Abstract
Purpose
To explore feasibility, tolerability, dosimetry and probable efficacy of intravenous endoradiotherapy with carrier-added 4-[131I]iodo-L-phenylalanine (c.a. 131I-IPA) in refractory high-grade glioma.
Methods
Two male patients (45 and 50 years), with long-standing, extensively pre-treated gliomas and evidence of progression underwent single intravenous injections of 2 and 4 GBq of c.a. 131I-IPA, respectively. Tumour targeting was verified by 131I-IPA single-photon emission computed tomography (SPECT). Metabolic and morphological changes indicative of tumour response were assessed by sequential [18F]fluoroethyltyrosine (18F-FET) positron emission tomography (PET) and contrast-enhanced magnetic resonance imaging (MRI) following therapy. Further monitoring included clinical state, safety laboratory, quality of life and dosimetry. Absorbed mean organ and whole-body doses were determined according to the Medical Internal Radiation Dose (MIRD) scheme using OLINDAEXM based on serial planar scintigraphy.
Results
Both patients tolerated the treatment well. No evidence of acute or delayed organ toxicity was observed. 131I-IPA accumulated in the tumour recurrences identified by MRI/18F-FET. In patient 1, PET showed progressively decreasing maximum standardised uptake values (SUVmax) over 10 months, indicating metabolic response, paralleled by reduced contrast enhancement and tumour volume on MRI. Progression occurred 18 months after therapy. Treatment was repeated using 6.6 GBq of 131I-IPA, to which no response was observed. Patient 2, followed-up for 3 months after therapy, showed stable disease on MRI and PET. Mean absorbed whole body doses ranged from 0.13 to 0.17 mSv/MBq, with the highest absorbed organ doses to kidneys, bladder and heart (0.86-1.23; 0.49-0.6 and 0.45-0.56 mSv/MBq).
Conclusion
Systemic endoradiotherapy using up to 6.6 GBq of c.a.131I-IPA is not associated with clinically detectable toxicity. Measurable anti-tumour effects in gliomas were observed. 131I-IPA warrants further evaluation as glioma therapy.
Keywords: Carrier-added 4-[131I]iodo-L-phenylalanine, Intracellular, Molecular endoradionuclide therapy, Dosimetry, Glioma, Human
Introduction
Gliomas are among the most fatal tumour entities, with median survival rates of only 12–15 months for high-grade gliomas [1]. Low-grade gliomas, when not treated radically by surgery at diagnosis, typically progress to high-grade gliomas either spontaneously or as a result of clonal selection from antineoplastic treatment. In a recent long-term follow-up in 314 low-grade gliomas, malignant transformation was observed in 65-72% of cases, independently from treatment modalities [2]. A median time of 1.8–3.9 years to malignant transformation has been reported by various groups using radiological criteria [3, 4].
External beam radiation therapy (EBRT), either alone or in combination with chemotherapy, continues to represent the standard of care for non-resectable tumours, although the relatively low sensitivity of gliomas to external irradiation is well known [5]. Overall survival rates have improved only marginally over the last 20 years [6], underlining the need for new treatment modalities.
Endoradiotherapy is defined as the systemic administration of a tracer or a pharmaceutical which is labelled with a therapeutic radionuclide and exploits pharmacological drug (or probe) properties for tissue targeting and radioactivity as antineoplastic principle. To direct therapeutic activity to the tumour, endoradiotherapy techniques use either extracellular receptors, targeted by labelled antibodies or peptides [7], or cellular transport mechanisms, enabling tissue-selective accumulation of radionuclides inside the target cells. Radioiodine therapy for thyroid cancer, the classical endoradiotherapy paradigm, introduced by Seidlin et al. [8] in 1946, demonstrates feasibility, safety and efficacy of a transporter-mediated intracellular endoradiotherapy approach. Long-term remission rates of approximately 80% following iodine-131 therapy in differentiated thyroid carcinoma emphasise the therapeutic potential of this strategy [9]. Though, as for most tumours, sufficiently tissue-selective transport mechanisms have not been identified to date, transporter-mediated intracellular endoradiotherapy has remained restricted to a few indications. Besides radioiodine therapy with elemental iodine-131, only [131I]meta-iodobenzylguanidine (131I-MIBG), a substrate of the noradrenalin transporter (NAT), over-expressed in a variety of neuroendocrine tumours, has become a clinically established therapy [10] as well as 131I-labelled anti-CD20 monoclonal antibodies for radioimmunotherapy of non-Hodgkin’s lymphoma.
The membrane-bound L-amino acid transporter 1 (LAT1) is over-expressed in gliomas and the degree of expression correlates with the histological tumour grade [11]. LAT1, therefore, constitutes an attractive target to mediate molecular therapy. Indeed, radiolabelled amino acid derivatives substrate to the LAT1 transporter, such as 11C-labelled methionine (11C-MET), [123I]iodo-α-methyltyrosine (123I-IMT) and [18F]fluoroethyltyrosine (18F-FET) [12–15], are established in clinical PET and single-photon emission computed tomography (SPECT) imaging of glioma. While efficiently targeting glioma cells in vivo, a retention in tumour tissue for less than 90 min [16], or a chemical structure unsuitable for radiohalogenation, prevent them to be used as scaffolds for therapeutic iodine-131 labelling.
