Abstract
Purpose
Recent studies have been conducted on the relationship between fluorodeoxyglucose (FDG) uptake in F-18 FDG PET/CT and prognosis in patients with pancreatic cancer, but these studies have been carried out in small numbers of patients. The aim of this retrospective study was to determine in a large number of patients whether glucose metabolism as assessed by F-18 FDG PET/CT provides prognostic information independent of established prognostic factors in patients with pancreatic cancer.
Methods
We reviewed retrospectively the medical records of 165 patients (men 105, women 60, mean age 67 ± 10 years) with a diagnosis of pancreatic cancer that had undergone F-18 FDG PET/CT as part of a pretreatment workup from January 2004 to December 2009. Subsequently, all patients underwent surgery, cyberknife, radiotherapy, and/or chemotherapy. For the analysis, patients were classified by age, demographic data, maximum standardized uptake value (SUVmax), size, location, serum level of CA19-9, type of treatment, and AJCC stage. The relationship between FDG uptake and survival was analyzed using the Kaplan-Meier with log-Rank test and Cox’s proportional-hazard regression methods.
Results
Median survival for all 165 study subjects was 290 days and median SUV by PET/CT was 5.8 (range: 0–25.1). Patients were allocated to high (> 4.1) and low (≤4.1) SUV groups, and median survivals of these patients were 229 days and 610 days, respectively, which were significantly different (p < 0.0001). Furthermore, SUVmax was found to be significantly related to survival in each stage, i.e., there were 1267 days in stage I, 440 days in stage II, 299 days in stage III, and 143 days in stage IV (p < 0.0001). The median survival was also found to be significantly related to tumor size (p = 0.001), site (p = 0.0298), serum level of CA19-9 (p = 0.0017), distant metastasis (p < 0.0001), and type of treatment (p < 0.0001). Multivariate analysis study revealed that the patients with a low SUV (p = 0.0298), a lower serum level of CA19-9 (p = 0.0071), a lower stage (p = 0.0017), and no distant metastasis (p < 0.0001) had longer survivals. In addition, SUVmax values were found to have a similar hazard ratio of distant metastasis; it was well known predictor. Furthermore, SUVmax values showed a higher hazard ratio than that of other clinicopathologic predictors.
Conclusion
The present study shows that SUVmax on F-18 FDG PET/CT can provide a prognostic information in patients with pancreatic cancer.
Keywords: Survival, FDG, PET/CT, Pancreatic cancer
Introduction
Pancreatic cancer accounts for 2.4 % of the incidence rate of all cancers, and is the ninth most common cancer with recorded incidence rate of 8 per 100,000. In Korea and the USA, pancreatic cancer has incidence rankings and five-year survival rates of 8th and 4th, and 8.0 % and 5.6 %, respectively [1, 2]. It is well known that pancreatic cancer has a poor prognosis because of the difficulty of detecting the primary tumor in early stage and the aggressive characteristics of the disease. Most patients with advanced stage disease are unsuitable for curative surgery, and thus, it is important that subgroups of patients that may benefit from aggressive therapy (surgery, chemotherapy, radiotherapy) be accurately identified [3–5]. Accordingly, prognostic factors that permit the identification of patients likely to benefit from treatment are of clinical relevance. 18-Fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) is a relatively recent, noninvasive imaging technique based on the increased glucose uptake by malignant cells [6, 7]. PET has been proposed for the diagnosis and staging of various malignancies, including pancreatic carcinoma. Furthermore, F-18 FDG PET can detect tumors earlier than conventional imaging, and can be used to evaluate tumor aggressiveness and predict prognosis. Recent studies have been conducted on the relationship between FDG uptake in F-18 FDG PET/CT and prognosis, but these studies have limited statistical power due to small patient numbers. Accordingly, the aim of this study was to determine in a large series of patients whether glucose metabolism assessed by F-18 FDG PET/CT provides prognostic information independent of established prognostic factors in patients with pancreatic cancer.
