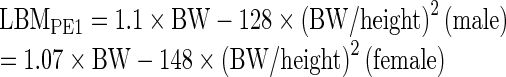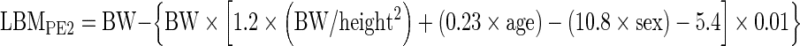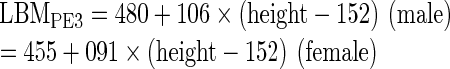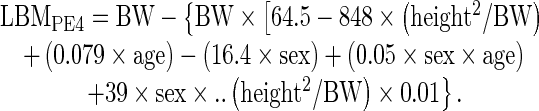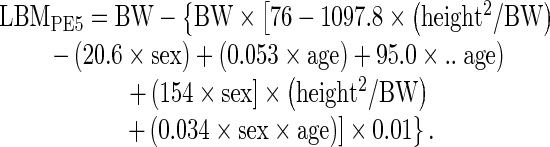Abstract
Purpose
Standardized uptake values (SUVs) normalized by lean body mass (LBM) determined by CT were compared with those normalized by LBM estimated using predictive equations (PEs) in normal liver, spleen, and aorta using 18F-FDG PET/CT.
Methods
Fluorine-18 fluorodeoxyglucose (F-FDG) positron emission tomography/computed tomography (PET/CT) was conducted on 453 patients. LBM determined by CT was defined in 3 ways (LBMCT1-3). Five PEs were used for comparison (LBMPE1-5). Tissue SUV normalized by LBM (SUL) was calculated using LBM from each method (SULCT1-3, SULPE1-5). Agreement between methods was assessed by Bland-Altman analysis. Percentage difference and percentage error were also calculated.
Results
For all liver SULCTs vs. liver SULPEs except liver SULPE3, the range of biases, SDs of percentage difference and percentage errors were −0.17-0.24 SUL, 6.15-10.17 %, and 25.07- 38.91 %, respectively. For liver SULCTs vs. liver SULPE3, the corresponding figures were 0.47-0.69 SUL, 10.90-11.25 %, and 50.85-51.55 %, respectively, showing the largest percentage errors and positive biases. Irrespective of magnitudes of the biases, large percentage errors of 25.07-51.55 % were observed between liver SULCT1-3 and liver SULPE1-5. The results of spleen and aorta SULCTs and SULPEs comparison were almost identical to those for liver.
Conclusion
The present study demonstrated substantial errors in individual SULPEs compared with SULCTs as a reference value. Normalization of SUV by LBM determined by CT rather than PEs may be a useful approach to reduce errors in individual SULPEs.
Keywords: Standardized uptake value, Normalization, Lean body mass, PET/CT
Introduction
Standardized uptake value (SUV) is a commonly used semiquantitative parameter in PET/CT studies valuable for diagnosis of various diseases and monitoring tumor response to therapy [1]. Many investigators have extensively studied biological and physical factors affecting numerators of the SUV equation such as region of interest (ROI) size, definition of ROI, image resolution and reconstruction algorithm, and uptake period [2]. Several studies have concerned denominator factors (i.e., normalization factors) such as body weight (BW), body surface area, lean body mass (LBM), and blood glucose level [3–9], in which LBM was estimated by various predictive equations (PEs). SUV normalized by BW (SUVBW) is known to be highly dependent on BW [3, 9]. SUV normalized by LBM (SUL) is typically more consistent across patients than SUVBW, as patients with high body mass indices have a relatively large amount of body fat or adipose tissue (AT) in which 18F-FDG does not significantly accumulate in the fasting state [3, 5]. Thus, the use of SUL has been recommended for PET response criteria in solid tumors [10]. However, the estimation of LBM relies on indirect measurements using various PEs, which are based on anthropometric parameters such as age, sex, body weight, and height. We chose five PEs which are commonly used in the PET community, and nutrition and obesity fields. These PEs were originally developed to estimate body fat mass based on reference methods including hydrodensitometry (underwater weighing), total body water measurements by isotope dilution technique, total body potassium counting, and total bone mineral measurement.
LBM (i.e., adipose tissue-free body mass) can be directly determined by CT in 18F-FDG PET/CT studies [11]. The CT method for quantifying AT volume or mass has been validated in animals [12], human cadavers [13, 14], and living humans [15, 16]. It is now considered the most accurate method available for direct measurement of AT volume in vivo [17, 18]. This CT measurement method has been widely used in obesity and nutrition research fields, but not yet in 18F-FDG PET/CT studies. The purpose of the present study was to compare SUVs normalized by LBM determined by CT with those normalized by LBM estimated using PEs in normal liver, spleen, and aorta in 18F-FDG PET/CT studies.