4-[123I]Iodo-L-phenylalanine (123I-IPA) is a new SPECT tracer, recently described in a validation study which was conducted in 35 patients with histologically confirmed glioma (WHO grade I–IV, primary tumours as well as recurrences) and demonstrated retention in glioma tissue for up to 24 h, high metabolic stability, and uptake by >85% of gliomas WHO grade I–IV [17] which previously had not been reported for any of the iodinated amino acid derivatives. These characteristics prompted the evaluation of the 131I-labelled analogue 131I-IPA as a therapeutic agent for endoradiotherapy of glioma [18, 19].
Here we report the first human data on the feasibility, tolerability, dosimetry and (preliminary) efficacy of a systemic endoradiotherapy using carrier-added (c.a.) 131I-IPA.
Materials and Methods
Radiosynthesis
C.a. 131I-IPA was prepared by isotopic radio-iodination in analogy to the method described previously [19]. Briefly, a mixture consisting of sodium [131I]iodide (2–5 GBq) in 100–500 μl 0.05 N NaOH (GE Healthcare, Germany) and 10 μl aqueous Na2S2O5 (4.0 mg Na2S2O5/ml) was evaporated to dryness by passing a stream of nitrogen through a 5-m conical Reactivial (Alltech Associates, Deerfield, IL, USA) at 90°C, followed by addition of 500 μl of a solution of 4-iodo-L-phenylalanine (2.5 mg/ml of 0.1 M H3PO4), 15 μl 30% HCl (suprapur; Merck, Germany), 200 μl L-ascorbic acid (10 mg/ml water), and 10 μl aqueous Cu(II) sulphate (0.10 mol/l). The reaction vessel was heated for 60 min at 165°C and cooled, and the mixture was diluted with water (1,000 μl).
131I-IPA was separated from radioactive impurities and by-products by high-performance liquid chromatography (HPLC), using a reversed phase RP-C18 column (250 × 4 mm, Nucleosil-100 5 μm) and water:ethanol:acetic acid (89:10:1; v/v) as eluent with pre-column 20 x 4 mm at 1.0 ml/min. The fraction containing 131I-IPA was collected into a sterile tube and ascorbic acid was added to a final concentration of 10 mg/ml and then the solution was filtrated through a 0.22-μm sterile membrane (Millex GS, Millipore) in an evacuated sterile vial. Immediately prior to injection, this solution was buffered with an equal volume of 0.6 M PBS (pH 7.0, Braun, Melsungen) and diluted with 0.9% NaCl (Braun, Melsungen) to a total volume of 50 ml, yielding an isotonic and injectable radiopharmaceutical (ethanol concentration <5% , pH 7.0). 131I-IPA was obtained in 88 ± 10% radiochemical yield. The specific activity of 131I-IPA used for the in vivo studies amounted to 1–1.5 GBq/μmol.
Patients and Treatment
The first patient (45 years, male), initially diagnosed with a left temporal gemistocytic astrocytoma (WHO grade II) 8 years earlier, presented with left hemispheral sensoric seizures and headaches of increasing frequency. He had initially undergone stereotactic radiation therapy with 15 MeV photons plus fractionated conformal radiation therapy, and further radio-chemotherapy (temozolomide) 6 months earlier, attaining a total focal dose of 115.6 Gy. Contrast-enhanced MRI (T1, T2, FLAIR) revealed an increasing tumour volume and extensive blood-brain barrier (BBB) disruption, compared with an examination performed 7 months earlier. In the same period, pathological amino acid uptake determined by 18F-FET PET increased in volume and intensity (SUVmax increased from 4.6 to 6.6), suggesting progressive tumour disease with histological up-grading.
The second patient (50 years, male), initially diagnosed with a right parietal oligodendroglioma (WHO grade II) 11 years earlier, initially underwent subtotal resection, followed by extensive chemotherapy regimens every other year (two courses of temozolomide, and PCV each), as well as proton beam therapy with 45 CGE (cobalt gray equivalents), 11 months ago. Restaging 2 months earlier had demonstrated tumour progression (increased volume and contrast enhancement, edema on MRI, and 18F-FET PET SUVmax rise from 2.3 to 3.3 in the residual tumour tissue).
In the absence of other established treatment options, the patients consented to undergo endoradiotherapy with c.a. 131I-IPA. The study was approved by the institutional review board and interdisciplinary tumour boards. The patients were extensively informed about possible adverse effects and signed written informed consent. Following thyroid blocking with potassium perchlorate (Irenate; Bayer, Germany), 131I-IPA was administered intravenously in 50 ml NaCl as short infusion over 15 min into an antecubital vein. The first patient was treated with 2.0 GBq, and the second patient with 4.0 GBq 131I-IPA. The former patient underwent repeated treatment with 6.6 GBq of c.a. 131I-IPA at 18 months after the first therapy cycle. After 4 days of hospitalisation, patient radioactvity had diminished below control levels, allowing discharge.
