Abstract
Purpose
This study aims to examine the findings of 99mTc-diphosphonate bone scans in cancer patients with a history of HIFU treatment.
Methods
Bone scan images of patients with a history of HIFU treatment for primary or metastatic cancer from January 2006 to July 2010 were retrospectively reviewed. Cases of primary bone tumor or HIFU treatment reaching only the superficial soft tissue layer were excluded.
Results
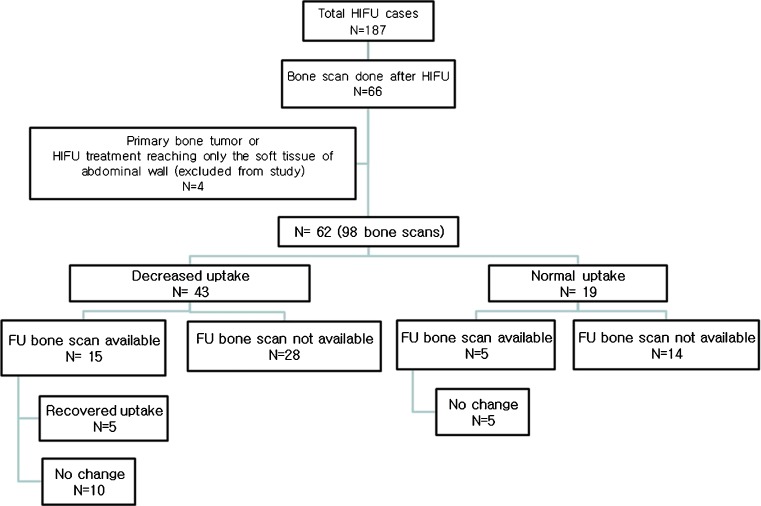
Bone scan images of 62 patients (26 female, 36 male; mean age 57 ± 9 years) were studied. HIFU treatment was performed in the liver (n = 40), pancreas (n = 16), and breast (n = 6). Mean interval time between HIFU treatment and bone scan was 106 ± 105 days (range: 1–572 days). Of 62 scans, 43 showed diffusely decreased uptake of bone within the path of HIFU treatment: antero-axillary and/or posterior arcs of right 5th to 11th ribs in 34 cases after treatment of hepatic lesions; anterior arcs of 2nd to 5th ribs in 5 cases after treatment for breast tumors; and posterior arcs of left 9th to 11th ribs or thoraco-lumbar vertebrae in 4 cases after treatment for pancreas tumor. Of 20 patients who had bone scans more than twice, five showed recovered uptake of the radiotracer in the involved ribs in the follow-up bone scan.
Conclusion
Of 62 bone scans in patients with a history of HIFU treatment for primary or metastatic cancer, 69% presented diffusely decreased uptake in the bone in the path of HIFU treatment.
Keywords: High-intensity focused ultrasound, Whole-body bone scan, Photon defect
Introduction
High-intensity focused ultrasound (HIFU) treatment is a type of non-invasive treatment using focused ultrasound beams that cause coagulative necrosis in the target lesion through intact skin without surgical procedures [1]. The clinical application of HIFU is for the treatment of benign and malignant solid tumors such as hepatocellular carcinoma (HCC), pancreatic cancer, breast cancer, or bone tumor. HIFU has the advantage of being non-invasive. However, several complications are reported: skin and/or subcutaneous edema, pain, skin burns, infection of the procedure site, and injury to the adjacent organs [2–5]. Higher attenuation of the HIFU beams increases the risk of damage in the ribs and overlying tissues, including the skin in the path of the beam. Bone has a greater ultrasound absorption rate than soft tissue, and reflection of the ultrasound energy at the bone-tissue interface may lead to heating of the ribs and overlying tissue [6]. This study aims to examine findings of 99mTc-diphosphonate bone scans in cancer patients with a history of HIFU treatment.
Materials and Methods
Subjects
This study was designed retrospectively. Bone scans of patients with histories of HIFU treatment for primary or metastatic cancer from January 2006 to July 2010 were reviewed. Through our electronic medical record (EMR) system we reviewed patients’ charts. Cases with primary bone tumor or HIFU treatment reaching only the soft tissue layer of the abdominal wall were excluded. Bone scan images of 62 patients were enrolled in this study. Thirty-six patients were male and 26 female. The age range was between 30 and 73. Mean interval time between the HIFU treatment and bone scan was 106 ± 105 days (range: 1–572 days).