Methods
Patients and Methods
From January 2004 to December 2009, 165 patients underwent a PET scan as part of a preoperative workup for pancreatic cancer. Informed consent was obtained from all patients. Mean patient age was 67 years (range 29 to 90 years), and the male:female ratio was 105:60. In 85 patients the presence of pancreatic ductal adenocarcinoma was histologically proven using surgical specimens or by percutaneous fine needle biopsy. Pathologic diagnoses and tumor classifications were made according to the American Joint Committee on Cancer (AJCC) staging system. The other 80 patient were diagnosed clinically using serum CA19-9 level and by imaging, such as CT, MRI, and/or EUS. The clinical and pathologic records of all patients were reviewed, and the following were analyzed: age, sex, TNM stage, SUVmax, tumor size and location, type of treatment (surgery, cyberknife, chemotherapy or radiation therapy), and CA 19-9 serum levels before treatment (RIA; serum reference 37 U/ml).
PET/CT Scanning
All patients fasted for at least six h before F-18 FDG administration, which was injected intravenously at 10–12 mCi (370–444 MBq) one h prior to imaging. Blood sugar levels were measured prior to injecting F-18 FDG. A non-enhanced low-dose CT scan was obtained for attenuation correction because all patients underwent a contrast enhanced abdominopelvic CT scan before the FDG PET/CT scan. The CT portion of the Discovery LS consisted of a multidetector helical scanner (LightSpeed Plus; General Electric Medical Systems) and a 6 slice CT (Biograph 6). Imaging parameters were as follows for acquisition in 5–7 bed positions: Discovery LS; 140 kV, 80 mA, 0.8 s per CT rotation, a pitch of six, a table speed of 22.5 mm/s, 722.5–1011.5 mm coverage, and a 31.9 s–37 s acquisition time; and for Biograph 6; 130 kV, 30 mA, 0.6 s per CT rotation, a pitch of 1.5, and an acquisition time of 20.89 s. The CT scan was performed before emission PET scans. CT tube current was adjusted according to patient weight, and CT data were resized from a 512 × 512 matrix to a 128 × 128 matrix to match the PET data in order to generate a CT transmission map and to fuse images. PET emission data were acquired for 5–7 bed positions, typically from the base of the skull through the upper thigh. Emission data were acquired for six min in each bed position. Each bed had 35 (for Discovery LS) or 39 (for Biograph 6) scanning planes with a 14.6 cm (for Discovery LS) or 16.2 cm (for Biograph 6) longitudinal field of view and a 1-slice overlap. PET images were reconstructed using CT for attenuation correction using the ordered-subsets expectation maximization algorithm (two iterations, eight subsets) and a 5-mm Gaussian filter using a 128 × 128 matrix.
Image Analysis
All PET/CT scans were examined retrospectively by three observers on an interactive computer display using fusion software (Xeleris; General Electric Medical Systems and Syngo; Siemens Medical Solutions). This software allows the review of PET, CT, and fused data using transaxial, sagittal, and coronal displays. To perform quantitative analysis, the standardized uptake values (SUV) were calculated in suspected neoplastic foci (SUV tissue tracer concentration/injected dose/body weight). For SUV analysis, a circular region of interest was placed over the area of maximal focal FDG uptake suspected to be a tumorous focus, and the maximal values were obtained.
Statistical Analysis
Statistical analysis was performed using Medcalc software v. 11.3. All values are expressed as means ± SDs. Patients were stratified and analyzed by univariate analysis with respect to age, sex, pre-treatment serum cancer antigen (CA)19-9 level, tumor size, tumor location, type of treatment, AJCC stage and the SUVmax of the primary lesion. Patients were classified into low SUVmax and high SUVmax subgroups by log-rank test. Survival time was defined as time from the pre treatment FDG-PET study and death. Overall cumulative survival was analyzed using the Kaplan-Meier method, and differences in survival between subgroups were compared using the log-rank test.