Safety
Safety assessments were performed at baseline, during infusion, at 1, 8, 24, 48 and 72 h post therapy, and after discharge in increasing intervals, including physical examination, vital signs, and safety laboratory parameters. Health-related quality of life (HRQL) was assessed using the validated EORTC-QLQ-C30 for general, and the EORTC-QLQ-BN-20 questionnaire for a brain-tumour-specific HRQL.
Imaging
Planar Imaging
To characterise dosimetry, planar whole-body 131I-IPA scans were acquired at 4–6 time points post therapy on a gamma camera (MEDISO, SPRIRIT DH-V). Scans were acquired, starting immediately after injection, at 3 h, 24 h, 48 h and then daily for up to 120 h. The camera parameters were the following: HeGP collimator, 20% energy window, peak at 365 keV, scan speed 12 cm/min. SPECT was used to assess tumour targeting of 131I-IPA. In the first patient, SPECT scans at all planar imaging time points were acquired with a Mediso DHV Spirit SPECT Scanner (high-energy collimator, 64 views, scan time 30 s per view, matrix 64 × 64), in the second only at 96 h post injection, due to the higher injected dose. Here and at the second therapy course of the first patient, a SPECT-CT system Siemens Symbia T (medium energy collimator, matrix 128 × 128, 60 views/30 s per view, CT based attenuation correction) was used.
MRI
Native and contrast-enhanced MR images were acquired on a 1.5-T scanner (SIEMENS, model) using clinical routine protocols (T1, T2, FLAIR).
18F-FET PET/CT was used to characterise amino acid uptake before and after therapy. Scans were acquired in 3D mode, 55 and 65 min after injection of 250 MBq 18F-FET using a PET/CT scanner (Siemens Biograph LSO Duo). Protocol: brain PET (one bed position, 15-min scan time). PET images were reconstructed using FORE-OSEM, six iterations, 16 subsets, with CT-based attenuation correction (CT parameters: 5-mm slice, coll. 2 × 4.0 mm Recon Kernel H30s medium very smooth).
PET/MRI image fusion was performed using a prorietary validated neuroimaging software suite (PMOD, Zurich, Switzerland). Contours for delineation of gross tumour volume (GTV) on PET were determined using a fixed threshold of 40% of SUVmax [20].
Dosimetry
Organ dosimetry data were obtained according to the Medical Internal Radiation Dose (MIRD) scheme from the 2D planar whole-image sets [21]. For the estimation of mean absorbed organ doses, we used the following dosimetry protocol. Because the patient was not allowed to empty the bladder before the first scan, the whole-body counts acquired immediately after the injection were defined to be 100% of the administered activity. Scintigraphies were analysed by the use of regions of interest (ROIs). To quantify the ROI data, the geometric mean of anterior and posterior counts was applied and the time-dependent time-activity curves were obtained and fitted to mono- or bi-exponential functions (software ORIGIN PRO 8.1 G). The residence time and cumulated activity as well as the uptake and effective half-life, were then calculated and the mean absorbed doses were estimated by using the OLINDAEXM software [22].
Additionally, venous blood samples obtained at 12–18 time points post injection (3 min to 92 h p.i.) were considered to estimate the mean absorbed dose to red marrow.
Results
Tumour Targeting
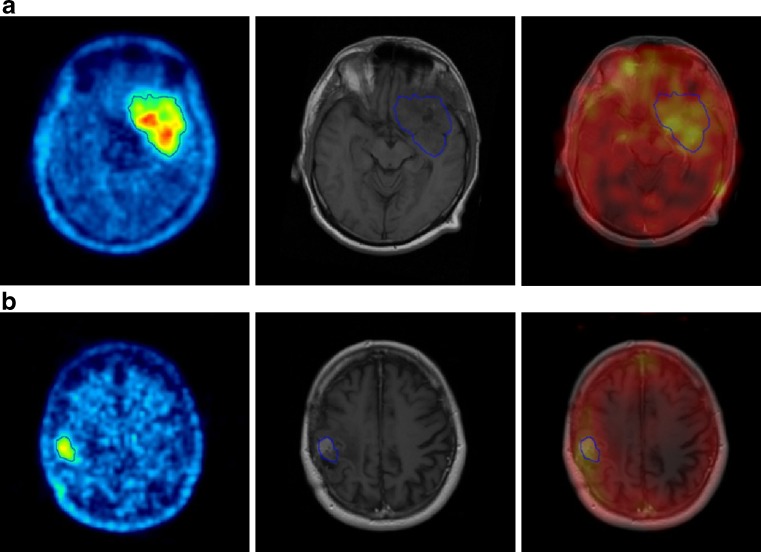
SPECT images obtained post injection (patient 1: 24 h, patient 2: 96 h, due to the higher injected activity), demonstrated in both patients accumulation of c.a. 131I-IPA inside the tumour lesion seen on MRI and 18F-FET PET (Fig. 1). Up to 24 h post injection, increased 131I-IPA activity is also seen in nasal mucosa, which gradually decreased with time.
