxillofac Surg. 2010;68:2232–2240. doi: 10.1016/j.joms.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Raje N, Woo SB, Hande K, Yap JT, Richardson PG, Vallet S, et al. Clinical, radiographic, and biochemical characterization of multiple myeloma patients with osteonecrosis of the jaw. Clin Cancer Res. 2008;14:2387–2395. doi: 10.1158/1078-0432.CCR-07-1430. [DOI] [PubMed] [Google Scholar]
- 13.Maurer P, Sandulescu T, Kriwalsky MS, Rashad A, Hollstein S, Stricker I, et al. Bisphosphonate-related osteonecrosis of the maxilla and sinusitis maxillaris. Int J Oral Maxillofac Surg. 2011;40:285–291. doi: 10.1016/j.ijom.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Meidan Z, Weisman S, Baron J, Binderman I. Technetium 99 m-MDP scintigraphy of patients undergoing implant prosthetic procedures: a follow-up study. J Periodontol. 1994;65:330–335. doi: 10.1902/jop.1994.65.4.330. [DOI] [PubMed] [Google Scholar]
- 15.Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2005;353:595–603. doi: 10.1056/NEJMcp043801. [DOI] [PubMed] [Google Scholar]
- 16.Wilde F, Steinhoff K, Frerich B, Schulz T, Winter K, Hemprich A, et al. Positron-emission tomography imaging in the diagnosis of bisphosphonate-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:412–419. doi: 10.1016/j.tripleo.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Damle N, Kumar R, Kumar P, Jaganathan S, Patnecha M, Bal C, et al. SPECT/CT in the diagnosis of skull base osteomyelitis. Nucl Med Mol Imaging. 2011;45:212–216. doi: 10.1007/s13139-011-0086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dore F, Filippi L, Biasotto M, Chiandussi S, Cavalli F, Di Lenarda R. Bone scintigraphy and SPECT/CT of bisphosphonate-induced osteonecrosis of the jaw. J Nucl Med. 2009;50:30–35. doi: 10.2967/jnumed.107.048785. [DOI] [PubMed] [Google Scholar]