P values of <0.05 were considered to be statistically significant. Variables affecting survival with a P value of < 0.05 by univariate analysis were included in the multivariate analysis, which was performed using a Cox proportional hazard model.
Results
Patient Characteristics
Characteristics of the patients are summarized in Table 1. The mean age of the 165 patients was 67 ± 10 years (range 29-90 years; 105 males and 60 females). Overall median survival study was 290 days, and mean SUVmax was 5.8 (range : 0–25.1). The median CA 19–9 level for the all patients was 174.5 U/mL, and mean tumor size in which patients was 3 cm. In terms of AJCC stage, there were 19 patients in stage I, 28 in stage II, 65 in stage III, and 53 in stage IV. Among 165 patients, 28 underwent surgery, 35 cyberknife, 40 chemotherapy, 19 radiotherapy and 43 non-anticancer treatment (pain control or supportive care).
Table 1.
Standardized uptake values and clinicopathologic factors
| low SUV (< 4.1) | high SUV (≥ 4.1) | P value | |
|---|---|---|---|
| SUV* (mean ± SD) | 2.9 ± 1.3 | 6.3 ± 3.2 | |
| Median survival (days) | 229 | 610 | |
| Age (mean ± SD, years) | 66 ± 10.5 | 67 ± 9.8 | 0.26 |
| Sex | |||
| Male | 30 | 74 | 0.17 |
| Female | 11 | 50 | |
| Stage | |||
| I | 13 | 6 | |
| II | 8 | 20 | <0.0001 |
| III | 13 | 52 | |
| IV | 7 | 46 | |
| Site | |||
| head | 25 | 65 | 0.67 |
| body & tail | 16 | 59 | |
| Pathologic proven | 29 | 60 | |
| Clinical diagnosis | 12 | 64 | |
| CA19-9 (mean ± SD, U/ml) | 55 ± 5941 | 240 ± 10469 | <0.0001 |
| Size (mean ± SD, cm) | 2.5 ± 1.23 | 3.2 ± 1.14 | 0.0001 |
| Treatment | |||
| operation | 15 | 13 | |
| cyberknife | 6 | 29 | 0.0004 |
| radiotherapy | 4 | 15 | |
| chemotherapy | 3 | 37 | |
| Etc. | 13 | 30 |
*SUV = Standardized uptake value
Comparison of Survivals According to SUVmax
Cutoff value of the SUVmax for the 165 patients was 4.1 (41 patients had a SUV of ≦ 4.1 and 124 had a SUV of > 4.1). Median survivals for patients with a SUVmax of ≦ 4.1 or > 4.1 were 610 and 229 days, respectively (p < 0.0001). Furthermore, these two groups differed significantly with regard to tumor size, distant metastasis, tumor stage, and type of treatment. In addition, patient of stages 2 to 4 had a higher mean SUVmax than those of stage I. Similar numbers of patients with SUVmax of < or > 4.1 underwent surgery, but patients that did not undergo surgery had much higher SUVmax than patients who underwent surgery. We were able to find a statistical difference in survival and SUVmax between surgical and non-surgical groups (p < 0.0001)
Survival Analysis
Kaplan-Meier curves were drawn for patients with SUVmax of < or > 4.1. By univariate analysis, age (p = 0.029), SUVmax (p < 0.0001), tumor stage (p < 0.0001), serum level of CA19–9 (p = 0.0004), tumor size (p = 0.001), type of treatment (p < 0.0001) and distant metastasis (p < 0.0001) were significantly related to overall survival (Table 2 and Fig. 1). However, multivariate analysis showed that only SUVmax (p = 0.0008), age (p = 0.027), serum level of CA19–9 (p = 0.021), tumor stage (p = 0.0016), and distant metastasis (p < 0.0001) were independently related to overall survival (Table 3). In particular, the hazard ratios of SUVmax and distant metastasis for overall survival were 2.1 (95 % CI 1.3745–3.3090) and 2.28 (95 % CI 1.5428–3.3634), respectively.