Materials and Methods
18F-FDG PET/CT Imaging
In total, 453 patients underwent 18F-FDG PET/CT examination (Biograph 16, Siemens, Knoxville, TN, USA) for routine clinical purposes. Patient characteristics are shown in Table 1. Approval by an institutional review board was not required for this routine clinical study. After 6-hour fasting, each patient was injected with approximately 7.4 MBq kg-1 of 18F-FDG after ensuring that blood glucose did not exceed 180 mg dL-1 (99.4 ± 17.4 mg dL-1). Patients were positioned feet first and supine with their arms laid beside the body, and imaging began 60 min after injection of 18F-FDG (58.8 ± 3.9 min). Most PET acquisition was performed from skull base to mid-thigh, and the following acquisition parameters were used: image matrix size of 168 × 168; reconstruction algorithm of ordered subset expectation maximization with four iterations and eight subsets, and post-smoothing with a Gaussian filter of 5 mm full width at half maximum (FWHM); and acquisition time of 2.5 min per bed position with 28 % overlap. The PET/CT system used in the present study allows 3D mode acquisition, 5.0 mm FWHM, and 16.2 cm trans-axial field of view.
Table 1.
Characteristics of Patients Studied in SUL Comparison (n = 453)
| Characteristics | Value (Range) |
|---|---|
| Men : Women | 233 : 220 |
| Age (yr) | 59.3 ± 13.4 ( 22 ~ 88) |
| Weight (kg) | 59.3 ± 10.74 (34 ~ 93) |
| Height (cm) | 160.3 ± 8.5 (137 ~ 185) |
| BMI (kg m-2) | 23.05 ± 3.43 (14.37 ± 37.03) |
BMI = body mass index; SUL = standardized uptake value normalized by lean body mass; Values are expressed as mean ± SD.
CT acquisition parameters were as follows: tube voltage of 120 kVp, tube current of 60 mAs (care dose), tube rotation speed of 0.5 s, table feed of 36 mm s-1, beam collimation of 1.5 mm, image matrix size of 512 × 512, and 5 mm slice thickness of reconstructed images with 3 mm spacing. Scans were acquired from the head to the feet. Neither intravenous nor oral contrast agent was administered. Blank CT scanning of the scanner table including head rest and abdominal binder was conducted three times to determine the number of voxels erroneously assigned to AT depending on patient height (500–700 cm3), and this volume was subtracted from patients’ total AT volume. Patient BW was corrected for hospital gown weight (approximately 610 g).
Measurements and Data Analysis
In the present study, AT was defined as voxels identified and measured by CT having a CT number between −140 and −30 Hounsfield units. A built-in software package was used to calculate total AT volume. An AT density of 0.95 kg L–1 and an AT fat content (fraction) of 80 % were applied to convert the AT volume to AT or fat mass [19].
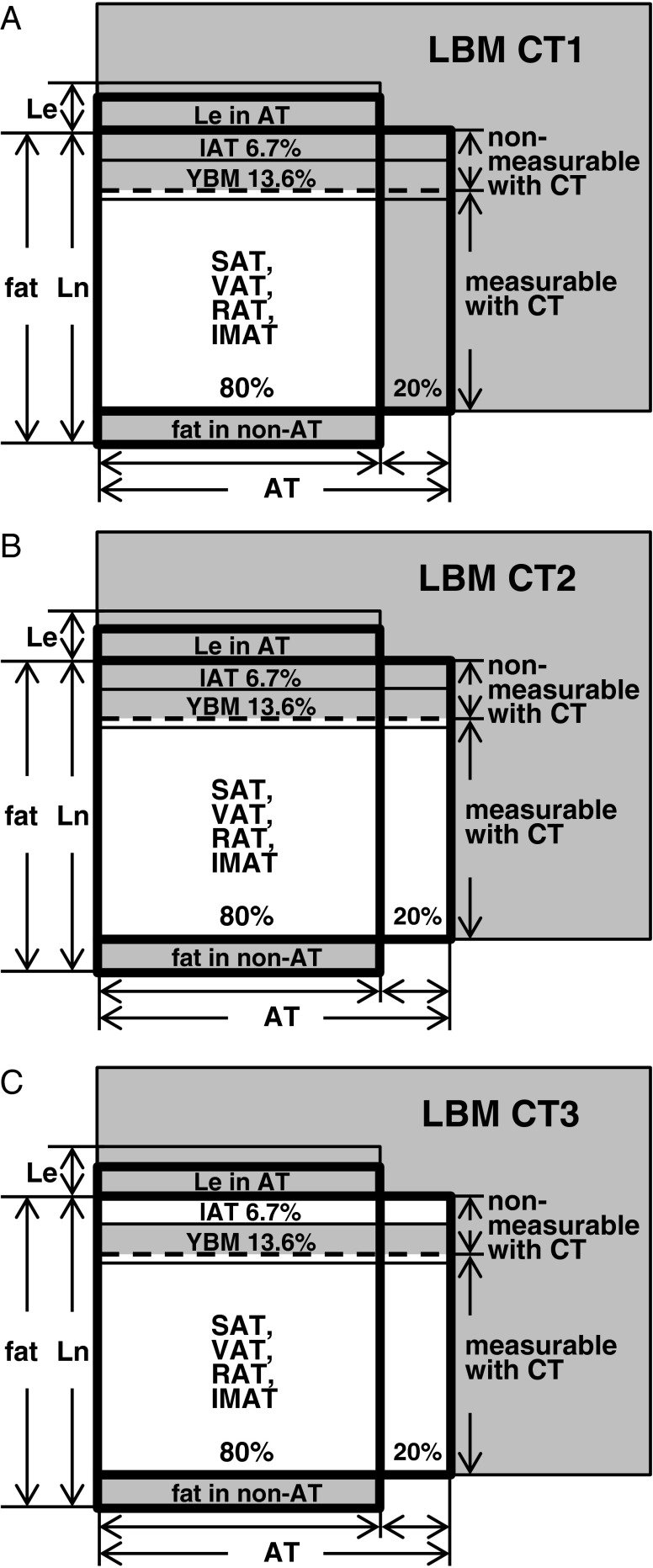
LBMs determined by CT were defined in the following 3 ways (Fig. 1):
Fig. 1.
