Abstract
Purpose
Dual-time-point (DTP) FDG PET/CT has been shown to be useful for lymph node (LN) staging in patients with non-small-cell lung cancer (NSCLC). The aim of this study was to evaluate the LN staging ability of DTP FDG PET/CT in the predominant area of pulmonary tuberculosis.
Methods
Sixty-nine NSCLC patients underwent DTP PET/CT. Regions of interest were placed on each LN of each station, and the maximum SUVs were measured. Three variables were obtained: (1) the SUV on the early scan (SUVearly), (2) the SUV on the delayed scan (SUVdelayed), and (3) the retention index of the SUV (RI). Each patient had one final LN stage and three other LN stages according to the cutoff values of SUVearly, SUVdelayed, and RI.
Results
In the LN-based analysis, the area under the ROC curve of SUVdelayed (0.884) was significantly larger (P < 0.01) than those of SUVearly (0.868) and RI (0.717). Among the three variables, SUVdelayed was more accurate (P < 0.01) for detecting the mediastinal LN metastasis than SUVearly and RI. In the patient-based analysis, SUVdelayed had correctly determined LN stages in 55 of 69 patients (sensitivity, specificity, and accuracy = 88.7 %, 50.0 %, and 79.7 %), whereas SUVearly and RI correctly determined LN stages in 53 and 52 patients, respectively.
Conclusions
In this study, comparing the diagnostic efficacy of SUVearly, SUVdelayed, and RI for LN staging in patients with NSCLC, SUVdelayed was the most accurate variable for LN staging. DTP PET/CT could provide improved diagnostic accuracy for the LN staging of NSCLC.
Keywords: Non-small-cell lung cancer, Fluorodeoxyglucose, Positron emission tomography, Dual-time-point imaging, Lymph node staging
Introduction
Non-small-cell lung cancer (NSCLC) is one of the leading causes of cancer deaths worldwide. Accurate lymph node (LN) staging is critical for determining the treatment strategy and predicting the outcome for NSCLC patients [1]. Lately, positron emission tomography/computed tomography (PET/CT) using F-18 fluorodeoxyglucose (FDG), a glucose analog, has been demonstrated to be useful in differentiating benign from malignant processes.
However, FDG PET/CT false-positive results may still occur [2–4]. Benign processes such as infection, inflammation, and granulomatous diseases may cause enhanced FDG uptake. This is mainly due to increased glucose utilization by activated macrophages and inflammatory cells [5]. Intrathoracic inflammations, such as tuberculosis, pneumonia, sarcoidosis, etc., are known to cause false-positive results. Therefore, the local prevalence of granulomatous pulmonary disease may lessen the ability of FDG PET/CT to differentiate malignancy from benign lesions and the accuracy of LN staging of FDG PET/CT in pulmonary malignancy.
A semi-quantitative approach using standardized uptake values (SUVs) has been widely used to help discriminate benign from malignant lesions. Moreover, in several studies, it has been proposed that dual-time-point (DTP) FDG PET/CT may improve the accuracy of LN staging in NSCLC [6–8]. However, other studies reported that single-time-point (STP) FDG PET/CT at 1 h after FDG injection was sufficiently useful for LN staging in NSCLC, and the usefulness of DTP PET/CT is still controversial [9–11].
The aim of this study was to evaluate the LN staging ability of DTP FDG PET/CT in the predominant area of pulmonary tuberculosis.
Materials and Methods
Patients
Among NSCLC patients who had undergone FDG PET/CT scans for primary staging at our institution from December 2007 to December 2008, we excluded those with bronchial alveolar carcinoma, with hyperglycemia (blood glucose levels > 125 mg/dl at the time of FDG injection), without any visible mediastinal lymph nodes on early PET scans, or with known malignancies other than lung cancer. According to the results, 69 NSCLC patients (54 male and 15 female; mean age 68.4 ± 9.3 years) were included in this study. Histopathological tumor types were adenocarcinoma in 33, squamous cell carcinoma in 31, large cell carcinoma in 1, and poorly differentiated NSCLC in 4. LN staging of all patients was done according to the 7th edition of the TNM staging system for lung cancer, which was published by the International Union Against Cancer and the American Joint Committee on Cancer [12].
