Abstract
Purpose
To assess the value of PET/CT for detecting local or distant recurrence in patients who undergo surgery for colorectal cancer (CRC) and to compare the accuracy of PET/CT to that of conventional imaging studies (CIS).
Methods
Tumor surveillance PET/CT scans done between March 2005 and December 2009 of disease-free patients after surgery with or without adjuvant chemotherapy for CRC were retrospectively studied. CIS (serial enhanced CT from lung base to pelvis and plain chest radiograph) were performed within 1 month of PET/CT. We excluded patients with distant metastasis on initial staging, a known recurrent tumor, and a lack of follow-up imaging. The final diagnosis was based on at least 6 months of follow-up with colonoscopy, biopsy, and serial imaging studies in combination with carcinoembryonic antigen levels.
Results
A total of 262 PET/CT scans of 245 patients were included. Local and distant recurrences were detected in 27 cases (10.3%). On case-based analysis, the overall sensitivity, specificity, and accuracy were 100, 97.0, and 97.3% for PET/CT and 85.1, 97.0, and 95.8% for CIS, respectively. On lesion-based analysis, PET/CT detected more lesions compared to CIS in local recurrence and lung metastasis. PET/CT and CIS detected the same number of lesions in abdominal lymph nodes, hepatic metastasis, and peritoneal carcinomatosis. PET/CT detected two more metachronous tumors than did CIS in the lung and thyroid gland.
Conclusion
PET/CT detected more recurrences in patients who underwent surgery for CRC than did CIS and had the additional advantage of evaluating the entire body during a single scan.
Keywords: Colorectal cancer, PET/CT, Surveillance
Introduction
Colorectal cancer (CRC) is the third most common cancer in both sexes in South Korea and constitutes 12.7% of all cancers [1]. Although radical resection followed by chemotherapy and/or radiotherapy is an effective treatment, unexpected recurrence occurs in 30–50% of patients during follow-up [2–5]. It has often been noted that earlier detection of local recurrence or distant metastasis would allow for more adequate treatment in patients with CRC. Identification of a secondary primary tumor as well as early detection of recurrence or metastasis is also crucial for determining the most appropriate therapeutic management in patients with cancer. In clinical practice, the postoperative status of patients with CRC is evaluated by physical examination, colonoscopy, carcinoembryonic antigen (CEA) level, and imaging studies including abdominal CT, MRI, and chest radiographs. Of these imaging modalities, abdominal CT is widely used, especially for locoregional recurrence, abdominopelvic lymph node metastasis, or hepatic metastasis, but occult lesions may be difficult to visualize. In addition, its field of view is limited to the pulmonary basal portion for the detection of lung metastasis.
The clinical usefulness of 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) or PET/CT has been well established in patients with CRC for staging and follow-up [6–8]. Several studies have demonstrated that the diagnostic performance of PET/CT is superior to that of other imaging techniques [9–11]. However, these studies focused mainly on patients with either suspected recurrence or a conspicuous finding using other imaging modalities. According to the National Comprehensive Cancer Network (NCCN), clinical practice guidelines in oncology, routine PET, or PET/CT scans are not recommended for routine surveillance in patients with CRC [12].
Very few studies have considered whether PET/CT in postoperative surveillance programs affects the rate of detection in disease-free patients with CRC. In a prospective study by Sobhani et al. [13] in which 130 patients who had undergone curative surgery were randomized to undergo either PET or a conventional workup during follow-up, among all patients with recurrence, the time until detection of recurrence was significantly shorter in the group that had undergone additional PET scanning than in the group that had undergone only the conventional workup.
In the present study, we assessed the value of PET/CT as a routine surveillance tool for detecting local and distant recurrence and secondary primary tumors in patients who undergo surgery for CRC and compared the accuracy of PET/CT to that of conventional imaging studies (CIS).