Three LBMCT models for male were constructed based on collective data combined with reference values of the Reference Male and Reference Female. (a) LBMCT1 model underestimated body fat. (b) The amount of AT measurable with CT and total body fat estimated with LBM model CT2 was similar. (c) LBM model CT3 was corrected for IAT, which was not measured by CT. Areas of LBM CTs are actually larger than those illustrated. AT = adipose tissue; CT = computed tomography; IAT = interstitial AT; IMAT = intermuscular AT; LBM = lean body mass; Le = essential lipid; Ln = non-essential lipid; RAT = retroperitoneal AT; SAT = subcutaneous AT; VAT = visceral AT; YBM = yellow bone marrow
The following 5 different PEs were used for comparison PEs [20–23]:
where sex = 1 for male and 0 for female, BW is in kg, and height is in m in PE2, 4 and 5, and in cm in PE1 and 3.
ROIs were drawn in the liver at the level of liver segment 6 (3 cm circular ROI), in the spleen at the level of the splenic hilum (2 cm circular ROI), and in the aorta at the level of the pancreas (1.5 cm circular ROI), and each SUVBW was measured. Using LBMCTs and LBMPEs obtained as described above, each SUL was calculated by multiplying SUVBW by the LBM divided by BW.
Bland-Altman analysis was performed to test the agreement between SULCTs and SULPEs [24]. The differences between SULCT1 and SULPE1 in each organ were calculated in each patient (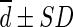 ), with bias defined as the mean difference (
), with bias defined as the mean difference (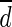 ). The limit of agreement was defined as
). The limit of agreement was defined as 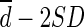 to
to 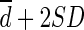 . To compare the magnitudes of the limit of agreement, the percentage error was calculated and defined as 100 × 4SD of differences divided by the average of global mean of SULCT1 and SULPE1 [25]. The percentage (relative) differences were calculated to compare the magnitudes of difference, which were defined as 100 × the difference between SULCT1 and SULPE1 divided by the mean of the two methods in each patient.
. To compare the magnitudes of the limit of agreement, the percentage error was calculated and defined as 100 × 4SD of differences divided by the average of global mean of SULCT1 and SULPE1 [25]. The percentage (relative) differences were calculated to compare the magnitudes of difference, which were defined as 100 × the difference between SULCT1 and SULPE1 divided by the mean of the two methods in each patient.
Analyses of SULCT1 vs. SULPE2-5 were conducted similarly. For SULCT2 vs. SULPE1-5 and SULCT3 vs. SULPE 1–5, the same analyses were also repeated.
Statistical Analysis
Statistical analysis was performed using SPSS version 18.0. P < 0.05 was considered statistically significant. The normal distribution of differences was tested using the Kolmogorov-Smirnov test. Most of the differences between SULCTs and SULPEs were approximately normally distributed, but differences showed somewhat skewed distribution. However, the overall interpretation of the results was not considered to be seriously disturbed.