Whole-Body Bone Scan
The bone scans were performed using a double-head γ-camera (ECam; Siemens Medical Solutions, Erlangen, Germany) equipped with low-energy, high-resolution collimators. Whole-body images were obtained 2–4 h after the intravenous injection of 740∼1,100 MBq (20∼30 mCi) of 99mTc-methylene diphosphonate (MDP) or 99mTc-hydroxymethylene diphosphonate (HDP) in the anterior and posterior projections. Additional spot views were acquired whenever a suspicious area was detected.
HIFU Treatment
HIFU was performed using an extracorporeal, ultrasound-guided, focused Model-JC tumor therapy system (Haifu Technology Co., Chongqing, China). This system consists of three selectable therapeutic transducers and a real-time imaging transducer having overlapping beams to allow targeting. The transducers were mounted in a water reservoir with the beam axis directed upward, and the patients were positioned above the transducers in a prone or lateral decubitus position with the skin overlying the lesion placed inside the water. The therapeutic transducers focus ultrasound beams into clinically relevant focus, which induces high temperatures in a well-localized small volume of interest. The area of destroyed cells is referred to as an ablation zone. Real-time ultrasound was done under the guidance of a 3.5–5.0-MHz diagnostic ultrasound transducer (Toshiba Medical System, Otawara, Japan). All patients had general anesthesia to ensure immobilization during the lengthy procedure and to prevent superficial skin pain.
Image Analysis
Two nuclear medicine physicians retrospectively evaluated the bone scan images together by reaching consensus.
Results
Of the total 62 bone scans, HIFU treatment was performed in the liver (n = 40), pancreas (n = 16), and breast (n = 6). Of 40 cases treating the liver, 20 cases were HCC, and 20 others were metastatic lesions. The primary cancer sites included the pancreas, stomach, colon, bile duct, esophagus, rectum, kidney, and retroperitoneum. Among the cases treating the pancreas, 1 was a metastatic lesion from colon cancer, and 15 others were primary pancreas lesions. All six breast cases were primary breast cancer lesions (Table 1).
Table 1.
The cancer distribution according to HIFU target sites
| HIFU target sites | |||
|---|---|---|---|
| Liver | Pancreas | Breast | |
| Primary cancer | 20 | 15 | 6 |
| Metastatic cancer | 20a | 1b | 0 |
| Total (n = 62) | 40 | 16 | 6 |
aThe sites of primary cancer: 6 pancreas, 3 stomach, 4 colon, 3 bile duct, and 1 each of esophagus, rectum, kidney, and retroperitoneum
bThe site of primary cancer: colon
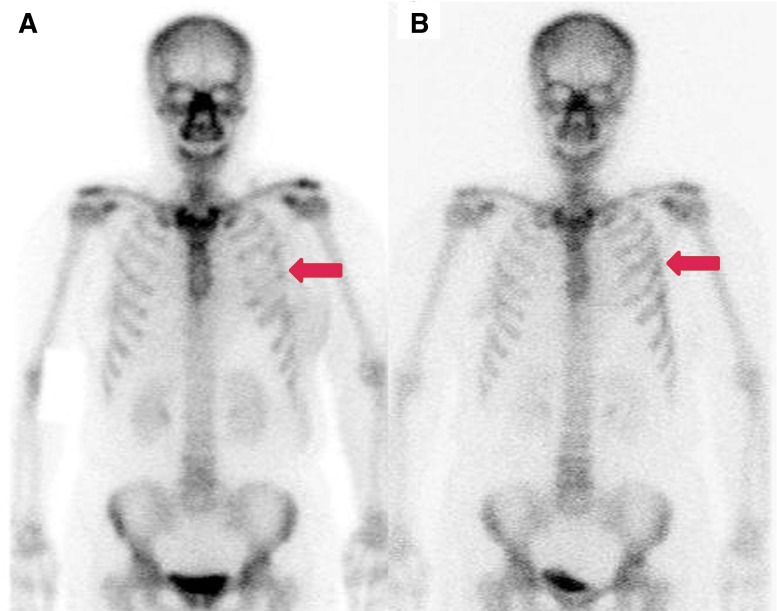
Of 62 bone scans, 43 scans (69%) showed decreased bone 99mTc-MDP or 99mTc-HDP uptake in the HIIFU treatment path. Right antero-axillary and/or posterior ribs, ranging from the 5th to 11th ribs, showed decreased uptake in 34 out of 40 cases after treatment of hepatic lesions (Fig. 1). Diffusely decreased bone uptake was noted in the left 9th to 11th posterior ribs in 1 case (Fig. 2), and T11 to L2 vertebrae showed decreased uptake in 4 out of 16 cases after treatment for pancreas tumor. Anterior arcs of the left 2nd to 5th ribs showed decreased uptake in five cases after treatment for breast tumors (Fig. 3, Table 2). In all of the breast tumor cases, treatment focuses were incidentally left sided because of left breast cancer.