Fig. 1.
18F-FET-PET fused to MRI (left column), MRI (middle column), and 131I-IPA SPECT fused to MRI (right column) from (a) patient 1 and (b) patient 2: SPECT 8 h and 96 h post injection of c.a. 131I-IPA demonstrates accumulation in the gross tumour volume, defined by 18F-FET-PET (blue line)
Antineoplastic Activity
Patient 1: Gemistocytic Astrocytoma, Transformed Into High-Grade Glioma by Radiological Criteria
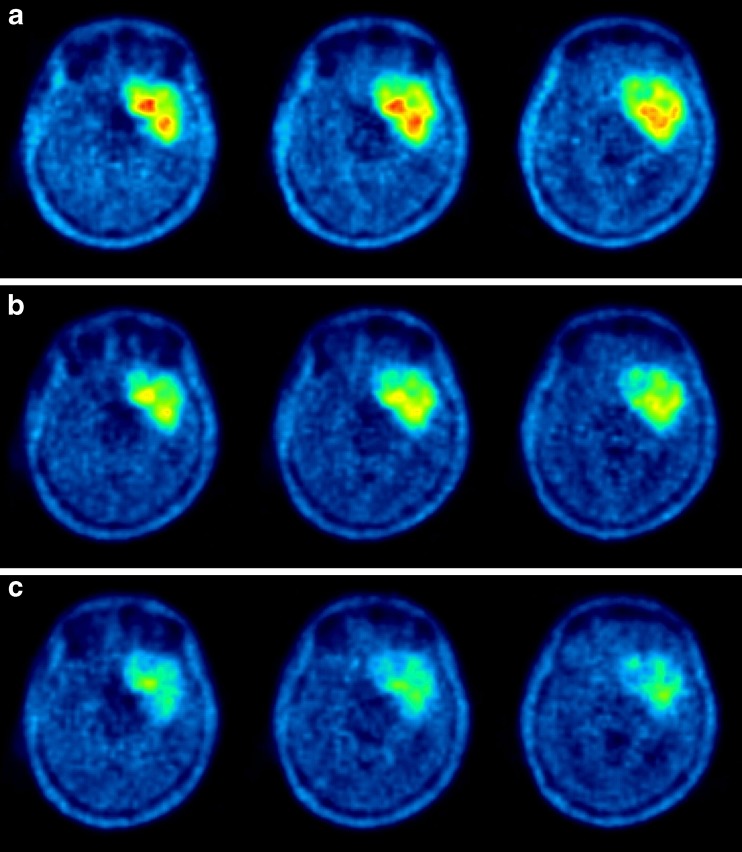
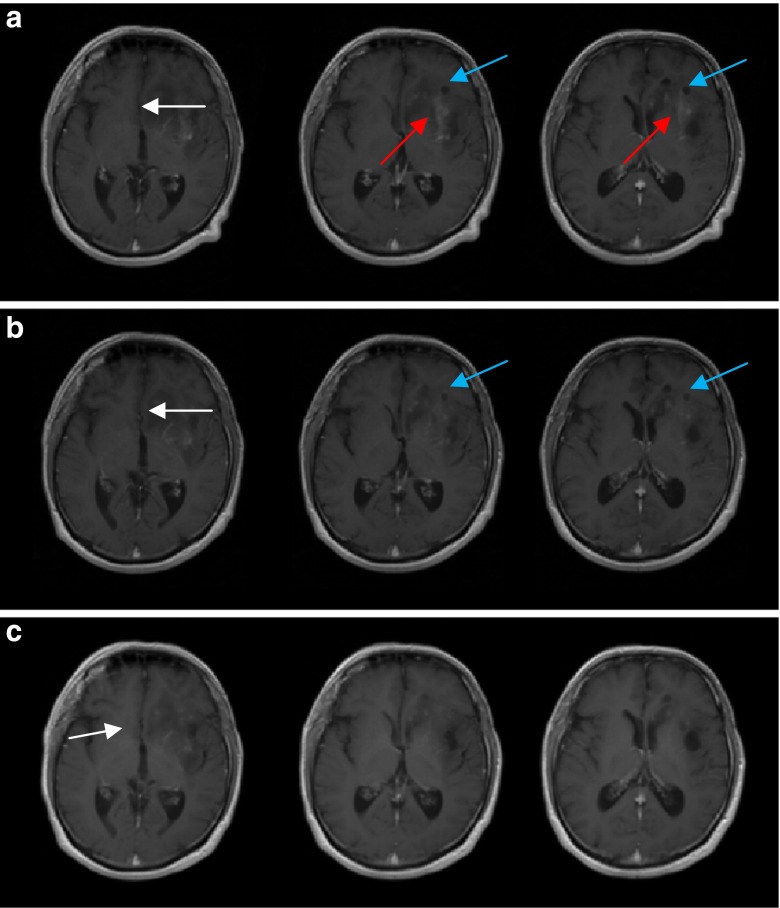
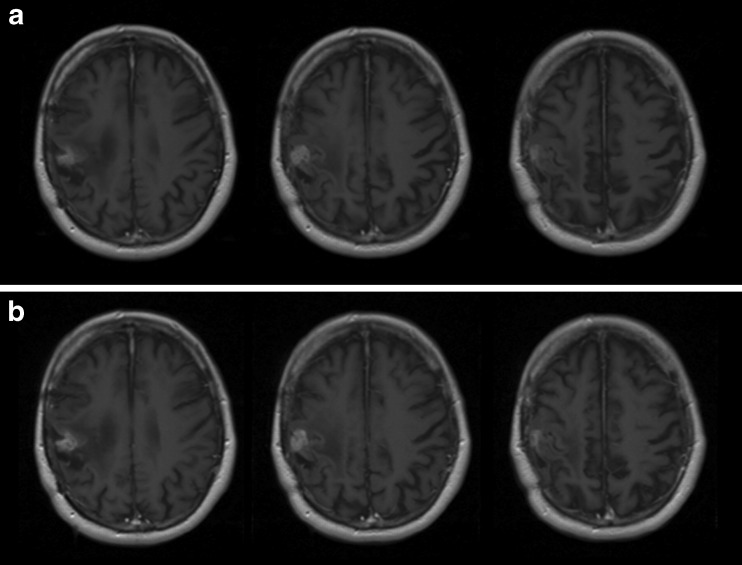
Following endoradiotherapy with 2 GBq c.a. 131I-IPA, 18F-FET tumour uptake regressed in intensity (SUV 6.6, 5.3 and 3.3, at baseline, week 6 and month 10, respectively) and volume (Fig. 2). On MRI, cystic tumour parts decreased in size, as did contrast-enhancing tumour portions. A slightly reduced midline displacement was noted at month 10 (Fig. 3).
Fig. 2.
18F-FET (a) top row: baseline (b) middle row: 6 weeks and (c) bottom row: 10 months after single dose of 2.0 GBq c.a. 131I-IPA. Note sustained reduction of pathological amino acid uptake and gross tumour volume
Fig. 3.
MRI, T1-weighed, with contrast agent (a) top row: baseline (b) middle row: 6 weeks and (c) bottom row: 10 months after therapy. Reduction of cystic tumour parts (blue arrows) in size, reduced contrast enhancement (red arrows), and midline displacement (white arrows)