Results
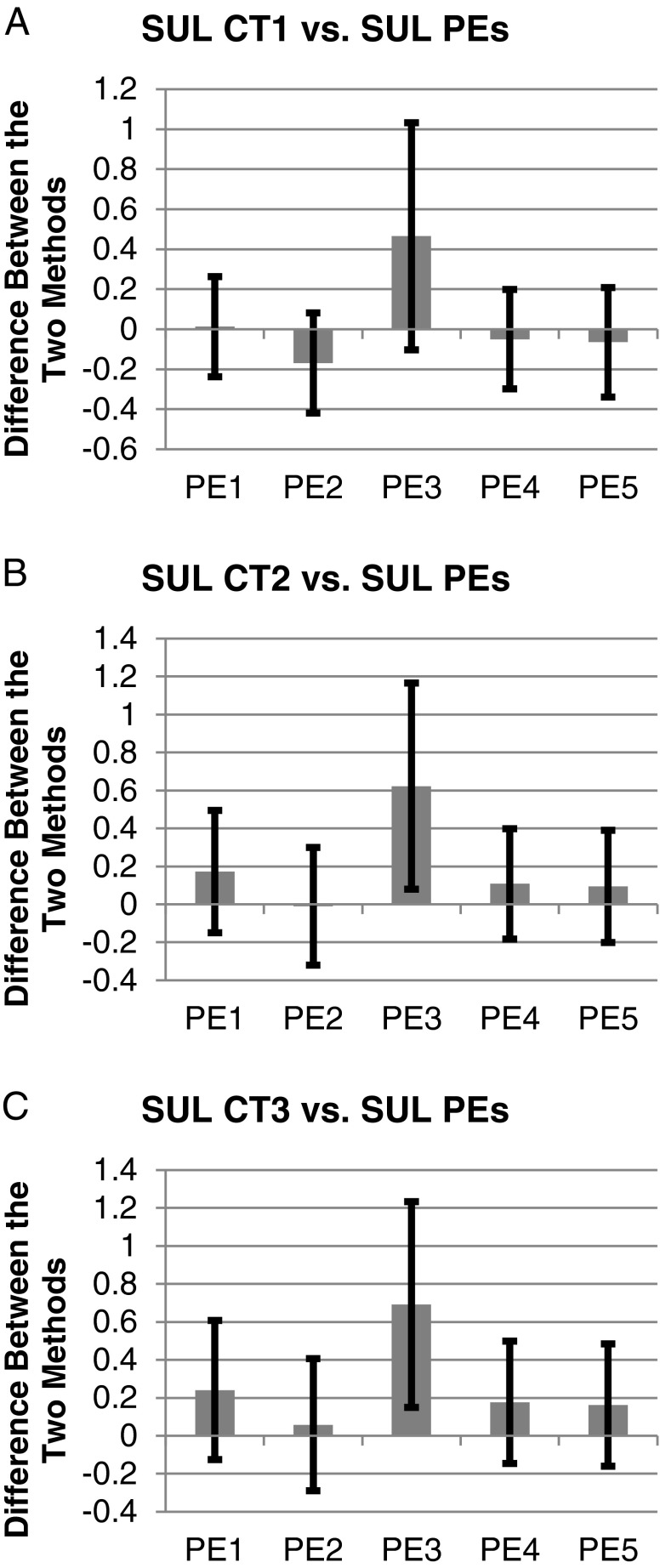
For all liver SULCTs vs. liver SULPEs except liver SULPE3, the range of biases, SDs of percentage difference and percentage errors were −0.17-0.24 SUL, 6.15-10.17 %, and 25.07-38.91 %, respectively, indicating large percentage errors and differing magnitudes of biases in liver SULPEs (Table 2; Fig. 2). For liver SULCTs vs. liver SULPE3, the corresponding figures were 0.47-0.69 SUL, 10.90-11.25 %, and 50.85-51.55 %, respectively, showing the largest percentage errors and positive biases compared with other liver SULPEs (Table 2; Fig. 2). Thus, liver SULPE3 was unsuitable for any of the SULCT models. Irrespective of the magnitudes of the biases, however, large percentage errors of 25.07-51.55 % were observed between all liver SULCTs vs. all liver SULPEs (Fig. 2).
Table 2.
Comparison of Liver SULCTs and Liver SULPEs (n = 453)
| Liver SUVbw = 2.65 ± 0.41 | SULPE1 | SULPE2 | SULPE3 | SULPE4 | SULPE5 |
|---|---|---|---|---|---|
| SULPEs | 2.00 ± 0.29 | 1.82 ± 0.29 | 2.45 ± 0.41 | 1.94 ± 0.30 | 1.92 ± 0.30 |
| SULCT1 (1.99 ± 0.30) | |||||
Difference (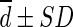 , SUL) , SUL) |
0.013 ± 0.125 | −0.169 ± 0.125 | 0.465 ± 0.284 | −0.050 ± 0.124 | −0.065 ± 0.137 |
| Limit of agreement (SUL) | −0.237 ~ 0.263 | −0.420 ~ 0.081 | −0.103 ~ 1.032 | −0.297 ~ 0.197 | −0.339 ~ 0.208 |
| Percentage error (%) | 25.07 | 26.34 | 51.15 | 25.18 | 28.00 |
| Percentage difference (%) | 0.80 ± 6.15 | −9.04 ± 6.65 | 20.61 ± 11.25 | −2.64 ± 6.20 | −3.42 ± 6.88 |
| SULCT2 (1.83 ± 0.30) | |||||
Difference (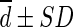 , SUL) , SUL) |
0.172 ± 0.161 | −0.010 ± 0.155 | 0.623 ± 0.272 | 0.108 ± 0.145 | 0.094 ± 0.148 |
| Limit of agreement (SUL) | −0.150 ~ 0.495 | −0.320 ~ 0.299 | 0.079 ~ 1.168 | −0.181 ~ 0.398 | −0.203 ~ 0.390 |
| Percentage error (%) | 33.69 | 33.94 | 50.85 | 30.72 | 31.58 |
| Percentage difference (%) | 9.32 ± 8.63 | −0.51 ± 8.56 | 29.00 ± 10.90 | 5.89 ± 7.66 | 5.11 ± 7.81 |
| SULCT3 (1.76 ± 0.31) | |||||
Difference (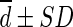 , SUL) , SUL) |
0.241 ± 0.183 | 0.058 ± 0.174 | 0.692 ± 0.271 | 0.177 ± 0.161 | 0.162 ± 0.161 |
| Limit of agreement (SUL) | −0.124 ~ 0.606 | −0.290 ~ 0.406 | 0.149 ~ 1.235 | −0.145 ~ 0.500 | −0.160 ~ 0.484 |
| Percentage error (%) | 38.85 | 38.91 | 51.55 | 34.90 | 34.96 |
| Percentage difference (%) | 13.29 ± 10.17 | 3.48 ± 9.92 | 32.89 ± 11.18 | 9.88 ± 8.90 | 9.10 ± 8.84 |
CT = computed tomography; Limit of agreement = 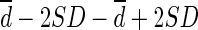 ; Percentage difference = difference / mean of two methods; Percentage error = 4SD / average of global mean of two methods; PE = predictive equation; SUL = standardized uptake value normalized by lean body mass; SUVbw = standardized uptake value normalized by body weight; Values are expressed as mean ± SD.