Fig. 1.
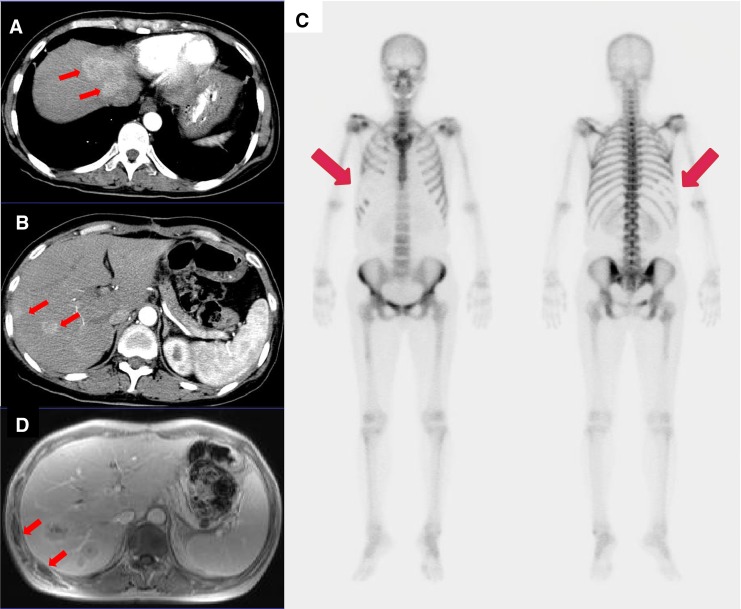
Bone scan was performed in a 53-year-old woman with a history of pancreatic cancer with liver metastasis. (a, b) Pancreatectomy was done 3 years ago, and multiple metastatic lesions (arrows) were seen in hepatic segments IV to VIII on follow-up CT. (c) Four months after HIFU treatment of the right hepatic lobe, diffusely decreased uptake is seen in the right 5th to 10th anterior and posterior ribs in the bone scan image (arrows). (d) MRI after HIFU treatment demonstrates bone necrosis in corresponding ribs (arrows)
Fig. 2.
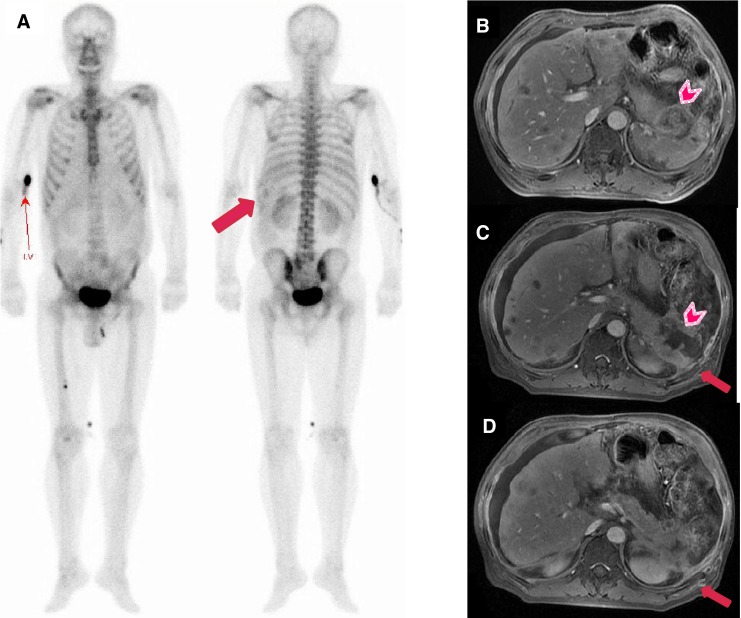
Decreased uptake is noted in this 58-year-old man who received HIFU treatment for pancreatic tail cancer. (a) Bone scan images obtained 7 days after HIFU show diffusely and unevenly decreased uptake in the left 9th to 11th posterior ribs (arrow). (b) Pre-HIFU MRI shows lobulated heterogeneous masses in the tail of the pancreas with invasion of the spleen (arrowhead). (c, d) MRI done 3 weeks after HIFU shows favorable ablation of pancreatic tail cancer (arrowhead), but bone necrosis is noted in overlying ribs (arrows)
Fig. 3.