The final confirmation for the presence or absence of metastasis was made histopathologically in nine patients. In the remaining 60 patients, the presence or absence of metastasis was decided by the findings on imaging studies of the patients, who were followed up for at least a 1.5-year interval (mean interval of patient follow-up, 276 ± 221 days). These imaging studies included FDG PET/CT, contrast-enhanced chest CT, and magnetic resonance imaging (MRI). All LNs that showed any radiographic findings representing metastasis during the period were considered as metastatic LNs. These final confirmations could provide the final LN stage for all patients.
FDG PET/CT Imaging
All patients fasted overnight or for at least 6 h before examinations. Their blood glucose levels were within normal limits before the injection of 4.0 MBq/kg of FDG per kilogram of body weight. FDG was prepared using a cyclotron and automated synthesis apparatus. The radiochemical and chemical purity of the product was assayed by analytic high-performance liquid chromatography and thin-layer chromatography, and was consistently > 90 % with both assays.
Images were acquired twice: an early scan including the head, thorax, abdomen, pelvis, and thigh at 60 min after injection, and a delayed scan including the thorax at about 120 min. The mean interval between the two scans was 79 ± 24 min. An integrated PET/CT (Biograph Sensation 16, Siemens Medical Systems) was used for image acquisition. The axes of both systems are mechanically aligned to coincide optimally. CT data were acquired first, and the following parameters were used: tube rotation time 0.5 s per revolution, 120 kV, 140 mAs, and reconstructed slice thickness 3 mm. No contrast medium was used for the CT examinations. After the acquisition of CT data had been completed, the table top with the patient on it automatically advanced into the PET-sensitive field of view, and acquisition of PET data was started in the three-dimensional mode with the patient in exactly the same position on the table. Scanning was performed in one bed position for 3 min. The attenuation correction was automatically completed using the corresponding CT data.
Image Analysis of FDG PET/CT
Regions of interest were placed on each mediastinal LN, and the maximum SUVs in each LN station were measured. According to the results, three variables were obtained for each LN station: (1) the SUV on the early scan (SUVearly), (2) the SUV on the delayed scan (SUVdelayed), and (3) the retention index of the SUV (RI) (%) calculated from the formula: [(SUVdelayed - SUVearly) / SUVearly] × 100. The optimal cutoff values of SUVearly, SUVdelayed, and RI in predicting mediastinal LN metastases were determined using receiver-operating characteristic (ROC) curve analysis. The results showed that each patient had three kinds of LN stage according to the SUVearly, SUVdelayed, and RI.
Statistical Analysis
Statistical analysis was performed using SPSS for Windows, version 18.0 (SPSS Inc., Chicago, IL). To make comparisons among the diagnostic performances of SUVearly, SUVdelayed, and RI, we performed the ROC curve analysis. The areas under the ROC curves were calculated and compared to the others statistically. To analyze the correlation among the LN stages, bivariate correlation was used. Comparisons between SUVs of benign LNs and those of malignant ones were performed using two-tailed paired and unpaired Student's t tests. LN-based sensitivity, specificity, and accuracy were calculated using crosstab tables and compared using chi-Square test. To compare the agreements between the final LN stage and SUV-derived LN stages, kappa statistics were used. Where applicable, data are presented as mean ± SD. Data were analyzed using descriptive statistics, and tests with P < 0.05 were considered significant.
Ethical Statement
The ethics committee of our institute approved the study (no. 7303–62), and informed consent was obtained from all patients.
Results
A total 268 nodal stations of 69 patients were reviewed. Mean SUVearly, SUVdelayed, and RI of primary lung cancer lesions were 10.3 ± 5.3, 12.5 ± 6.5, and 20.7 ± 10.7 %, respectively. LN metastases were present in 209 nodal stations (78 %) of 57 patients (83 %). The final LN stages of NSCLC were N0 in 12, N1 in 10, N2 in 16, and N3 in 31 patients.