Table 2.
Univariate analysis of clinicopathologic factors and survival
| Variable | No of patient | median survival ± SD (days) | P value |
|---|---|---|---|
| Age | |||
| <67 | 82 | 319 | 0.029 |
| ≥67 | 83 | 272 | |
| sex | |||
| male | 104 | 299 | 0.526 |
| female | 61 | 286 | |
| SUVmax* | |||
| <4.1 | 41 | 610 | <0.0001 |
| ≥4.1 | 124 | 229 | |
| Stage | |||
| I | 19 | 1267 | |
| II | 28 | 440 | <0.0001 |
| III | 65 | 299 | |
| IV | 53 | 143 | |
| CA19-9 | |||
| <174.5 | 82 | 401 | 0.0004 |
| ≥174.5 | 83 | 225 | |
| Size | |||
| <3.0 | 56 | 454 | 0.001 |
| ≥3.0 | 109 | 229 | |
| Treatment | |||
| operation | 28 | 1267 | |
| cyberknife | 35 | 313 | <0.0001 |
| radiotherapy | 19 | 290 | |
| chemotherapy | 40 | 171 | |
| Etc. | 43 | 221 | |
| M factor† | |||
| no | 112 | 374 | <0.0001 |
| yes | 53 | 143 |
*SUVmax = Maximum standardized uptake value
†M factor = Distant metastasis
Fig. 1.
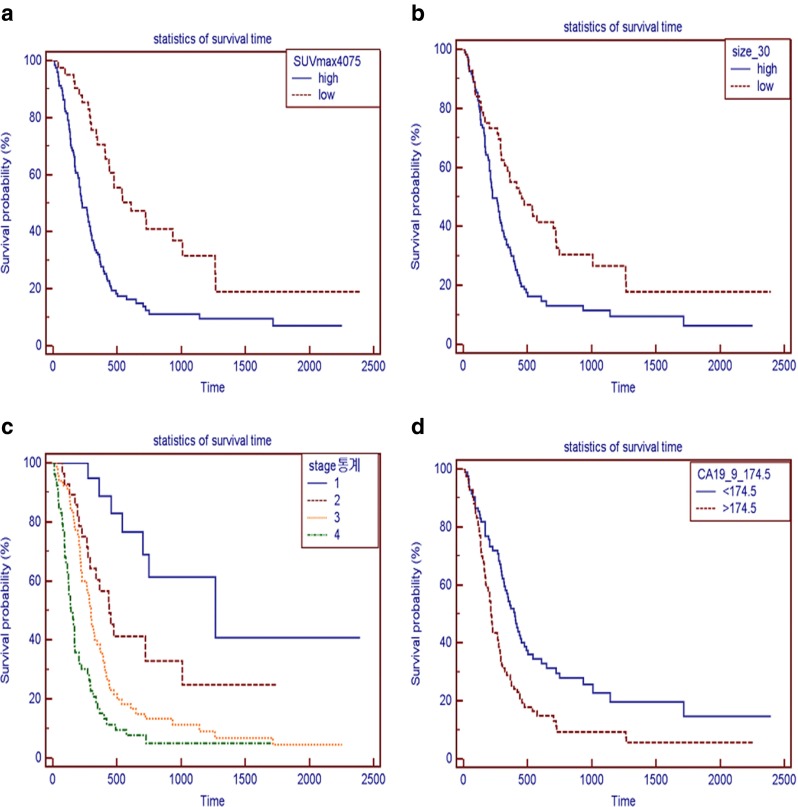

a. Survival curves of patients with standardized uptake values (SUV) of > 4.1 (124 patients, unbroken line) or ≦ 4.1 (41 patients, broken line). * P value < 0.0001. b Survival curves of patients with a tumor size > 3.0 cm (109 patients, unbroken line) or ≦ 3.0 cm (56 patients, broken line). * P value < 0.001. c Survival curves for patients of tumor stage I (19 patients, unbroken line), II (28 patients, broken line), III (65 patients, dotted line), or IV (53 patients, broken and dotted line) * p value < 0.0001. d Survival curves of patients with serum CA19-9 levels of > 174.5 (83 patients, broken line) or ≦ 174.5 (82 patients, unbroken line). * P value < 0.004. e Survival curves of patients with (n = 53, broken line) or without (n = 113, unbroken line) distant metastasis * p value < 0.0001. f Survival curves of patients with age > 67 (82 patients, broken line) or ≦ 67 (83 patients, unbroken line). * p-value < 0.029. g Survival curves by treatment type: surgery (28 patients, broken line), cyberknife surgery (35 patients, unbroken line), radiotherapy (19 patients, broken and one dotted line), chemotherapy (35 patients, dotted line), and other treatments (48 patients, broken and two dotted line) * p-value < 0.0001
Table 3.