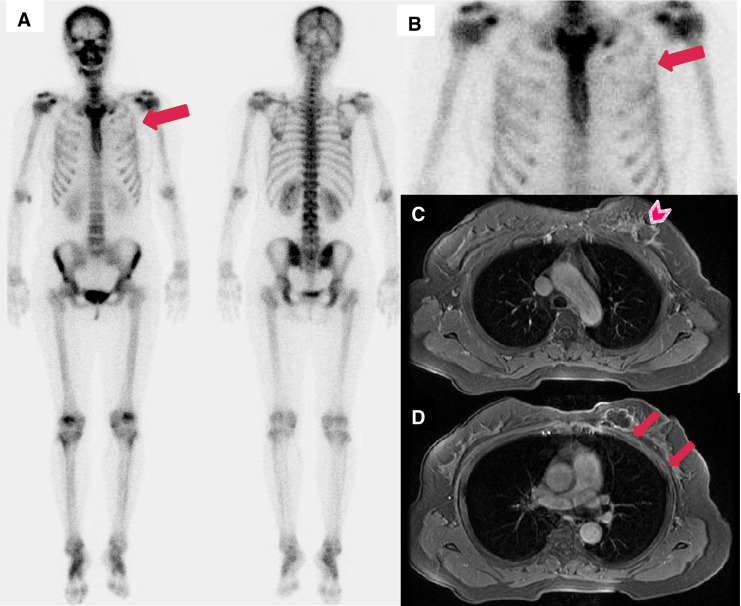
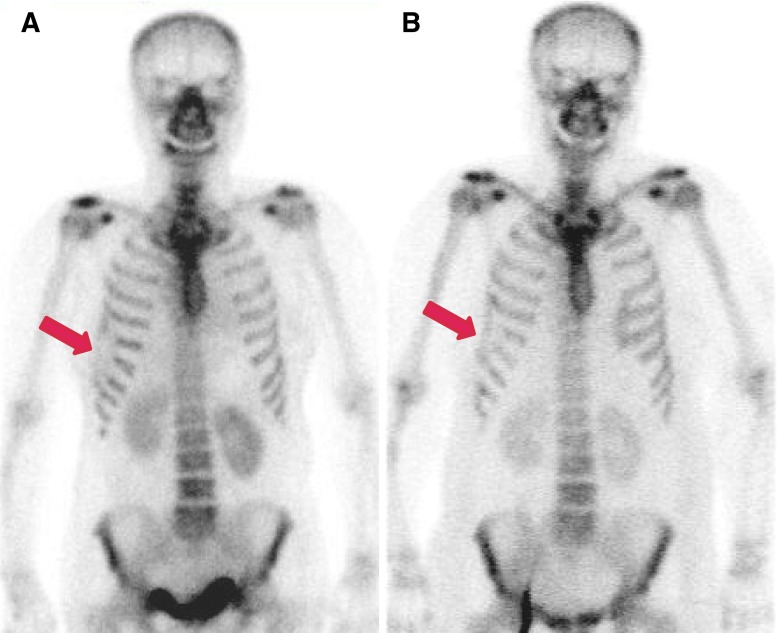
(a) Bone scan images of a 47-year-old female patient who had left breast cancer and HIFU treatment 5 months ago show mildly and diffusely decreased uptake in the left 2nd to 4th anterior ribs (arrow). (b) Enlarged bone scan spot image. (c, d) MRI after HIFU treatment shows favorable ablation of left breast cancer (arrowhead), but bone necrosis is noted in the adjacent ribs (arrows)
Table 2.