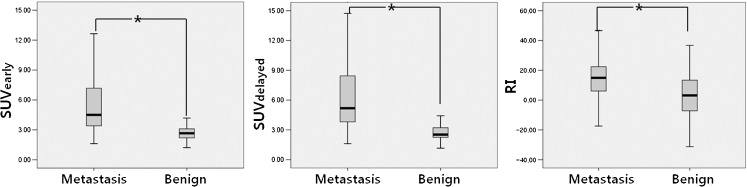
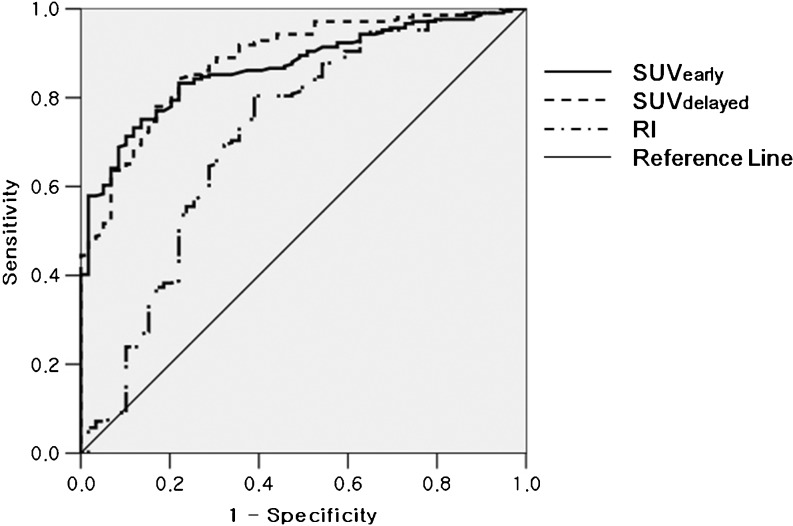
In LN-based analysis, SUVearly (5.9 ± 4.2 vs. 2.7 ± 0.7, P < 0.005), SUVdelayed (6.8 ± 5.0 vs. 2.8 ± 1.0, P < 0.005), and RI (15.1 ± 12.9 vs. 4.1 ± 16.5, P < 0.005) of metastatic LNs were significantly higher than those of non-metastatic LNs (Fig. 1). Figure 2 shows the ROC curves of three variables. The area under the ROC curve was largest in SUVdelayed, which was 0.884 (SUVearly, 0.868; RI, 0.717; P < 0.01). The optimal cutoff values of SUVearly, SUVdelayed, and RI were 3.4, 3.3, and 4.3, respectively. The diagnostic accuracy of three variables for diagnosing LN metastasis in patients with NSCLC was compared using the optimal cutoff values. SUVdelayed (accuracy, 82.8 %) was more accurate for detecting mediastinal LN metastases than SUVearly (77.6 %, P < 0.01) and RI (76.1 %, P < 0.01), and this was statistically significant (Table 1).
Fig. 1.
SUVs on the early scan (SUVearly, P < 0.001), SUVs on the delayed scan (SUVdelayed, P <0.001, and the retention index of SUVs (RI, P < 0.046) were compared according to the presence of metastasis in mediastinal lymph nodes
Fig. 2.
Receiver-operating characteristic (ROC) curves of SUVs on the early scan (SUVearly), SUVs on the delayed scan (SUVdelayed), and the retention index of SUVs (RI) are shown
Table 1.
Diagnostic performances of SUV parameters for detecting individual metastatic lymph nodes using the optimal cutoff values of 3.4, 3.3, and 4.3 for SUVearly, SUVdelayed, and RI, respectively
| Sensitivity (%) | Specificity (%) | Accuracy (%) | |
|---|---|---|---|
| SUV on early scan (SUVearly) | 75.1* | 86.4* | 77.6* |
| SUV on delayed scan (SUVdelayed) | 84.7 | 76.3 | 82.8 |
| Retention index of SUVs (RI) | 80.4 | 61.0* | 76.1* |
*P < 0.01 vs. SUVdelayed using McNemar test
In patient-based analysis, positive criteria for LN metastasis were 3.4, 3.3, and 4.3 for SUVearly, SUVdelayed, and RI, respectively. SUVdelayed had correctly determined LN stages in 55 of 69 patients (sensitivity, specificity, and accuracy = 88.7 %, 50.0 %, and 79.7 %), whereas SUVearly and RI correctly determined LN stages in 53 and 52 patients, respectively (Table 2). On the kappa statistic analysis, the final LN stage showed higher agreement with the LN stage of SUVdelayed (0.699, P < 0.001) than those of SUVearly (0.666) and RI (0.629).
Table 2.