Multivariate analysis using Cox proportional hazard regression model
| covariable | hazard ratio | 95 % confidence interval | P value |
|---|---|---|---|
| SUVmax† | 2.1326 | 1.3745 to 3.3090 | 0.0008 |
| age | 1.5078 | 1.0502 to 2.1648 | 0.0268 |
| CA19-9 | 1.5131 | 1.0668 to 2.1462 | 0.0208 |
| M factor* | 2.2779 | 1.5428 to 3.3634 | <0.0001 |
| stage | 1.7975 | 0.6812 to 0.9786 | 0.0016 |
| treatment | 1.0842 | 0.9598 to 1.2248 | 0.1960 |
*M factor = Distant metastasis
†SUVmax = Maximum standardized uptake value
Comparison of Survivals by Treatment Type
We analyzed the relationship between SUVmax and type of treatment (surgery, cyberknife, radiotherapy, and chemotherapy) to examine the efficacies of treatments. The median survival times for patients that underwent surgery, cyberknife, chemotherapy, and radiotherapy were 2267, 313, 171, and 290 days, respectively. The low and high SUVs were unable to separate patients with a significantly different survival with regard to non-surgical type of treatments. (Table 4)
Table 4.
Subgroup survival analysis by treatment type and SUV
| Low SUV (< 4.1) Median survival (days) | High SUV (≥ 4.1) median survival (days) | P value (< 0.05) | |
|---|---|---|---|
| operation | 1267 | 430 | 0.35 |
| cyberknife | 442.5 | 303 | 0.48 |
| cyberknife and radiotherapy | 347.5 | 291 | 0.48 |
| chemotherapy | 299 | 170 | 0.2 |
Discussion
Generally, pancreatic cancer patients undergo abdominal CT or EUS with cytology at initial evaluations, and based on the results obtained, patients deemed resectable undergo surgery and those deemed unresectable undergo chemotherapy and/or radiotherapy. Initial staging and selecting therapeutic options in pancreatic cancer patients using these conventional imaging has been reported to have a sensitivity of up to 94 %. However, these anatomical imaging modalities evaluate structural rather than tumor metabolic changes. In addition, conventional imaging modalities require that patients be scanned several times, and thus, patients are exposed to high levels of radiation. Furthermore, the use of diagnostic EUS-FNA is relative invasive although it is sensitive (84 %), specific (97 %), and accurate (84 %) and has a high positive predictive value (99 %) with only rare major complications. On the other hand, its negative predictive value is low at only 64 %. In addition, FNA may fail to differentiate malignant pancreatic diseases and other benign processes in up to 40 % of cases and may cause spread to adjacent organs.