; Percentage difference = difference / mean of two methods; Percentage error = 4SD / average of global mean of two methods; PE = predictive equation; SUL = standardized uptake value normalized by lean body mass; SUVbw = standardized uptake value normalized by body weight; Values are expressed as mean ± SD.
Fig. 2.
Irrespective of biases (bars, mean differences), large limit of agreement (error bars, mean difference - 2SD to mean difference + 2SD) were observed between liver SULCT1 vs. liver SULPE1-5 (a), liver SULCT2 vs. liver SULPE1-5 (b) and liver SULCT3 vs. liver SULPE1-5 (c). CT = computed tomography; PE = predictive equation; SUL = standardized uptake value normalized by lean body mass
The results of comparison between SULCTs and SULPEs in the spleen and aorta were almost identical to those for liver SUL as follows: For all spleen SULCTs vs. spleen SULPEs, the range of biases, SDs of percentage difference and percentage errors were −0.13-0.54 SUL, 6.15-11.25 % and 25.42-55.93 %, respectively (Table 3). Corresponding figures for the aorta were −0.13-0.53 SUL, 6.15-11.25 % and 24.51-52.11 %, respectively, showing large percentage errors in individual SULPEs (Table 4).
Table 3.
Comparison of Spleen SULCTs and Spleen SULPEs (n = 453)
| Spleen SUVbw = 2.04 ± 0.32 | SULPE1 | SULPE2 | SULPE3 | SULPE4 | SULPE5 |
|---|---|---|---|---|---|
| SULPEs | 1.54 ± 0.23 | 1.40 ± 0.23 | 1.90 ± 0.35 | 1.50 ± 0.24 | 1.48 ± 0.25 |
| SULCT1 (1.53 ± 0.23) | |||||
Difference (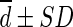 , SUL) , SUL) |
0.013 ± 0.098 | −0.130 ± 0.096 | 0.364 ± 0.233 | −0.037 ± 0.096 | −0.048 ± 0.107 |
| Limit of agreement (SUL) | −0.183 ~ 0.208 | −0.322 ~ 0.063 | −0.101 ~ 0.829 | −0.230 ~ 0.156 | −0.261 ~ 0.166 |
| Percentage error (%) | 25.42 | 26.24 | 54.30 | 25.48 | 28.33 |
| Percentage difference (%) | 0.80 ± 6.15 | −9.04 ± 6.65 | 20.61 ± 11.25 | −2.64 ± 6.20 | −3.42 ± 6.88 |
| SULCT2 (1.41 ± 0.23) | |||||
Difference ( , SUL) , SUL) |
0.135 ± 0.128 | −0.008 ± 0.121 | 0.486 ± 0.227 | 0.086 ± 0.115 | 0.075 ± 0.119 |
| Limit of agreement (SUL) | −0.122 ~ 0.392 | −0.249 ~ 0.234 | 0.033 ~ 0.940 | −0.145 ~ 0.316 | −0.162 ~ 0.312 |
| Percentage error (%) | 34.78 | 34.33 | 54.89 | 31.70 | 32.80 |
| Percentage difference (%) | 9.32 ± 8.63 | −0.51 ± 8.56 | 29.00 ± 10.90 | 5.89 ±7.66 | 5.11 ± 7.81 |
| SULCT3 (1.36 ± 0.23) | |||||
Difference (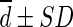 , SUL) , SUL) |
0.188 ± 0.146 | 0.045 ± 0.136 | 0.539 ± 0.227 | 0.139 ± 0.129 | 0.128 ± 0.130 |
| Limit of agreement (SUL) | −0.103 ~ 0.479 | −0.227 ~ 0.318 | 0.085 ~ 0.994 | −0.119 ~ 0.396 | −0.131 ~ 0.387 |
| Percentage error (%) | 40.15 | 39.45 | 55.93 | 36.08 | 36.47 |
| Percentage difference (%) | 13.29 ± 10.17 | 3.48 ± 9.92 | 32.88 ± 11.18 | 9.88 ± 8.90 | 9.10 ± 8.84 |
CT = computed tomography; Limit of agreement = 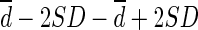 ; Percentage difference = difference / mean of two methods; Percentage error = 4SD / average of global mean of two methods; PE = predictive equation; SUL = standardized uptake value normalized by lean body mass; SUVbw = standardized uptake value normalized by body weight; Values are expressed as mean ± SD.