Materials and Methods
Patient Population and Study Design
Tumor surveillance PET/CT scans of disease-free patients from March 2005 to December 2009 after surgery with or without adjuvant chemotherapy for CRC were retrospectively studied. Patients were eligible if they underwent CIS within 1 month of PET/CT. CIS included an enhanced CT from the lung base to the pelvis and a plain chest radiograph. We excluded patients with distant metastasis on initial staging, a known recurrent tumor, and a lack of follow-up imaging. Patient characteristics are detailed in Table 1. The final postoperative TNM stage was based on the Seventh American Joint Committee on Cancer Staging guidelines. This study was approved by the institutional review board at our institution. Informed consent was waived because of the retrospective design of this study.
Table 1.
Clinicopathologic characteristics of the patients with CRC in our study
| Characteristics | Value |
|---|---|
| Male/female (n) | 143/102 |
| Mean ± SD (range) age (years) | 60 ± 11 (32–86) |
| Location of primary CRC (n) | |
| Colon | 134 |
| Rectum | 111 |
| Stage of primary CRC (n) | |
| Stage I | 27 |
| Stage II | 50 |
| Stage III | 107 |
| Unknown | 61 |
| Mean interval time from operation to PET/CT scan (months) | 32.6 (3–297) |
18F- FDG PET/CT Scan and Interpretation
All patients fasted for at least 6 h before the PET/CT study. 18F-FDG was injected intravenously (370–555 MBq) and scanning began 60 min later. None of the patients had blood glucose levels >130 mg/dL before the injection. No intravenous contrast agent was administered. Studies were acquired on combined PET/CT inline systems, either Biograph Duo or Biograph TruePoint (Siemens Medical Solutions, Knoxville, TN). The acquisition time was 2–3 min per bed position. All patients were in a supine position with their arms raised. CT began at the orbitomeatal line and progressed to the upper thigh (130 kVp, 80 mA, and 5-mm slice thickness; 120 kVp, 50 mA, and 5-mm slice thickness). PET followed immediately over the same body region. The CT data were used for attenuation correction, and images were reconstructed using a standard ordered-subset expectation maximization (OSEM) algorithm. The axial spatial resolution was 6.5 or 4.5 mm at the center of the field of view.
Interpretation and Analysis
All PET/CT images were reviewed at a workstation with fusion software (Syngo; Siemens Medical Solutions, Knoxville, TN) that provided multiplanar reformatted images and displayed PET images after attenuation correction, CT images, and PET/CT fusion images. Both PET/CT and CIS images were closely reviewed retrospectively for the presence of local or distant recurrence by consensus of two nuclear medicine physicians who are board-certified in both nuclear medicine and radiology.
The following PET/CT findings were considered recurrence: (1) newly developed lesion that showed significant, nonphysiologic radiotracer accumulation relative to the surrounding tissue, (2) increase in nonphysiologic activity compared to the prior exam, (3) newly developed lung lesion regardless of increased radiotracer uptake. In the same manner, newly developed abnormal density or opacity on the enhanced abdominal CT or plain chest radiograph was considered recurrence.
The presence of local recurrence or metastatic disease was assessed in the following body regions: (1) abdominal and/or pelvic lymph node, (2) liver, (3) peritoneum, (4) lung, (5) local tumor site, and (6) other. We performed case-based and tumor site-based analyses for both PET/CT and CIS.
Final Diagnosis
In all patients, the standard of reference was based on histopathologic results and at least 6 months of follow-up with colonoscopy or serial imaging studies in combination with CEA levels. The imaging follow-up comprised PET/CT, abdominal CT, MRI, a plain chest radiograph, and CT. For cases in which histopathologic confirmation was not possible, the final diagnosis was made by follow-up colonoscopy or imaging studies. For example, a lesion with suspicion of malignancy was considered positive for malignant involvement if it resolved after chemotherapy or demonstrated progression on follow-up imaging. A lesion suspicious for malignancy that remained unchanged for at least 6 months of follow-up imaging was presumed to be negative for malignancy. Lesions that spontaneously resolved without further therapy were also considered benign.
Statistical Analysis
The sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of PET/CT and CIS for the detection of local recurrence or distant metastasis were calculated in a case-based analysis. The McNemar test was used to compare the diagnostic accuracy of PET/CT to that of CIS. A p-value of <0.05 was considered to be statistically significant. Statistical analysis was performed using MedCalc 11.1 (MedCalc Software).