Upon repeat treatment with 6.6 GBq c.a. 131I-IPA, no anew tumour stabilisation was observed on MRI and 18F-FET PET imaging at month 3. The patient underwent other experimental therapy.
Patient 2: Progessive Oligodendroglioma
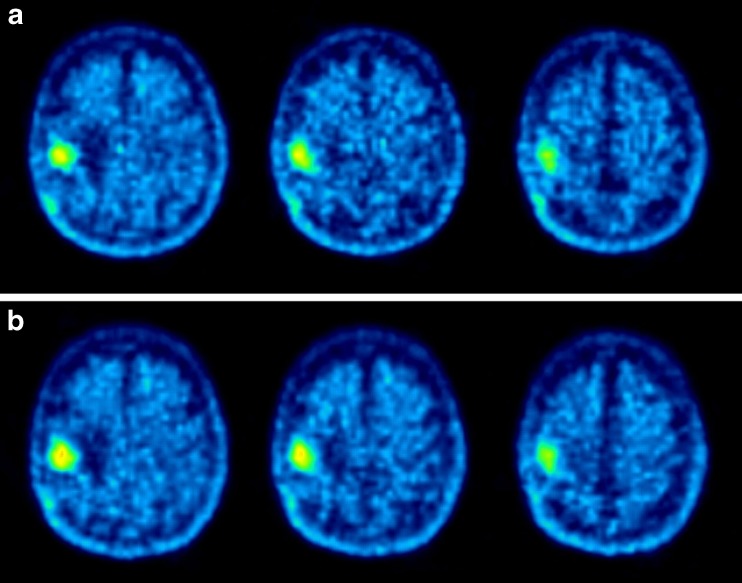
Following therapy with 4 GBq c.a. 131I-IPA, 18F-FET tumour uptake essentially remained unchanged in intensity (SUV 3.3 and 3.7, at baseline, month 3, respectively) and volume (Fig. 4). Also on MRI, stable disease was observed (Fig. 5).
Fig. 4.
18F-FET a) top row: baseline b) bottom row: 3 months after single dose of 4.0 GBq c.a. 131I-IPA. Note essentially unchanged pathological uptake and gross tumour volume
Fig. 5.
MRI, T1-weighted, with contrast agent (a) top row: baseline and (b) bottom row: 3 months after therapy, indicating disease stabilisation
Safety and Tolerance
Laboratory parameters, and the EORTC-QLQ-C30 and EORTC-QLQ-B20 instruments, verified in weekly intervals did not show any evidence of toxicity until month 3 in both patients. Especially, no haematological, nephrotoxicity or neurotoxicity was determined at the dose levels used.