Summary of whole-body bone scan findings according to the HIFU target site
| HIFU target sites | |||
|---|---|---|---|
| Liver | Pancreas | Breast | |
| Number of cases (n = 62) | 40 | 16 | 6 |
| Cases with decreased bone uptake (n = 43) | 34 | 4 | 5 |
| Bones with decreased uptake | Right antero-axillary and/or posterior 5th∼12th ribs | 1 case: left posterior 9th∼11th ribs | Left anterior 2nd∼5th ribs |
| 3 cases: T11∼L2 vertebrae | |||
The mean interval time between the HIFU treatment and bone scan was 135 days (range 1∼572 days) in the group with abnormal decreased bone uptake and 41 days (range 6∼179 days) in the group with normal bone uptake in the HIFU treatment path.
Five of the bone scans performed after treating breast tumors and five bone scans performed after treating hepatic lesions showed mildly decreased bone uptake. The other 33 cases showed flank photon defect areas. Of the 43 cases that showed decreased bone uptake, 10 were accompanied by hot spots denoting fractures adjacent to the decreased uptake areas.
Of 20 patients who had bone scans more than twice, 5 showed recovered uptake of the radiotracer in the involved ribs in the follow-up bone scans, and the 15 patients showed no change (Fig. 4). Of the five patients with recovered follow-up bone scans, four patients were treated for breast cancer, and the bone scans showed partially or completely recovered bone uptake in the bone scans done 6 months, 12 months, 19 months, and 27 months after the abnormal post-HIFU bone scans. One patient treated for HCC showed partially recovered bone uptake 24 months after the initial abnormal post-HIFU bone scan.
Fig. 4.
Among the 66 patients with bone scans after HIFU treatment, a total of 98 bone scans of 62 patients were reviewed. Forty-three patients showed decreased uptake in the initial bone scan, and of the 15 patients with a follow-up bone scan, recovered bone uptake was noted in 5 patients. In the 19 patients with normal uptake in the initial bone scan, 5 had follow-up bone scans, which showed no change
Among the cases with recovered uptake on follow-up bone scan, a bone scan taken 5 months after HIFU treatment of the left breast showed focally decreased uptake in the left 3rd anterior rib, which was partially recovered on follow-up bone scans 6 months later. In another case, decreased bone scan uptake was seen in the left 4th to 5th anterior ribs 10 days after left breast treatment, and then completely recovered bone uptake was seen on follow-up bone scans 1 year later (Fig. 5). In the cases with unchanged follow-up bone scans, diffusely decreased uptake was noted in the right 6th to 9th anterior ribs 2 months after HIFU treatment at segments V and VIII due to HCC, and the bone scan remained unchanged in the follow-up bone scan 2 years later (Fig. 6).
Fig. 5.
Decreased bone uptake is noted in this 60-year-old woman who had HIFU treatment for a tumor in the mid-portion of the left breast. (a) Bone scan images obtained 10 days after HIFU show diffusely decreased uptake in the left 3rd to 5th anterior ribs (arrow). (b) Follow-up study 1 year later show normalized uptake in the previously involved ribs (arrow)
Fig. 6.
Bone scans were performed in a 63-year-old woman with HCC. (a) Two months after HIFU treatment of the lesion in hepatic segments V and VIII, diffusely decreased uptake is noted in the right 6th to 9th anterior ribs (arrow). (b) In the follow-up bone scan done 2 years later, the decreased uptake area is essentially unchanged (arrow)
Mild local pain was experienced in 35 (56%) of the 62 patients after being treated with HIFU. The pain was controlled by intramuscular or oral analgesics for 2 to 3 days. Among the 35 patients who had mild local pain, 10 also had skin redness and edema. These minor complications were resolved in all cases without any further special care. One patient who had a bone scan 1 day after the HIFU treatment had mild pain in the treatment region, but in the rest of the patients, no complications related to HIFU treatment were noted when the bone scans were taken.
Of the 43 patients with bone scans showing decreased bone uptake, CT containing the HIFU procedure site was performed within 1 month from the bone scan in 31 patients, MRI in 25 patients, and both CT and MRI in 18 patients. The rib showing decreased uptake in the bone scan showed hypointense bone marrow on T1-weighted images, suggesting osteonecrosis. No discrete abnormal finding could be identified in the corresponding bone setting CT images.
Discussion
The mechanism of HIFU treatment is to cause coagulative necrosis and immediate cell death by increasing the temperature in a highly selective tissue volume to above 55°C for 1 s or longer [7, 8]. The complete non-invasiveness and clearly defined treatment margins of HIFU treatment lead to very low complication rates. However, there are several complications reported after HIFU treatment due to the high-energy US waves reflected on gas or bony structures. Skin burn can be caused by poor acoustic coupling between the skin and the therapeutic window or a scar from a previous operation [9–11].