F-18 FDG PET had the advantage of providing scans of the whole body in one session, and the merits of allowing initial staging, including distant metastasis and early detection. 18-Fluorodeoxyglucose (FDG) positron emission tomography (PET) is a relatively recent, noninvasive imaging technique that is based on the principle of the specific tissue metabolism because F-18 FDG is selectively uptaken and retained by malignant cells. In fact, PET has been proposed for the diagnosis, staging, and for determining the effectivenesses of treatments of different malignancies, including pancreatic carcinoma. Many studies have addressed the relationship between FDG uptake and prognosis for a variety of cancers, such as, non-small cell lung cancer [8], head and neck cancer [9], and malignant lymphoma [10].
Recently, studies have been conducted to evaluate the relationship between FDG uptake by F-18 FDG PET/CT and prognosis in pancreatic cancer, but unfortunately, these studies were carried out on small numbers of patients. Nakata et al. published two consecutive studies on 14 and 37 patients on the values of SUVs obtained before treatment to predict outcome in pancreatic cancer. They reported that those with a SUV of < 3.0 had longer survival than those with a SUV of > 3.0 [11, 12]. In 52 patients with pancreatic cancer, Zimny et al. found that survival was significantly influenced by SUV (cutoff 6.1) and CA 19-9 serum level, by univariate and multivariate analysis [13]. However, as mentioned above these three studies involved small numbers of patients, and thus, statistical analyses were underpowered. Furthermore, they did not show a significant value in any factors of TNM. Sperti et al. reported a similar relation between prognosis and SUV at a cutoff of 4.0, and their multivariate analysis revealed that SUV and UICC 1997 tumor stage were the only independent predictors of survival. They also separated patients into three groups according to treatment methods, that is, resection, bypass, and palliative therapy, and found that in each group the median survival time of low SUV patients was longer than that of high SUVmax patients [14]. Schellenberg et. al. evaluated 55 locally advanced pancreatic cancer patients undergoing radiotherapy, and also showed that clinical SUVmax independently predicts overall survival and progression-free survival [5].
The aim of the present retrospective study was to determine in a large-scale study whether glucose metabolism as assessed by F-18 FDG PET provides prognostic information independent of established prognostic factors in patients with pancreatic cancer. In our study, we used SUVmax, which represents the amount of metabolic activity at a pixel, as a parameter of FDG PET. Because it is a widely used semi-quantitative value that can be easily assessed by a formula that uses the amount of FDG injected and the patient’s weight, this simplicity is useful in the clinical setting and contrasts with the complexity of full quantitative assessment or additional program settings to measure metabolic volume.
Survival analysis showed that survival was significantly influenced by SUVmax, tumor stage, pretreatment CA19-9, age, and distant metastasis. Furthermore, SUVmax was found to significantly affect survival time in the SUVmax < and > 4.1 groups by univariate and multivariate analysis. In fact, multivariate analysis showed that the hazard ratio of the higher SUVmax group was more than 2.1 times that of the lower SUVmax group and this relation was not influenced by other prognostic factors. According to our results, SUVmax on F-18 FDG-PET/CT predict prognosis well in pancreatic cancer and could allow patients likely to benefit from intensive surgical treatment at different stages of the disease to be accurately identified. In former studies, SUV cut-off values 4.0 were found to shown significantly correlation between SUV and tumor stage. Similarly, in the present study a cutoff value 4.1 was found to predict overall outcome better that the other clinicopathologic factors examined. In addition, the statistical power of the present study was enhanced by including a larger number of patients. Because survival periods according to the tumor stage had significant differences in each groups, tumor stage can influence decision of resectability and prediction of prognosis in pancreatic cancer patients [15]. Fujino et al. reported that liver and peritoneal metastasis are prognostic factors in unresectable pancreatic cancer patients, and Benassai et al. concluded that the presence of lymph node metastasis significantly reduces the likelihood of survival in patients with otherwise potentially curable pancreatic carcinoma [16, 17].