Results
Overall, a total of 262 PET/CT scans of 245 patients were included. Repeat PET/CT scans were also included for 16 patients. All patients were evaluated using CIS such as enhanced abdominal CT from the lung base to the pelvis and plain chest radiography within 1 month of PET/CT.
Case-based Analysis
In the final analysis, local recurrence or distant metastasis was confirmed in 27 (10.3%) of 262 cases. Of these, 20 cases were confirmed by pathologic examination and 7 by follow-up imaging studies. As indicated in Table 2, 27 (100%) of the 27 cases with metastases were detected by PET/CT, and 23 (85.1%) of the 27 cases were detected by CIS. Four metachronous tumors were classified as true lesions. The results of our study show that the sensitivity, specificity, and accuracy of PET/CT (100, 97, and 97.3%, respectively) for the diagnosis of local recurrence or distant metastasis in patients with CRC were superior to those of CIS (85.1, 97, and 95.8%, respectively). However, no significant difference was found between the diagnostic performances of PET/CT and CIS (p = 0.1172). For PET/CT, 27 cases were true positive, 228 were true negative, and the remaining 7 were false positive. For CIS, 23 cases were true positive, 228 were true negative, 7 were false positive, and 4 were false negative. In terms of tumor stage, among the patients with recurrent CRC, 14 patients had stage III (52%), 2 had stage II (7%), 1 had stage I (4%), and the remaining 10 had an unknown stage (37%).
Table 2.
Case-based diagnostic accuracy of PET/CT and CIS for detection of recurrent or metastatic disease
| Performance value | PET/CT (%) | CIS (%) |
|---|---|---|
| Sensitivity | 100 (27/27) | 85.1 (23/27) |
| Specificity | 97.0 (228/235) | 97.0 (228/235) |
| Positive predictive value | 79.4 (27/34) | 76.6 (23/30) |
| Negative predictive value | 100 (228/228) | 98.2 (228/232) |
| Accuracy | 97.3 (255/262) | 95.8 (251/262) |
Lesion-based Analysis
Overall, 36 suspicious tumor sites were identified with either PET/CT or CIS. Twenty-four of these sites had true positive findings for recurrent disease, and four had true positive findings for metachronous tumors. Table 3 shows the diagnostic accuracy of PET/CT and CIS by body region. Recurrent tumor sites were located in the region of the colorectal anastomosis site (n = 6), metastatic abdominal or pelvic lymph nodes (n = 3), liver (n = 8), peritoneum (n = 4), and lung (n = 3). False-positive and false-negative findings of PET/CT and CIS according to body region are shown in Table 4. PET/CT detected two more recurrent tumors compared to CIS in the abdominal and extra-abdominal areas. That in the abdomen was histologically confirmed to be a local recurrence in the rectal anastomosis site (Fig. 1), and the remaining tumor in the extra-abdominal area was a pulmonary metastasis in the right upper lobe (Fig. 2). No tumors were correctly identified by CIS that had been missed by PET/CT. Both PET/CT and CIS showed false-positive findings in eight lesions: acute inflammation in the presacral space and small bowel mesentery in three cases, reactive hyperplasia of the abdominal or pelvic lymph nodes in three cases, inflammation of the spleen in one case (Fig. 3), and an inflammatory pulmonary nodule in one case (Fig. 4). The incidentally detected metachronous tumors were thyroid cancer, hepatocellular carcinoma, bronchioalveolar carcinoma, and pancreatic cancer. Of four metachronous tumors, two (thyroid cancer and bronchioalveolar carcinoma) were revealed only by PET/CT.
Table 3.
Distribution of the recurrences and accuracy of PET/CT and CIS
| Lesion sites | No. of recurrences (n = 28) | Sensitivity of PET/CT | Sensitivity of CIS |
|---|---|---|---|
| Lymph nodes | 3 | 100% (3/3) | 100% (3/3) |
| Liver | 8 | 100% (8/8) | 100% (8/8) |
| Peritoneal seeding | 4 | 100% (4/4) | 100% (4/4) |
| Lung | 3 | 100% (3/3) | 67% (2/3) |
| Local recurrence | 6 | 100% (6/6) | 83% (5/6) |
| Metachronous tumor | 4 | 100% (4/4) | 50% (2/4) |
Table 4.