In HIFU cases with liver as the target, 34 (85%) showed decreased uptake on bone scans. Among the 34 cases, photon defect areas were noted in 29 cases (85%). The involved lesions had a relatively wide range from the right 5th to 12th ribs antero-axillary and/or posterior arcs. The decrease in bone uptake is deemed to be caused either directly by high-energy US waves that pass the focal therapeutic zone or indirectly by elevated temperatures of the overlying ribs. The beam may have moved up and down as the liver changed positions with the patient’s respiratory motion. Or in cases of multiple tumors, the range of the beam overlapping with the rib cage may have become greater. There were six bone scans with normal findings after liver treatment, and in all six cases, the lesion was located in the left hepatic lobe. Compared to the right hepatic lobe lesions, HIFU treatment of the left lobe could more easily avoid beams overlapping with the rib cage.
In cases targeting breast lesions, five out of six cases showed mildly decreased bone uptake, and the range of involved bone was relatively limited from the 2nd to 5th anterior ribs. When the treated lesion is located superficially, as with breast cancer, ribs posterior to breast tissue can be injured by reduced energy US waves.
In cases targeting the pancreas, three (19%) showed decreased uptake in the vertebrae. However, these patients also received external radiation therapy on the same area as HIFU treatment. In another pancreas treatment case, decreased uptake was noted in posterior arcs of the left 9th to 11th ribs, probably because the cancer was in the pancreas tail and the patient was in the left decubitus position for the HIFU treatment. The 12 pancreas treatment cases with normal bone scans all had target lesions in the pancreas head or body, and the patients were in prone position. Technically the beam path may slightly overlap with the vertebrae in the prone position, but the bone scans did not show abnormal findings.
There are many possible causes for localized cold lesions appearing on bone scans: overlying attenuation artifacts, radiation therapy effects, local vascular compromise such as infarctions and intrinsic vascular lesions or necrosis [12, 13]. In cases following HIFU treatment, high-energy US waves can cause direct thermal injury to the ribs along the ultrasound beam path. HIFU can lead to vessel wall disruption and vascular occlusion, and the vascular damage could cause bone changes such as bone atrophy. Fractures can also occur at the sites of weakened bones [14–17]. In 11 cases from this study, decreased bone uptake at the HIFU treatment path accompanied adjacent bone fractures.
There are some limitations to this study. Of the total 62 cases, further follow-up bone scans were available in only 20 patients, and the follow-up interval was irregular in these. Thus, the exact time point when the bone uptake becomes normal again could not be assessed. When the HIFU target was in the liver, normalized bone scan uptake was not observed before 24 months. In addition, the interval from HIFU treatment to the bone scan also varied, and we could not tell when the bone uptake started to appear as a photon defect. However, in one case with a bone scan done a day after the HIFU therapy, the bone uptake was already “cold.” The radiopharmaceutical was not uniform in the compound and the injected dose varied, but the decreased uptake areas were evident in most cases and could easily be identified visually. Although this study has some limitations, to the best of our knowledge no prior study demonstrating bone scan findings after HIFU treatment has been published.
Conclusion
Of 62 bone scans in patients with a history of HIFU treatment for primary or metastatic cancer, 43 scans (69%) presented decreased uptake in bones in the HIFU treatment path. Of 20 patients who had bone scans more than twice after HIFU, 5 showed recovered radiotracer uptake in the involved ribs in the follow-up bone scans. The findings of the current study have implications for reading bone scans of cancer patients; abnormal photon defects in the path of HIFU treatment on bone scans are most likely due to bone necrosis rather than direct bone invasion. In addition, judging from the fact that the patients were symptom free despite persistent cold defects in the bone scans, the HIFU treatment-related decreased bone uptake is of little clinical relevance.
Acknowledgments
Conflicts of interest
The authors declare that there are no conflicts of interest in this study.