; Percentage difference = difference / mean of two methods; Percentage error = 4SD / average of global mean of two methods; PE = predictive equation; SUL = standardized uptake value normalized by lean body mass; SUVbw = standardized uptake value normalized by body weight; Values are expressed as mean ± SD.
Table 4.
Comparison of Aorta SULCTs and Aorta SULPEs (n = 453)
| Aorta SUVbw = 2.00 ± 0.33 | SULPE1 | SULPE2 | SULPE3 | SULPE4 | SULPE5 |
|---|---|---|---|---|---|
| SULPEs | 1.53 ± 0.24 | 1.39 ± 0.23 | 1.88 ± 0.34 | 1.48 ± 0.25 | 1.47 ± 0.25 |
| SULCT1 (1.52 ± 0.25) | |||||
Difference (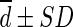 , SUL) , SUL) |
0.010 ± 0.093 | −0.131 ± 0.097 | 0.357 ± 0.218 | −0.039 ± 0.093 | −0.050 ± 0.103 |
| Limit of agreement (SUL) | −0.177 ~ 0.197 | −0.324 ~ 0.062 | −0.080 ~ 0.793 | −0.226 ~ 0.147 | −0.256 ~ 0.156 |
| Percentage error (%) | 24.51 | 26.56 | 51.45 | 24.86 | 27.51 |
| Percentage difference (%) | 0.80 ± 6.15 | −9.04 ± 6.65 | 20.61 ± 11.25 | −2.64 ± 6.20 | −3.42 ± 6.88 |
| SULCT2 (1.40 ± 0.25) | |||||
Difference ( , SUL) , SUL) |
0.131 ± 0.122 | −0.010 ± 0.118 | 0.478 ± 0.210 | 0.082 ± 0.109 | 0.071 ± 0.111 |
| Limit of agreement (SUL) | −0.112 ~ 0.374 | −0.245 ~ 0.226 | 0.057 ~ 0.898 | −0.135 ~ 0.299 | −0.152 ~ 0.294 |
| Percentage error (%) | 33.26 | 33.78 | 51.35 | 30.18 | 31.06 |
| Percentage difference (%) | 9.32 ± 8.63 | −0.51 ± 8.56 | 29.00 ± 10.90 | 5.89 ±7.66 | 5.11 ± 7.81 |
| SULCT3 (1.35 ± 0.26) | |||||
Difference (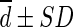 , SUL) , SUL) |
0.183 ± 0.138 | 0.043 ± 0.132 | 0.530 ± 0.210 | 0.134 ± 0.121 | 0.123 ± 0.121 |
| Limit of agreement (SUL) | −0.093 ~ 0.460 | −0.221 ~ 0.306 | 0.110 ~ 0.950 | −0.108 ~ 0.376 | −0.119 ~ 0.365 |
| Percentage error (%) | 38.42 | 38.57 | 52.11 | 34.24 | 34.41 |
| Percentage difference (%) | 13.29 ± 10.17 | 3.48 ± 9.92 | 32.88 ± 11.18 | 9.88 ± 8.90 | 9.10 ± 8.84 |
CT = computed tomography; Limit of agreement = 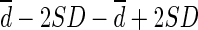 ; Percentage difference = difference / mean of two methods; Percentage error = 4SD / average of global mean of two methods; PE = predictive equation; SUL = standardized uptake value normalized by lean body mass; SUVbw = standardized uptake value normalized by body weight; Values are expressed as mean ± SD.
; Percentage difference = difference / mean of two methods; Percentage error = 4SD / average of global mean of two methods; PE = predictive equation; SUL = standardized uptake value normalized by lean body mass; SUVbw = standardized uptake value normalized by body weight; Values are expressed as mean ± SD.
Discussion
The purpose of the present study was to determine LBM by CT with a subsequent normalization of SUV and compare it with SUV normalized by LBM estimated by five different PEs. To test agreement between the two methods, Bland-Altman analysis was performed, which is known to be one of the most appropriate analytical methods for comparing clinical measurement methods [24]. The difference between the two methods and the limit of agreement in each patient were calculated. In addition, the percentage differences and percentage errors were calculated.