In the present study, 76 of the 165 patient were diagnosed clinically, 14 patients had stage I or II and the remainder had stage III or IV. Patients of stages III or IV underwent definitive treatments, such as, chemotherapy, radiotherapy, or cyberknife surgery.
The early diagnosis of pancreatic cancer is difficult due to its retroperitoneal location and cancer dissemination to lymph nodes, major vessels, and liver at time of diagnosis. For this reason, some non-resectable patients with advanced pancreatic cancer undergo more frequent medical or radiologic treatment (cyberknife, radiotherapy, and chemotherapy) than patients with another gastrointestinal malignancy [18, 19]. Oya et al. reported that systemic treatments are necessary to improve therapeutic outcome in pancreatic cancer patients, but treatment method has been demonstrated to improve prognosis to date. [20] In the present study, we investigated the relation between SUV and survival time by dividing the cohort into four groups (surgical, cyberknife, chemotherapy, and cyberknife plus radiotherapy groups). In our univariate analysis of all patients, overall survival time in surgical patients was found to be longer than that in non-surgical patients, presumably because patients underwent surgery had little local invasion or distant metastasis. We were able to find a statistically difference in survival and SUVmax between surgical and non-surgical groups. However, we were unable to find a significant survival difference in each surgical and non-surgical groups. This may have been due to the small number of surgical and non-surgical patients after stratifying by treatment type, to the small number of early-stage cancers (stage I/II 2 of 60), and to the retrospective nature of this study.
Our study has another limitation that possibly influence the results. Enrolled tumor size had variable ranges (0.5–7 cm). The smaller tumor had relatively greater underestimation of the FDG uptake value because of partial volume effect and had a tendency to longer survival. Consequently, there are possibilities to overestimate a survival prediction in patients with smaller tumor size. Larger and prospective studies with selected size criteria are needed to determine whether PET/CT FDG uptake can be used to select therapeutic options and to predict prognosis in patients with pancreatic cancer.
In conclusion, SUVmax measured by FDG PET was found to be significantly related to survival and it could be useful to predict prognosis in patients with pancreatic cancer.
Acknowledgments
Conflict of Interest
We declare that we have no conflict of interest
References
- 1.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. In: SEER Cancer Statistics Review 1975-2008. National Cancer Institute. Bethesda, http://seer.cancer.gov/csr/1975_2008/ based on November 2010 SEER data submission, posted to the SEER web site, 2011.
- 2.In: Cancer Statistics in Korea, National Cancer information center, http://www.Cancer.go.kr/ncic/cicis_f/01/012/index.html, 2012
- 3.Morganti AG, Brizi MG, Macchia G, Sallustio G, Costamagna G, Alfieri S, et al. The prognostic effect of clinical staging in pancreatic adenocarcinoma. Ann Surg Oncol. 2005;12:145–151. doi: 10.1245/ASO.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Maemura K, Takao S, Shinchi H, Noma H, Mataki Y, Kurahara H, et al. Role of positron emission tomography in decisions on treatment strategies for pancreatic cancer. J Hepatobillary Pancreat Surg. 2006;13(5):435–441. doi: 10.1007/s00534-006-1102-8. [DOI] [PubMed] [Google Scholar]