Dosimetry
Three administrations of 2, 4, and 6.6 GBq were analysed for organ and whole-body radiation absorbed doses. The biological half-life of c.a. 131I-IPA was determined as 30.1–39.2 h. Organs with the highest radiation absorbed doses were kidney, bladder and heart. Radiation absorbed organ doses are given in Table 1.
Table 1.
Mean radiation absorbed doses from c.a. 131I-IPA to organs and the whole body. Values from three administration cycles of 2.0, 4.0 and 6.6 GBq
| Organ | Mean absorbed dose in mSv/MBq | ||
|---|---|---|---|
| 2 GBq | 4 GBq | 6.6 GBq | |
| Adrenals | 0.15 | 0.16 | 0.20 |
| Brain | 0.11 | 0.10 | 0.13 |
| Breasts | 0.11 | 0.11 | 0.13 |
| Gallbladder wall | 0.15 | 0.16 | 0.19 |
| LLI Wall | 0.14 | 0.14 | 0.18 |
| Small intestine | 0.14 | 0.14 | 0.18 |
| Stomach wall | 0.14 | 0.13 | 0.17 |
| ULI wall | 0.14 | 0.14 | 0.18 |
| Heart wall | 0.45 | 0.50 | 0.56 |
| Kidneys | 0.86 | 0.97 | 1.23 |
| Liver | 0.26 | 0.28 | 0.26 |
| Lungs | 0.13 | 0.13 | 0.16 |
| Muscle | 0.12 | 0.12 | 0.15 |
| Ovaries | 0.14 | 0.14 | 0.18 |
| Pancreas | 0.15 | 0.15 | 0.19 |
| Red marrow | 0.20 | 0.27 | 0.35 |
| Osteogenic cells | 0.28 | 0.32 | 0.41 |
| Skin | 0.10 | 0.10 | 0.12 |
| Spleen | 0.14 | 0.14 | 0.18 |
| Testes | 0.12 | 0.11 | 0.15 |
| Thymus | 0.14 | 0.14 | 0.17 |
| Thyroid | 0.12 | 0.12 | 0.15 |
| Urinary bladder wall | 0.49 | 0.57 | 0.60 |
| Uterus | 0.15 | 0.15 | 0.19 |
| Total body | 0.13 | 0.13 | 0.17 |
Compared with the whole body, the blood kinetics showed longer half-lives (53 h, 47 h and 48 h) and the resulting mean absorbed dose to red marrow ranged from 0.2 to 0.35 mSv/MBq.
Discussion
The present report summarizes the first human experience of systemic endoradiotherapy with c.a. 131I-IPA in two patients with progressive gliomas, which were initially diagnosed as low-grade astrocytoma (WHO II) and oligodenroglioma (WHO II), respectively. For newly diagnosed low-grade gliomas a 65-72% likelihood [2], and a median interval of 1.8–3.9 years [3] until malignant transformation into high-grade glioma has been reported. With a history of 8 and 11 years, both patients reported here can be reasonably assumed to have had high-grade glioma at the time of their treatment, although no current histological confirmation could be obtained. Presence of secondary high-grade glioma is further supported by radiological criteria.
We demonstrate that glioma tissue may accumulate therapeutically active concentrations of c.a. 131I-IPA following single intravenous administration, able to induce a sustained tumour response, in spite of extensive prior radiation and chemotherapy. No acute or delayed–type radiotoxicity, especially no haematological, renal, or neurotoxicity was observed from c.a. 131I-IPA in the dose range investigated.
In the first patient, an 18F-FET SUV reduction from initially 6.6 to 3.3, together with a substantial reduction in the metabolic gross tumour volume, developed gradually over the course of 10 months following therapy. This was paralleled by a clear reduction in pathological contrast enhancement and a slight reduction in tumour volume on MRI. Considering that the patient had experienced an SUV increase from 4.6 to 6.6 in the preceding 6 months in spite of radio-chemotherapy, we rate this metabolic response as an antineoplastic effect from c.a. 131I-IPA. Tumour progression was assessed by MRI and FET-PET 18 months after initial therapy. A second therapy, with an increased dose of 6.6 GBq c.a. 131I-IPA, was therefore administrated. The second administration of c.a. 131I-IPA was also well tolerated, demonstrating the possibility of repeat treatments from a safety perspective.
In the second patient, MRI showed stable disease 3 months after therapy. Concordantly, 18F-FET-PET demonstrated an unchanged metabolic gross tumour volume, and SUVmax values remained nearly constant (from 3.3. to 3.6), which is in the range of intra-individual variability reported for 18F-FDG [23].