Diagnostic performances of SUV parameters for detecting patients with metastatic lymph nodes using the optimal cutoff values of 3.4, 3.3, and 4.3 for SUVearly, SUVdelayed, and RI, respectively
| Sensitivity (%) | Specificity (%) | Accuracy (%) | |
|---|---|---|---|
| SUV on early scan (SUVearly) | 80.0* | 64.3* | 76.8* |
| SUV on delayed scan (SUVdelayed) | 88.7 | 50.0 | 79.7 |
| Retention index of SUVs (RI) | 88.2 | 38.9* | 75.4* |
*P < 0.01 vs. SUVdelayed using McNemar test
Discussion
In this study, the area under the ROC curve of SUVdelayed was larger than those of SUVearly and RI. This indicated that SUVdelayed is necessary to differentiate LNs with metastasis from normal and benign tissue, and DTP PET/CT is useful. The results showed that the diagnostic accuracy of SUVdelayed was superior to those of SUVearly and RI.
In most FDG PET/CT studies, imaging is performed 50–60 min after FDG injection; however, the uptake of FDG in malignancies is expected to increase over 1.5-5 h [13]. In theory, images obtained 2–3 h after FDG injection should show improved contrast between tumor and normal tissues or benign processes, because uptake is increased in the tumor and decreased in the normal background [14].
Kubota et al. reported that most malignant lesions, including primary lung cancer, mediastinal LN metastasis, and lymphoma, showed higher FDG uptake at 2 h than at 1 h after injection of FDG, while normal tissue, including lung and mediastinum, and benign lesions, except for sarcoidosis, showed lower uptake at 2 h than 1 h after injection, and that a delayed FDG PET scan provided better sensitivity than an early scan [5]. Nishiyama et al. also evaluated the diagnostic performance of DTP PET/CT for the diagnosis of LN metastasis in patients with NSCLC. They reported that DTP PET showed better (although not statistically significant) specificity, positive predictive value, and accuracy than early or delayed PET alone for LN staging in NSCLC [6]. Other studies also have described the advantages of DTP PET/CT for the evaluation of mediastinal LN metastasis [7, 8]. The present study demonstrated similar results, showing that SUVdelayed is more accurate than SUVearly in staging LN metastasis for NSCLC patients. However, RI was less accurate than SUVearly and SUVdelayed.
On the contrary, several studies reported that, at 1 h after FDG injection, STP PET/CT was sufficient for evaluating mediastinal LN metastasis [9–11]. In these studies, SUVdelayed showed comparable or superior diagnostic performances to SUVearly. However, SUVdelayed was not considered a useful variable to detect LN metastasis for the following reasons: (1) DTP PET/CT requires additional efforts, such as undergoing one more scan and longer waiting times for the patients [11]. (2) Although DTP PET/CT improves the differentiation between metastatic and benign LNs compared with STP PET/CT, a biopsy procedure is still required for accurate assessment of LN status [10]. However, if the diagnostic performance of DTP PET/CT in staging LN metastasis for NSCLC patients is superior to STP PET/CT, this could compensate for the aforementioned concerns.
A large meta-analysis reported that the average sensitivity and specificity for LN staging were approximately 85 % and 90 %, respectively [15]. The present study showed comparable sensitivity (84.7 %). The improved diagnostic accuracy of SUVdelayed compared to that of SUVearly was mainly caused by increased sensitivity (from 75.1 % to 84.7 %). Because of the increased uptake by tumors along with a decreased normal background, the malignant lesions area is more easily detected with delayed PET imaging [5]. In the present study, significantly higher SUVdelayed in metastatic LNs and reduced blood pool activity of mediastinum with time may make it easier to recognize small metastatic LNs.
However, the specificity was relatively low (76.3 %). The lower specificity may be related to inflammatory conditions in the lung, such as pulmonary tuberculosis. Our previous study had showed that benign LNs also showed increased FDG uptake in delayed PET/CT imaging [16].
Our study had several limitations. First, LNs were not always evaluated histopathologically. However, our pilot study, which only included histopathologically evaluated LN, showed similar results. Second, cutoff values of SUVearly, SUVdelayed, and RI were obtained from patients from a single institution, and this might not be useful for other institutions.
In the present study, comparing the diagnostic efficacy of SUVearly, SUVdelayed, and RI for LN staging in patients with NSCLC, SUVdelayed was the most accurate variable for LN staging. DTP PET/CT could provide improved diagnostic accuracy for the LN staging of NSCLC.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (no. 2011–0018362).
Conflict of Interest Statement
We declare that we have no conflict of interest.