- 5.Schellenberg D, Quon A, Minn AY, Graves EE, Kunz P, Ford JM, et al. 18Fluorodeoxyglucose PET is prognostic of progression-free and overall survival in locally advanced pancreas cancer treated with stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77(5):1420–1425. doi: 10.1016/j.ijrobp.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 6.Delbeke D, Martin WH. Positron emission tomography imaging in oncology. Radiol Clin N Am. 2001;39:883–917. doi: 10.1016/S0033-8389(05)70319-5. [DOI] [PubMed] [Google Scholar]
- 7.Hustinx R, Benard F, Alavi A. Whole-body imaging in the management of patients with cancer. Sem Nucl Med. 2002;32:35–46. doi: 10.1053/snuc.2002.29272. [DOI] [PubMed] [Google Scholar]
- 8.Higashi K, Ueda Y, Arisaka Y, Sakuma T, Nambu Y, Oguchi M, et al. 18 F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med. 2002;43:39–45. [PubMed] [Google Scholar]
- 9.Minn H, Lapela M, Klemi PJ, Grenman R, Leskinen S, Lindholm P, et al. Prediction of survival with fluorine-18-fluoro-deoxyglucose and PET in head and neck cancer. J Nucl Med. 1997;38:1907–1911. [PubMed] [Google Scholar]
- 10.Geus-Oei LF, Oyen WJ. Predictive and prognostic value of FDG-PET. Cancer Imaging. 2008;8:70–80. doi: 10.1102/1470-7330.2008.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakata B, Chung YS, Nishimura S, Nishihara T, Sakural Y, Sawada T, et al. 18 F-fluorodeoxyglucose positron emission tomography and the prognosis of patients with pancreatic adenocarcinoma. Cancer. 1997;79:695–699. doi: 10.1002/(SICI)1097-0142(19970215)79:4<695::AID-CNCR6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Nakata B, Nishimura S, Ishikawa T, Ohira M, Nishino H, Kawabe J, et al. Prognostic predictive value of 18 F-fluoro-deoxyglucose positron emission tomography for patients with pancreatic cancer. Int J Oncol. 2001;19:53–58. doi: 10.3892/ijo.19.1.53. [DOI] [PubMed] [Google Scholar]
- 13.Zimmy M, Fass J, Bares R, Cremerius U, Sabri O, Buechin P, Schumpelick V, et al. Fluorodeoxyglucose positron emission tomography and the prognosis of pancreatic carcinoma. Scand J Gastroenterol. 2000;35:883–888. doi: 10.1080/003655200750023273. [DOI] [PubMed] [Google Scholar]
- 14.Sperti C, Pasquali C, Chierichetti F, Ferronato A, Decet G, Pedrazzoli S. 18-Fluorodeoxyglucose positron emission tomography in predicting survival of patients with pancreatic carcinoma. J Gastrointest Surg. 2003;7:953–959. doi: 10.1016/j.gassur.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Hartwig W, Hackert T, Hinz U, Gluth A, Bergmann F, Strobel O, et al. Pancreatic cancer surgery in the new millennium: better prediction and of outcome. Ann Surg. 2011;254(2):311–319. doi: 10.1097/SLA.0b013e31821fd334. [DOI] [PubMed] [Google Scholar]
- 16.Fujino Y, Suzuki Y, Allki T, Tanioka Y, Kuroda Y. Predicting factors for survival of patients with unresectable pancreatic cancer : a management guideline. Hepatogastroenterology. 2003;50:250–253. [PubMed] [Google Scholar]
- 17.Benassai G, Mastrorilli M, Mosella F, Mosella G. Significance of lymph node metastases in the surgical management of pancreatic head carcinoma. J Exp Clin Cancer Res. 1999;18:23–28. [PubMed] [Google Scholar]
- 18.Wilkowski R, Wolf M, Heinemann V. Primary advanced unresectable pancreatic cancer. Recent Results Cancer Res. 2008;177:79–93. doi: 10.1007/978-3-540-71279-4_10. [DOI] [PubMed] [Google Scholar]
- 19.Wilkowski R, Thoma M, Bruns C, Wagner A, Heinemann V. Chemoradiotherapy with gemcitabine and continuous 5-FU in patients with primary inoperable pancreatic cancer. JOP. 2006;7(4):349–360. [PubMed] [Google Scholar]
- 20.Oya N. Chemoradiotherapy for pancreatic cancer: current status and perspectives. Int J Clin Oncol. 2004;9:451–457. doi: 10.1007/s10147-004-0449-6. [DOI] [PubMed] [Google Scholar]