Traditionally, the induction of DNA double-strand breaks in target cells directly hit by radioactive particles has been seen as key cytocidal mode of action in endoradiotherapy. Yet, considering the physical half-life of 8 days for iodine-131, the observation that a single injection of c.a. 131I-IPA induced regressive metabolic tumour changes, which continuously increased over a period of 10 months, suggests that the treatment triggers antineoplastic mechanisms, beyond the effects of direct ß-irradiation. Recently, radiation-induced biological bystander effects (RIBBE) have been recognised as a complementary cytocidal mechanism which may significantly contribute to the overall efficacy of endoradiotherapy [24]. Potent toxins, generated by cells directly exposed to radiation, and released into the intercellular environment, are thought to mediate cytocidal effects on cells not directly irradiated. In fact, experimental data from glioma cells in vitro indicate that following intracellular iodine-131 exposure, RIBBE may account for up to 70-80% of total radiation-induced cytotoxic activity, compared with only 30-40% following conventional external field irradiation [25]. In contrast, extracellular iodine-131 exposure in medium alone, did not elicit RIBBE in this model. These observations are in line with the concept that intracellular 131I-IPA exposure might activate proapoptotic pathways in glioma cells, which continue to be active after the physical impact of radiation itself has ceased.
Owing to the method of radiopharmaceutical preparation using an iodine exchange reaction, c.a. 131I-IPA contains a relevant proportion of non-radioactive IPA. Due to their structural and pharmacological identity, carrier and 131I-IPA are taken up by the tumour cells simultaneously in proportion to their molecular occurrence, defined by the specific activity. As non-radioactive IPA possesses both an intrinsic cytostatic and an independent radiosensitiser effect [19], c.a. 131I-IPA is likely to exert a multimodal antineoplastic activity at the cellular level, which differentiates it from other endoradiotherapy agents or chemotherapeutics used in glioma therapy.
In the context of systemic endoradiation therapies, unwanted exposure of healthy organs may practically limit the possibility to achieve sufficient tumour doses. In the patients reported here, neither acute nor delayed radiotoxicity to bone marrow, kidney, brain or other systems were observed to date, in spite of systematic screening. This result confirms our previous findings based on animal studies [18, 19]. Indeed, internal dosimetry, performed according to MIRD recommendations [21] and obtained in three treatment cycles, demonstrates that absorbed doses to radiation-critical organs, such as red marrow, kidney, liver and gonads, are comparable with those reported for established endoradiotherapy agents, like iodine-131 for the treatment of thyroid malignancies or 131I-MIBG for pheochromoticytoma or neuroblastoma [10, 26]. Administration of higher doses than reported here, therefore, seems feasible from a dosimetric perspective.
In summary, c.a. 131I-IPA is a promising agent for the systemic endoradiotherapy of glioma, which showed first evidence of antineoplastic activity in two extensively pre-treated patients with progressive glioma. Excellent clinical tolerability and a favourable whole-body dosimetry indicate that c.a. 131I-IPA seems to possess a high therapeutic index, with potential to use higher doses than reported here.
References
- 1.Quick A, Patel D, Hadziahmetovic M, et al. Current therapeutic paradigms in glioblastoma. Rev Rec Clin Trial. 2010;5:14–27. doi: 10.2174/157488710790820544. [DOI] [PubMed] [Google Scholar]
- 2.Van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985–90. doi: 10.1016/S0140-6736(05)67070-5. [DOI] [PubMed] [Google Scholar]
- 3.Caseiras GB, Ciccarelli O, Altmann D, et al. Low-grade gliomas: six-month tumour growth predicts patient outcome better than admission tumor volume, relative cerebral blood volume, and apparent diffusion coefficient. Radiology. 2009;253:505–12. doi: 10.1148/radiol.2532081623. [DOI] [PubMed] [Google Scholar]
- 4.Danchaivijitr N, Waldman AD, Tozer DJ, et al. Low-grade gliomas: do changes in rCBV measurements at longitudinal perfusion-weighted MR imaging predict malignant transformation? Radiology. 2008;247:170–8. doi: 10.1148/radiol.2471062089. [DOI] [PubMed] [Google Scholar]
- 5.Taghian A. duBois W, Budach W, et al. In vivo radiation sensitivity of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1995;32:99–104. doi: 10.1016/0360-3016(94)00494-6. [DOI] [PubMed] [Google Scholar]