False-positive and false-negative findings of PET/CT and CIS according to body region
| Lesion sites | PET/CT | CIS | ||
|---|---|---|---|---|
| False positive (n = 8) | False negative (n = 0) | False positive (n = 8) | False negative (n = 4) | |
| Abdominal lymph nodes | 3 | 0 | 3 | 0 |
| Lung | 1 | 0 | 1 | 1 |
| Local recurrence | 3 | 0 | 3 | 1 |
| Spleen | 1 | 0 | 1 | 0 |
| Metachronous tumor | 0 | 0 | 0 | 2 |
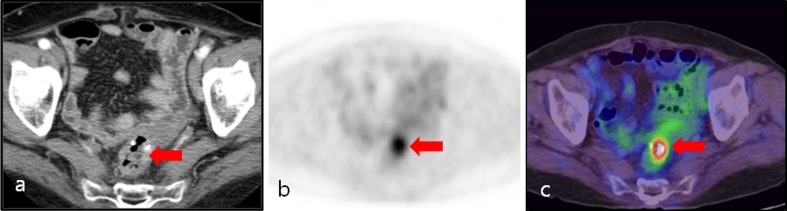
Fig. 1.
An 80-year-old male postoperative rectosigmoid colon cancer patient. a Contrast-enhanced axial CT image showed no evidence of abnormal wall thickening or a mass-like lesion at the anastomosis site. b, c Axial PET and PET/CT fusion images showed intense focal FDG uptake (arrow) around the rectal anastomosis site (SUVmax: 6.1). This finding was reported to be a recurrent tumor. Local tumor recurrence was confirmed by histopathological examination of the surgical specimen
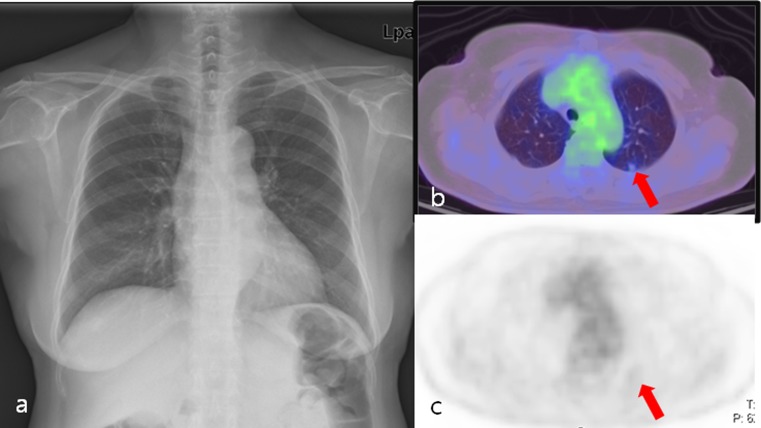
Fig. 2.
A 70-year-old female postoperative rectal cancer patient. a Chest X-ray showed no abnormal findings. b, c Axial PET/CT fusion and PET images showed a solitary nodule (arrow) with faintly perceptible FDG uptake in the left upper lobe (SUVmax: 0.8). Wedge resection confirmed metastatic adenocarcinoma
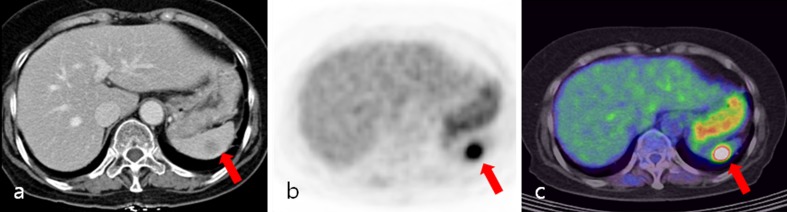
Fig. 3.