References
- 1.Park MY, Jung SE, Cho SH, Piao XH, Hahn ST, Han JY, et al. Preliminary experience using high intensity focused ultrasound for treating liver metastasis from colon and stomach cancer. Int J Hyperthermia. 2009;25(3):180–8. doi: 10.1080/02656730802641949. [DOI] [PubMed] [Google Scholar]
- 2.Jung SE, Cho SH, Jang JH, Han JY. High-intensity focused ultrasound ablation in hepatic and pancreatic cancer: complications. Abdom Imaging. 2011;36(2):185–95. doi: 10.1007/s00261-010-9628-2. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy JE, Wu F, ter Haar GR, Gleeson FV, Phillips RR, Middleton MR, et al. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics. 2004;42(1–9):931–5. doi: 10.1016/j.ultras.2004.01.089. [DOI] [PubMed] [Google Scholar]
- 4.Kim YS, Rhim H, Choi MJ, Lim HK, Choi D. High-intensity focused ultrasound therapy: an overview for radiologists. Korean J Radiol. 2008;9(4):291–302. doi: 10.3348/kjr.2008.9.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Zhu H, Jin C, Zhou K, Li K, Su H, et al. High-intensity focused ultrasound (HIFU): effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur Radiol. 2009;19(2):437–45. doi: 10.1007/s00330-008-1137-0. [DOI] [PubMed] [Google Scholar]
- 6.Marmor JB, Pounds D, Hahn GM. Clinical studies with ultrasound-induced hyperthermia. Natl Cancer Inst Monogr. 1982;61:333–7. [PubMed] [Google Scholar]
- 7.Dubinsky TJ, Cuevas C, Dighe MK, Kolokythas O, Hwang JH. High-intensity focused ultrasound: current potential and oncologic applications. AJR Am J Roentgenol. 2008;190(1):191–9. doi: 10.2214/AJR.07.2671. [DOI] [PubMed] [Google Scholar]
- 8.Haar GT, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia. 2007;23(2):89–104. doi: 10.1080/02656730601186138. [DOI] [PubMed] [Google Scholar]
- 9.Furusawa H, Namba K, Thomsen S, Akiyama F, Bendet A, Tanaka C, et al. Magnetic resonance-guided focused ultrasound surgery of breast cancer: reliability and effectiveness. J Am Coll Surg. 2006;203(1):54–63. doi: 10.1016/j.jamcollsurg.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Stewart EA, Rabinovici J, Tempany CM, Inbar Y, Regan L, Gostout B, et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril. 2006;85(1):22–9. doi: 10.1016/j.fertnstert.2005.04.072. [DOI] [PubMed] [Google Scholar]
- 11.Li JJ, Xu GL, Gu MF, Luo GY, Rong Z, Wu PH, et al. Complications of high intensity focused ultrasound in patients with recurrent and metastatic abdominal tumors. World J Gastroenterol. 2007;13(19):2747–51. doi: 10.3748/wjg.v13.i19.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell MJ, Logan PM. Radiation-induced changes in bone. Radiographics. 1998;18(5):1125–36. doi: 10.1148/radiographics.18.5.9747611. [DOI] [PubMed] [Google Scholar]
- 13.Bell EG, McAfee JG, Constable WC. Local radiation damage to bone and marrow demonstrated by radioisotopic imaging. Radiology. 1969;92(5):1083–8. doi: 10.1148/92.5.1083. [DOI] [PubMed] [Google Scholar]
- 14.Delon-Martin C, Vogt C, Chignier E, Guers C, Chapelon JY, Cathignol D. Venous thrombosis generation by means of high-intensity focused ultrasound. Ultrasound Med Biol. 1995;21(1):113–9. doi: 10.1016/0301-5629(94)00095-6. [DOI] [PubMed] [Google Scholar]
- 15.Wu F, Chen WZ, Bai J, Zou JZ, Wang ZL, Zhu H, et al. Tumor vessel destruction resulting from high-intensity focused ultrasound in patients with solid malignancies. Ultrasound Med Biol. 2002;28(4):535–42. doi: 10.1016/S0301-5629(01)00515-4. [DOI] [PubMed] [Google Scholar]
- 16.Hynynen K, Chung AH, Colucci V, Jolesz FA. Potential adverse effects of high-intensity focused ultrasound exposure on blood vessels in vivo. Ultrasound Med Biol. 1996;22(2):193–201. doi: 10.1016/0301-5629(95)02044-6. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy JE, Ter Haar GR, Cranston D. High intensity focused ultrasound: surgery of the future? Br J Radiol. 2003;76(909):590–9. doi: 10.1259/bjr/17150274. [DOI] [PubMed] [Google Scholar]