- 6.Omay S, Vogelbaum M. Current concepts and newer developments in the treatment of malignant gliomas. Ind J Cancer. 2009;46:88–95. doi: 10.4103/0019-509X.49146. [DOI] [PubMed] [Google Scholar]
- 7.Bodei L, Pepe G, Paganelli G. Peptide receptor radionuclide therapy (PRRT) of neuroendocrine tumors with somatostatin analogues. Eur Rev Med Pharmacol Sci. 2010;14:347–51. [PubMed] [Google Scholar]
- 8.Seidlin S, Marinelli L, Oshry E. Radioactive iodine therapy; effect on functioning metastases of adenocarcinoma of the thyroid. JAMA. 1946;132:838–47. doi: 10.1001/jama.1946.02870490016004. [DOI] [PubMed] [Google Scholar]
- 9.Pacini F, Castagna M, Brilli L, et al. Differentiated thyroid cancer. ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl):iv142–6. doi: 10.1093/annonc/mdp156. [DOI] [PubMed] [Google Scholar]
- 10.Giammarile F, Chiti A, Lassmann M, et al. EANM procedure guidelines for 131I-meta-iodobenzylguanidine (131I-mIBG) therapy. Eur J Nucl Med Mol Imaging. 2008;35:1039–47. doi: 10.1007/s00259-008-0715-3. [DOI] [PubMed] [Google Scholar]
- 11.Nawashiro H, Otani N, Shinomiya N, et al. L-type amino acid transporter 1 as potential molecular target in human astrocytic tumours. Int J Cancer. 2006;119:484–92. doi: 10.1002/ijc.21866. [DOI] [PubMed] [Google Scholar]
- 12.Okubo S, Zhen H, Kawai N, et al. Correlation of L-methyl-11C-methionine (MET) uptake with L-type amino acid transporter 1 in human gliomas. J Neurooncol. 2010;99:217–25. doi: 10.1007/s11060-010-0117-9. [DOI] [PubMed] [Google Scholar]
- 13.Kopka K, Riemann B, Friedrich M, et al. Characterization of 3-[123I]iodo-L-α-methyl tyrosine transport in astrocytes of neonatal rats. J Neurochem. 2001;76:97–104. doi: 10.1046/j.1471-4159.2001.00048.x. [DOI] [PubMed] [Google Scholar]
- 14.Samnick S, Bader JB, Hellwig D, et al. Clinical value of iodine-123-α-methyl-L-tyrosine single photon emission tomography in the differential diagnosis of recurrent brain tumor in patients pretreated for glioma at follow-up. J Clin Oncol. 2002;20:396–404. doi: 10.1200/JCO.20.2.396. [DOI] [PubMed] [Google Scholar]
- 15.Pauleit D, Floeth F, Hamacher K, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128:678–87. doi: 10.1093/brain/awh399. [DOI] [PubMed] [Google Scholar]
- 16.Langen K, Coenen H, Rosen N, et al. SPECT studies of brain tumours with L-3-[123I]iodo-α-methyl–tyrosine: comparison with PET, 124IMT first clinical results. J Nucl Med. 1990;31:281–6. [PubMed] [Google Scholar]
- 17.Hellwig D, Ketter R, Romeike B, et al. Validation of brain tumour imaging with p-[123I]iodo-L-phenylalanine and SPECT. Eur J Nucl Med Mol Imaging. 2005;32:1041–9. doi: 10.1007/s00259-005-1807-y. [DOI] [PubMed] [Google Scholar]
- 18.Romeike B, Hellwig D, Heimann A, et al. Action and efficacy of p-[131I]iodo-L-phenylalanine on primary human glioma cell cultures and rats with C6-gliomas. Anticancer Res. 2004;24:3971–6. [PubMed] [Google Scholar]
- 19.Samnick S, Romeike B, Lehmann T, et al. Efficacy of systemic radionuclide therapy with p-131I-iodo-L-phenylalanine combined with external beam photon irradiation in treating malignant gliomas. J Nucl Med. 2009;50:2025–32. doi: 10.2967/jnumed.109.066548. [DOI] [PubMed] [Google Scholar]
- 20.Vees H, Senthamizhchelvan S, Miralbell R, et al. Assessment of various strategies for 18F-FET PET-guided delineation of target volumes in high-grade glioma patients. Eur J Nucl Med Mol Imaging. 2009;36:182–93. doi: 10.1007/s00259-008-0943-6. [DOI] [PubMed] [Google Scholar]
- 21.Bolch WE, Eckerman KF, Sgouros G, Thomas SR. MIRD Pamphlet 21: a generalized schema for radiopharmaceutical dosimetry—standardization of nomenclature. J Nucl Med 2009; 50:477–84 [DOI] [PubMed]
- 22.Stabin M, Sparks R, Crowe E, et al. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–7. [PubMed] [Google Scholar]
- 23.Paquet N, Albert A, Foidart J, Hustinx R. Within-patient variability of (18)F-FDG: standardized uptake values in normal tissues. J Nucl Med. 2004;45:784–8. [PubMed] [Google Scholar]
- 24.Mairs R, Fullerton N, Zalutsky M, et al. Targeted radiotherapy: microgray doses and the bystander effect. Dose Response. 2007;5:204–13. doi: 10.2203/dose-response.07-002.Mairs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyd M, Ross S, Dorrens J, et al. Radiation-induced biologic bystander effect elicited in vitro by targeted radiopharmaceuticals labeled with α-, ß-, and auger electron–emitting radionuclides. J Nucl Med. 2006;47:1007–15. [PubMed] [Google Scholar]
- 26.Taïeb D, Sebag F, Farman-Ara B, et al. Iodine biokinetics and radioiodine exposure aftr recombinant human thyrotropin-assisted remnant ablation in comparison with thyroid hormone withdrawal. J Clin Endocrinol Metab. 2010;95:3283–90. doi: 10.1210/jc.2009-2528. [DOI] [PubMed] [Google Scholar]