A 65-year-old female patient with cancer of the descending colon, postoperatively. a Contrast-enhanced axial CT image showed a 1.4 × 1.3 cm hypodense nodule (arrow) in the spleen. This finding was reported to be a metastatic tumor. b, c Axial PET and PET/CT fusion images showed intense focal FDG uptake (arrow) in the spleen (SUVmax: 10.8). This finding was reported to be a metastatic tumor. Splenectomy confirmed chronic inflammation
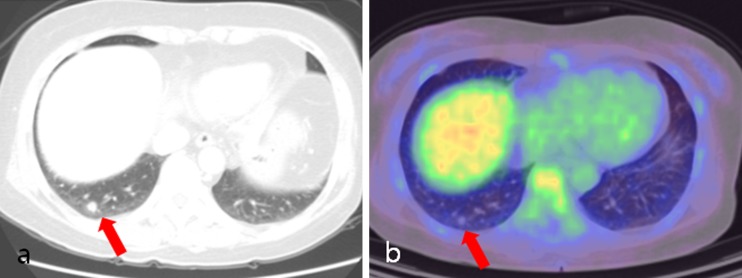
Fig. 4.
A 65-year-old female patient with cancer of the descending colon, postoperatively. a Axial lung setting image of abdominal CT showed a solitary nodule (arrow) in the right lower lobe. b Axial PET/CT fusion image showed a solitary nodule (arrow) without FDG uptake in the right lower lobe. Wedge resection confirmed organizing pneumonia
Discussion
Many studies have demonstrated the role of 18F-FDG PET or integrated PET/CT in the treatment and follow-up of patients with CRC [7, 14–16]. However, in most of these, the objective was to assess the ability of PET or PET/CT to visualize a recurrence that was clinically already highly suspected. The present study used PET/CT to monitor disease-free patients with CRC after surgery with or without chemotherapy. In our study, which comprised 245 patients and an 8.7% prevalence of disease (23 patients), PET/CT detected tumor recurrence in all 23 patients (100%). In four patients (1.5%), second primary tumors were incidentally detected by PET/CT.
Recently, the role of PET/CT for detection of recurrence in patients with CRC, and with elevated CEA levels in particular, has been discussed extensively in the literature [16–20]. The reported sensitivity has ranged from 89 to 98% and the specificity has ranged from 83 to 96%. The results of the present study showed that PET/CT was highly sensitive (100%) in the identification of local recurrence and distant metastasis. The difference between these results and those of previous studies may have arisen from the lower prevalence of recurrence (8.7%) and larger number of patients (n = 245) in our study. The relatively low disease prevalence in the present study compared to previous studies may be because this study included only disease-free patients with CRC and excluded patients with known or suspected recurrent CRC. We believe that the greater values of diagnostic performance in our study have important clinical value, although there was no significant difference in the sensitivity, specificity, and accuracy between PET/CT and CIS.
Lee et al. [20] evaluated 63 PET/CT cases involving patients with suspected recurrent CRC according to diverse diagnostic workups and reported that the sensitivity of PET/CT is superior to that of conventional imaging workups, including abdominal CT, for the detection of recurrent CRC (94.9 vs. 56.4%, respectively; p < 0.0001). In addition, Mester et al. [10] reported that PET/CT has a higher sensitivity than multidetector CT in patients with CRC and elevated CEA levels for the detection of recurrence (97.3 vs. 70.3%, respectively; p < 0.0001). However, we did not find a significant difference in sensitivity between PET/CT and CIS. Because these previous studies initially enrolled CRC patients with suspected recurrent CRC or increased CEA levels, the relatively high pretest probability of recurrence before PET/CT overestimated the sensitivity of PET/CT compared to a conventional imaging workup or multidetector CT. Taking into account the utility of surveillance PET/CT in our study, we can conclude that both PET/CT and CIS are applicable to disease-free patients with CRC for follow-up.