95 % of differences between SULCTs vs. SULPEs will lie between each mean difference 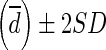 (more precisely,
(more precisely, 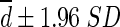 ). For example, liver SULPE1 was similar for patients A and B, i.e., 2.093 and 2.112, respectively. However, liver SULCT2 values for patients A and B were 1.684 and 2.254, respectively. The difference in liver SULCT2 between the two patients was 0.57. The difference between liver SULCT2 and SULPE1 for the whole group was 0.172 ± 0.161 (
). For example, liver SULPE1 was similar for patients A and B, i.e., 2.093 and 2.112, respectively. However, liver SULCT2 values for patients A and B were 1.684 and 2.254, respectively. The difference in liver SULCT2 between the two patients was 0.57. The difference between liver SULCT2 and SULPE1 for the whole group was 0.172 ± 0.161 (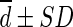 ; Table 2). Thus, the difference in liver SULCT2 between the two patients approached 4 SD (2 SD in both directions from the mean difference, i.e., percentage error of 33.69 %), as illustrated by Bland-Altman plot (Table 2; Fig. 3). The differences between liver SULCT2 and SULPE1 for patients A and B each lay, as expected, within 2 SD. In a contrasting case, liver SULPE1 values for patients C and D were 1.790 and 2.382, respectively. The difference in liver SULPE1 between the two patients was 0.592. Thus, the difference in liver SULPE1 between the two patients also approached 4 SD. However, liver SULCT2 values for patients C and D were almost identical: 1.893 and 1.894, respectively.
; Table 2). Thus, the difference in liver SULCT2 between the two patients approached 4 SD (2 SD in both directions from the mean difference, i.e., percentage error of 33.69 %), as illustrated by Bland-Altman plot (Table 2; Fig. 3). The differences between liver SULCT2 and SULPE1 for patients A and B each lay, as expected, within 2 SD. In a contrasting case, liver SULPE1 values for patients C and D were 1.790 and 2.382, respectively. The difference in liver SULPE1 between the two patients was 0.592. Thus, the difference in liver SULPE1 between the two patients also approached 4 SD. However, liver SULCT2 values for patients C and D were almost identical: 1.893 and 1.894, respectively.
Fig. 3.
Bland-Altman plot indicates the differences between the two methods against the mean of the two methods. The difference between the two methods for each patient lay within the 2 SD of the difference in 95 % of cases. However, the difference in LBM between 2 patients would approach 4 SD of the difference when the two patient values were 2 SD from the mean difference in opposite directions. CT = computed tomography; PE = predictive equation; SD = standard deviation; SUL = standardized uptake value normalized by lean body mass
Among SULPEs, SULPE3 was considerably overestimated, with a large percentage error compared with other SULPEs, because SULPE3 was normalized by ideal body weight (i.e., estimated by PE3). This observation suggests that SUV normalized by ideal body weight should be interpreted with caution.
More importantly, it should be noted that irrespective of the magnitudes of biases, large percentage errors ranging from 25.07 % to 51.55 % were observed between all SULCTs and all SULPEs.
SULCT1 was based on LBMCT1, which clearly underestimated body fat with a subsequent overestimation of LBM, because fat mass in AT measurable with CT is smaller than total AT fat mass including non-measurable AT (Fig. 1a). SULCT3 was based on LBMCT3, which was corrected for interstitial adipose tissue mass, but interstitial adipose tissue cannot be measured with CT, and large individual variability and uncertainty are associated with interstitial adipose tissue estimation (Fig. 1c). Hence, the SULCT2 model was considered most reasonable among SULCT models (Fig. 1b).
To our knowledge, the present study is the first report on the application of LBM determined by CT to normalize SUV in 18F-FDG PET/CT studies. Large errors in individual SULPEs are often encountered when applying PEs to individual patients, even though the magnitudes of mean difference (biases) are small over a whole population. Therefore, normalization of SUV by LBM determined by CT instead of PEs may be a useful approach for reducing errors found in individual SULPEs.
Conclusion
The present study demonstrated that substantial errors exist in individual SULPEs, when compared with SULCTs as a reference value. Normalization of SUV by LBM determined by CT rather than PEs may be a useful approach to reduce errors in individual SULPEs in quantitative 18F-FDG PET/CT examination.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2011–0018362).