References
- 1.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 2.Gupta NC, Tamim WJ, Graeber GG, Bishop HA, Hobbs GR. Mediastinal lymph node sampling following positron emission tomography with fluorodeoxyglucose imaging in lung cancer staging. Chest. 2001;120:521–527. doi: 10.1378/chest.120.2.521. [DOI] [PubMed] [Google Scholar]
- 3.Konishi J, Yamazaki K, Tsukamoto E, Tamaki N, Onodera Y, Otake T, et al. Mediastinal lymph node staging by FDG-PET in patients with non-small cell lung cancer: analysis of false-positive FDG-PET findings. Respiration. 2003;70:500–506. doi: 10.1159/000074207. [DOI] [PubMed] [Google Scholar]
- 4.Takamochi K, Yoshida J, Murakami K, Niho S, Ishii G, Nishimura M, et al. Pitfalls in lymph node staging with positron emission tomography in non-small cell lung cancer patients. Lung Cancer. 2005;47:235–242. doi: 10.1016/j.lungcan.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Kubota R, Kubota K, Yamada S, Tada M, Ido T, Tamahashi N. Microautoradiographic study for the differentiation of intratumoral macrophages, granulation tissues and cancer cells by the dynamics of fluorine-18-fluorodeoxyglucose uptake. J Nucl Med. 1994;35:104–112. [PubMed] [Google Scholar]
- 6.Nishiyama Y, Yamamoto Y, Kimura N, Ishikawa S, Sasakawa Y, Ohkawa M. Dual-time-point FDG-PET for evaluation of lymph node metastasis in patients with non-small-cell lung cancer. Ann Nucl Med. 2008;22:245–250. doi: 10.1007/s12149-007-0103-2. [DOI] [PubMed] [Google Scholar]
- 7.Shinya T, Rai K, Okumura Y, Fujiwara K, Matsuo K, Yonei T, et al. Dual-time-point F-18 FDG PET/CT for evaluation of intrathoracic lymph nodes in patients with non-small cell lung cancer. Clin Nucl Med. 2009;34:216–221. doi: 10.1097/RLU.0b013e31819a1f3d. [DOI] [PubMed] [Google Scholar]
- 8.Uesaka D, Demura Y, Ishizaki T, Ameshima S, Miyamori I, Sasaki M, et al. Evaluation of dual-time-point 18F-FDG PET for staging in patients with lung cancer. J Nucl Med. 2008;49:1606–1612. doi: 10.2967/jnumed.108.051250. [DOI] [PubMed] [Google Scholar]
- 9.Kasai T, Motoori K, Horikoshi T, Uchiyama K, Yasufuku K, Takiguchi Y, et al. Dual-time point scanning of integrated FDG PET/CT for the evaluation of mediastinal and hilar lymph nodes in non-small cell lung cancer diagnosed as operable by contrast-enhanced CT. Eur J Radiol. 2010;75:143–146. doi: 10.1016/j.ejrad.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 10.Suga K, Kawakami Y, Hiyama A, Sugi K, Okabe K, Matsumoto T, et al. Differential diagnosis between (18)F-FDG-avid metastatic lymph nodes in non-small cell lung cancer and benign nodes on dual-time point PET/CT scan. Ann Nucl Med. 2009;23:523–531. doi: 10.1007/s12149-009-0268-y. [DOI] [PubMed] [Google Scholar]
- 11.Yen RF, Chen KC, Lee JM, Chang YC, Wang J, Cheng MF, et al. 18F-FDG PET for the lymph node staging of non-small cell lung cancer in a tuberculosis-endemic country: is dual time point imaging worth the effort? Eur J Nucl Med Mol Imaging. 2008;35:1305–1315. doi: 10.1007/s00259-008-0733-1. [DOI] [PubMed] [Google Scholar]
- 12.Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–577. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- 13.Hamberg LM, Hunter GJ, Alpert NM, Choi NC, Babich JW, Fischman AJ. The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification? J Nucl Med. 1994;35:1308–1312. [PubMed] [Google Scholar]
- 14.Matthies A, Hickeson M, Cuchiara A, Alavi A. Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. J Nucl Med. 2002;43:871–875. [PubMed] [Google Scholar]
- 15.Gould MK, Kuschner WG, Rydzak CE, Maclean CC, Demas AN, Shigemitsu H, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med. 2003;139:879–892. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- 16.Kim DW, Kim CG. Dual-time point positron emission tomography findings of benign mediastinal lymph nodes in a tuberculosis-endemic region. Jpn J Radiol. 2011;29:682–687. doi: 10.1007/s11604-011-0613-7. [DOI] [PubMed] [Google Scholar]