In our study, no false-negative cases of PET/CT were found, whereas four cases, including two metachronous malignancies, were false negative for CIS. In one case, PET/CT detected local recurrence in the rectal anastomosis site, which was missed by enhanced abdominal CT imaging. This result is explained by the difficulty in identifying attenuation differences between recurrent tumors and postoperative changes in anastomosis sites on enhanced abdominal CT images. The remaining three lesions were located in the extra-abdominal areas, which was not included in the field of view on the abdominal CT. There was one metastatic nodule and two metachronous tumors (bronchioalveolar carcinoma and thyroid cancer). In clinical practice, PET/CT inspects the body from the head to the pelvic floor and has the advantage of imaging the entire body during a single scan. Therefore, besides the regions of primary interest, various lesions are incidentally detected in PET/CT. One example is pulmonary nodules with or without FDG-avidity. In our study, PET/CT detected pulmonary nodules that were suggestive of metastasis in five cases. Of these, four were confirmed to be metastasis. In two cases, abdominal CT missed the pulmonary nodules located in the upper lobe because of the field of view. The remaining two cases showed the pulmonary nodules in the basal portion, which were detected by both PET/CT and CIS.
The specificities of PET/CT and CIS in the detection of recurrent CRC were identical in case-based analysis (97%). Similarly, Mester et al. [10] did not find a significant difference in the specificity between PET/CT and multidetector CT (94.4 vs. 94.4%). They mentioned that resolving hematomas, fat necrosis, and granulation tissue accompanying increased FDG uptake caused false-positive findings on PET/CT. In a review of 262 PET/CT scans of the patients with CRC in our study, 23% (8/35) of suspicious lesions on PET/CT were confirmed to be benign lesions. Although the CT portion of PET/CT provides assistance in terms of anatomic detail, it can often be difficult to differentiate between recurrent tumors and inflammation or infectious conditions associated with increased FDG uptake. The most frequent location of false-positive PET/CT findings was the abdomen (n = 6), where it is well known that physiological or inflammatory bowel conditions or reactive lymph nodes can mimic malignant foci. The other lesion was located in the spleen (n = 1). False-positive uptake in the spleen can be associated with many inflammatory or hematopoietic diseases because of activation of the immune system in the white pulp or compensatory expansion of the red marrow [21–23]. Lastly, an inflammatory nodule in the lung (n = 1) was falsely interpreted to be a single metastatic nodule from CRC. This nodule was very small and had faintly perceptible FDG uptake. Small pulmonary nodules with little or no FDG uptake are known to cause difficulty in determining whether they have malignant potential. Regardless of the perceptibility of FDG uptake, pulmonary malignancy should be considered, especially if there is no accompanying benign lung lesion [24, 25].
Several studies have reported that PET findings have a significant impact on management of patients with suspected recurrent CRC [26–28]. Given the retrospective nature of our study, the direct clinical influence of the greater detection of tumor recurrence could not be accurately determined. However, early detection of either recurrence or a second primary tumor may result in changes in treatment intent and may potentially confer a survival advantage. Among the 23 patients with recurrent disease that PET/CT detected, 1 patient underwent surgical intervention with curative intent, 8 underwent chemotherapy and/or radiotherapy, and 13 underwent surgical resection followed by chemoradiation therapy. The one remaining patient was not treated with any modality because of refusal of chemotherapy. Of four patients with newly diagnosed second primary tumors, three were treated surgically and one was treated with transcatheter arterial chemoembolization and radiofrequency ablation for HCC. A total of 26 patients who were referred for a subsequent treatment strategy were evaluated correctly by PET/CT. Furthermore, of these 26 patients, 4 (15%) would have otherwise missed the chance for appropriate treatment had they undergone CIS without PET/CT. In particular, approximately 50% of the patients with recurrent CRC showed stage III disease. Of four recurrent tumors correctly identified by PET/CT but missed by CIS, three were stage III. Therefore, in patients with advanced-stage disease, surveillance PET/CT may be mandatory.
Limitations of our study include its retrospective nature. We could not accurately examine the impact of PET/CT on the management of patients with recurrent CRC. Second, histopathological confirmation of PET/CT-positive or CIS-positive lesions could not be performed in all cases.
Conclusion
Both PET/CT and CIS were effective for the detection of recurrences in patients who underwent surgery for CRC. However, in addition to evaluating the region of primary interest, PET/CT had the advantage of evaluating the entire body during a single scan and positively impacted the management of patients with CRC with unexpected recurrence.