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Park S, Ryu J-S, Oh S-J, Park S-I, Kim Y, Jung H-Y, et al. The Feasibility of 18F-Fluorothymidine PET for Prediction of Tumor Response after Induction Chemotherapy Followed by Chemoradiotherapy with S-1/Oxaliplatin in Patients with Resectable Esophageal Cancer. Nucl Med Mol Imaging. 2012;46:57–64. doi: 10.1007/s13139-011-0118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50(Suppl 1):11S–20S. doi: 10.2967/jnumed.108.057182. [DOI] [PubMed] [Google Scholar]
- 3.Zasadny KR, Wahl RL. Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose: variations with body weight and a method for correction. Radiology. 1993;189:847–50. doi: 10.1148/radiology.189.3.8234714. [DOI] [PubMed] [Google Scholar]
- 4.Yeung HW, Sanches A, Squire OD, Macapinlac HA, Larson SM, Erdi YE. Standardized uptake value in pediatric patients: an investigation to determine the optimum measurement parameter. Eur J Nucl Med Mol Imaging. 2002;29:61–6. doi: 10.1007/s00259-001-0662-8. [DOI] [PubMed] [Google Scholar]
- 5.Sugawara Y, Zasadny KR, Neuhoff AW, Wahl RL. Reevaluation of the standardized uptake value for FDG: variations with body weight and methods for correction. Radiology. 1999;213:521–5. doi: 10.1148/radiology.213.2.r99nv37521. [DOI] [PubMed] [Google Scholar]
- 6.Menda Y, Bushnell DL, Madsen MT, McLaughlin K, Kahn D, Kernstine KH. Evaluation of various corrections to the standardized uptake value for diagnosis of pulmonary malignancy. Nucl Med Commun. 2001;22:1077–81. doi: 10.1097/00006231-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer–a PET study. J Nucl Med. 1993;34:1–6. [PubMed] [Google Scholar]
- 8.Langen KJ, Braun U, Rota Kops E, Herzog H, Kuwert T, Nebeling B, et al. The influence of plasma glucose levels on fluorine-18-fluorodeoxyglucose uptake in bronchial carcinomas. J Nucl Med. 1993;34:355–9. [PubMed] [Google Scholar]
- 9.Kim CK, Gupta NC, Chandramouli B, Alavi A. Standardized uptake values of FDG: body surface area correction is preferable to body weight correction. J Nucl Med. 1994;35:164–7. [PubMed] [Google Scholar]
- 10.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim CG, Kim WH, Kim DW. Direct determination of individual lean body mass by using CT image data in 18F-FDG PET/CT studies: Comparison with lean body mass estimated by using various predictive equations. J Nucl Med. 2011;52(Suppl 1):391. [Google Scholar]
- 12.Ross R, Leger L, Guardo R, De Guise J, Pike BG. Adipose tissue volume measured by magnetic resonance imaging and computerized tomography in rats. J Appl Physiol. 1991;70:2164–72. doi: 10.1152/jappl.1991.70.5.2164. [DOI] [PubMed] [Google Scholar]
- 13.Rossner S, Bo WJ, Hiltbrandt E, Hinson W, Karstaedt N, Santago P, et al. Adipose tissue determinations in cadavers–a comparison between cross-sectional planimetry and computed tomography. Int J Obes. 1990;14:893–902. [PubMed] [Google Scholar]
- 14.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–22. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 15.Sjostrom L, Kvist H, Cederblad A, Tylen U. Determination of total adipose tissue and body fat in women by computed tomography, 40 K, and tritium. Am J Physiol. 1986;250:E736–45. doi: 10.1152/ajpendo.1986.250.6.E736. [DOI] [PubMed] [Google Scholar]
- 16.Seidell JC, Bakker CJ, van der Kooy K. Imaging techniques for measuring adipose-tissue distribution–a comparison between computed tomography and 1.5-T magnetic resonance. Am J Clin Nutr. 1990;51:953–7. doi: 10.1093/ajcn/51.6.953. [DOI] [PubMed] [Google Scholar]
- 17.Ross R. Advances in the application of imaging methods in applied and clinical physiology. Acta Diabetol. 2003;40(Suppl 1):S45–50. doi: 10.1007/s00592-003-0025-y. [DOI] [PubMed] [Google Scholar]
- 18.Mattsson S, Thomas BJ. Development of methods for body composition studies. Phys Med Biol. 2006;51:R203–28. doi: 10.1088/0031-9155/51/13/R13. [DOI] [PubMed] [Google Scholar]
- 19.Martin AD, Daniel MZ, Drinkwater DT, Clarys JP. Adipose tissue density, estimated adipose lipid fraction and whole body adiposity in male cadavers. Int J Obes Relat Metab Disord. 1994;18:79–83. [PubMed] [Google Scholar]
- 20.Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: age- and sex-specific prediction formulas. Br J Nutr. 1991;65:105–14. doi: 10.1079/BJN19910073. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 22.Morgan DJ, Bray KM. Lean body mass as a predictor of drug dosage. Implications for drug therapy. Clin Pharmacokinet. 1994;26:292–307. doi: 10.2165/00003088-199426040-00005. [DOI] [PubMed] [Google Scholar]
- 23.James WPT. Research on obesity : a report of the DHSS/MRC group. London: H.M.S.O; 1976. p. 9. [Google Scholar]
- 24.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–60. doi: 10.1191/096228099673819272. [DOI] [PubMed] [Google Scholar]
- 25.Hanneman SK. Design, analysis, and interpretation of method-comparison studies. AACN Adv Crit Care. 2008;19:223–34. doi: 10.1097/01.AACN.0000318125.41512.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]