References
- 1.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011;43(1):1–11. doi: 10.4143/crt.2011.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson AB., 3rd The hope for today—the promise for tomorrow: will oncologists meet the challenge? J Clin Oncol. 2007;25(16):2156–2158. doi: 10.1200/JCO.2006.09.9838. [DOI] [PubMed] [Google Scholar]
- 3.Desch CE, Benson AB, 3rd, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23(33):8512–8519. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 4.Ariyan CE, Salem RR. Evolution in the treatment of metastatic colorectal carcinoma of the liver. World J Gastroenterol. 2006;12(20):3253–3258. doi: 10.3748/wjg.v12.i20.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandes LC, Kim SB, Saad SS, Matos D. Value of carcinoembryonic antigen and cytokeratins for the detection of recurrent disease following curative resection of colorectal cancer. World J Gastroenterol. 2006;12(24):3891–3894. doi: 10.3748/wjg.v12.i24.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruers TJ, Langenhoff BS, Neeleman N, Jager GJ, Strijk S, Wobbes T, et al. Value of positron emission tomography with [F-18]fluorodeoxyglucose in patients with colorectal liver metastases: a prospective study. J Clin Oncol. 2002;20(2):388–395. doi: 10.1200/JCO.20.2.388. [DOI] [PubMed] [Google Scholar]
- 7.Flamen P, Hoekstra OS, Homans F, Van Cutsem E, Maes A, Stroobants S, et al. Unexplained rising carcinoembryonic antigen (CEA) in the postoperative surveillance of colorectal cancer: the utility of positron emission tomography (PET) Eur J Cancer. 2001;37(7):862–869. doi: 10.1016/S0959-8049(01)00049-1. [DOI] [PubMed] [Google Scholar]
- 8.Flanagan FL, Dehdashti F, Ogunbiyi OA, Kodner IJ, Siegel BA. Utility of FDG-PET for investigating unexplained plasma CEA elevation in patients with colorectal cancer. Ann Surg. 1998;227(3):319–323. doi: 10.1097/00000658-199803000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huebner RH, Park KC, Shepherd JE, Schwimmer J, Czernin J, Phelps ME, et al. A meta-analysis of the literature for whole-body FDG PET detection of recurrent colorectal cancer. J Nucl Med. 2000;41(7):1177–1189. [PubMed] [Google Scholar]
- 10.Metser U, You J, McSweeney S, Freeman M, Hendler A. Assessment of tumor recurrence in patients with colorectal cancer and elevated carcinoembryonic antigen level: FDG PET/CT versus contrast-enhanced 64-MDCT of the chest and abdomen. AJR Am J Roentgenol. 2010;194(3):766–771. doi: 10.2214/AJR.09.3205. [DOI] [PubMed] [Google Scholar]
- 11.Yoon H, Lee J, Kim Y, Kim S. FDG-PET/CT is superior to enhanced CT in detecting recurrent subcentimeter lesions in the abdominopelvic cavity in colorectal cancer. Nucl Med Mol Imaging. 2011;45(2):132–138. doi: 10.1007/s13139-011-0082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood EH. The National Comprehensive Cancer Network (NCCN) J Med Libr Assoc. 2004;92(3):382–383. [Google Scholar]
- 13.Sobhani I, Tiret E, Lebtahi R, Aparicio T, Itti E, Montravers F, et al. Early detection of recurrence by 18FDG-PET in the follow-up of patients with colorectal cancer. Br J Cancer. 2008;98(5):875–880. doi: 10.1038/sj.bjc.6604263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi O, Saito N, Koda K, Sarashina H, Nakajima N. Clinical assessment of positron emission tomography for the diagnosis of local recurrence in colorectal cancer. Br J Surg. 1999;86(7):932–937. doi: 10.1046/j.1365-2168.1999.01178.x. [DOI] [PubMed] [Google Scholar]
- 15.Valk PE, Abella-Columna E, Haseman MK, Pounds TR, Tesar RD, Myers RW, et al. Whole-body PET imaging with [18F]fluorodeoxyglucose in management of recurrent colorectal cancer. Arch Surg. 1999;134(5):503–511. doi: 10.1001/archsurg.134.5.503. [DOI] [PubMed] [Google Scholar]
- 16.Even-Sapir E, Parag Y, Lerman H, Gutman M, Levine C, Rabau M, et al. Detection of recurrence in patients with rectal cancer: PET/CT after abdominoperineal or anterior resection. Radiology. 2004;232(3):815–822. doi: 10.1148/radiol.2323031065. [DOI] [PubMed] [Google Scholar]
- 17.Votrubova J, Belohlavek O, Jaruskova M, Oliverius M, Lohynska R, Trskova K, et al. The role of FDG-PET/CT in the detection of recurrent colorectal cancer. Eur J Nucl Med Mol Imaging. 2006;33(7):779–784. doi: 10.1007/s00259-006-0072-z. [DOI] [PubMed] [Google Scholar]
- 18.Shamim SA, Kumar R, Halanaik D, Shandal V, Reddy RM, Bal CS, et al. Role of FDG-PET/CT in detection of recurrent disease in colorectal cancer. Nucl Med Commun. 2010;31(6):590–6. [DOI] [PubMed]
- 19.Kyoto Y, Momose M, Kondo C, Itabashi M, Kameoka S, Kusakabe K. Ability of 18F-FDG PET/CT to diagnose recurrent colorectal cancer in patients with elevated CEA concentrations. Ann Nucl Med. 2010;24(5):395–401. [DOI] [PubMed]
- 20.Lee JH, Park SG, Jee KN, Park DG, Namgung H, Song IH. Performance of FDG PET/CT in postoperative colorectal cancer patients with a suspected recurrence and a normal CEA level. Nucl Med Commun. 2010;31(6):576–82. [DOI] [PubMed]
- 21.Liu Y. Clinical significance of diffusely increased splenic uptake on FDG-PET. Nucl Med Commun. 2009;30(10):763–769. doi: 10.1097/MNM.0b013e32832fa254. [DOI] [PubMed] [Google Scholar]
- 22.Tomas MB, Tronco GG, Karayalcin G, Palestro CJ. 22. FDG uptake in infectious mononucleosis. Clin Positron Imaging. 2000;3(4):176. doi: 10.1016/S1095-0397(00)00088-1. [DOI] [PubMed] [Google Scholar]
- 23.Lustberg MB, Aras O, Meisenberg BR. FDG PET/CT findings in acute adult mononucleosis mimicking malignant lymphoma. Eur J Haematol. 2008;81(2):154–156. doi: 10.1111/j.1600-0609.2008.01088.x. [DOI] [PubMed] [Google Scholar]
- 24.Kernstine KH, Grannis FW, Rotter AJ. Is there a role for PET in the evaluation of subcentimeter pulmonary nodules? Semin Thorac Cardiovasc Surg. 2005;17(2):110–114. doi: 10.1053/j.semtcvs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 25.JH O, Yoo Ie R, Kim SH, Sohn HS, Chung SK. Clinical significance of small pulmonary nodules with little or no 18F-FDG uptake on PET/CT images of patients with nonthoracic malignancies. J Nucl Med. 2007;48(1):15–21. [PubMed] [Google Scholar]
- 26.Kalff V, Hicks RJ, Ware RE, Hogg A, Binns D, McKenzie AF. The clinical impact of (18)F-FDG PET in patients with suspected or confirmed recurrence of colorectal cancer: a prospective study. J Nucl Med. 2002;43(4):492–499. [PubMed] [Google Scholar]
- 27.Scott AM, Gunawardana DH, Kelley B, Stuckey JG, Byrne AJ, Ramshaw JE, et al. PET changes management and improves prognostic stratification in patients with recurrent colorectal cancer: results of a multicenter prospective study. J Nucl Med. 2008;49(9):1451–1457. doi: 10.2967/jnumed.108.051615. [DOI] [PubMed] [Google Scholar]
- 28.Meta J, Seltzer M, Schiepers C, Silverman DH, Ariannejad M, Gambhir SS, et al. Impact of 18F-FDG PET on managing patients with colorectal cancer: the referring physician’s perspective. J Nucl Med. 2001;42(4):586–590. [PubMed] [Google Scholar]