Abstract
Purpose
Although the three-phase bone scan (TBPS) is one of the widely used imaging studies for diagnosing complex regional pain syndrome type I (CRPS-1), there is some controversy regarding the TPBS image criteria for CRPS-1. In this study, we modified the image criteria using image pattern and quantitative analysis in the patients diagnosed using the most recent consensus clinical diagnostic criteria.
Materials and Methods
The study included 140 patients with suspected CRPS-1 (CRPS-1, n = 79; non-CRPS, n = 61; mean age 39 ± 15 years) who underwent TPBS. The clinical diagnostic criteria for CRPS-1 revised by the Budapest consensus group were used for confirmative diagnosis. Patients were classified according to flow/pool and delayed uptake (DU) image patterns, and the time interval between the initiating event and TPBS (TIevent-scan). Quantitative analysis for lesion-to-contralateral ratio (LCR) was performed. Modified TPBS image criteria were created and evaluated for optimal diagnostic performance.
Results
Both increased and decreased periarticular DU were significant image findings for CRPS-1 (CRPS-1 positive-rate = 73% in the increased DU group, 75% in the decreased DU group). The TIevent-scan did not differ significantly between the different image pattern groups. Quantitative analysis revealed an LCR of 1.43 was the optimal cutoff value for CRPS-1 and diagnostic performance was significantly improved in the increased DU group (area under the curve = 0.732). Given the modified image criteria, the sensitivity and specificity of TPBS for diagnosing CRPS-1 were 80% and 72%, respectively.
Conclusions
Optimally modified TPBS image criteria for CRPS-1 were suggested using image pattern and quantitative analysis. With the criteria, TPBS is an effective imaging study for CRPS-1 even with the most recent consensus clinical diagnostic criteria.
Keywords: Complex regional pain syndrome type I, Diagnosis, Three-phase bone scan, Optimal criteria
Introduction
Complex regional pain syndrome type I (CRPS-1), formerly called reflex sympathetic dystrophy, is a chronic pain syndrome that accompanies autonomic nervous dysfunction to cause sensory, vasomotor, sudomotor, or motor/trophic abnormalities [1]. As its name implies, this syndrome has a complex pathogenesis and manifestations. A diagnosis of CRPS-1 is usually based on clinical criteria, including symptoms and signs without an objective diagnostic test [2, 3]. Since the International Association for the Study of Pain (IASP) coded the name and diagnostic criteria for the syndrome in 1994, there has been much effort to develop more accurate and valid diagnostic criteria for CRPS [4]. The most recent modification to the clinical diagnostic consensus criteria was proposed at an expert meeting held in Budapest in 2003. In these modified clinical diagnostic criteria, at least one symptom in all four symptom categories and at least one sign in two or more sign categories are required to meet a CRPS diagnosis [3].
In addition to a patient’s symptom and physical examination, several diagnostic tests have been used to support diagnosis of CRPS-1. Among them, the three-phase bone scan (TPBS) is one of the widely used imaging studies [5]. Typical CRPS-1 shows increased blood flow, pool, and delayed periarticular uptake in affected limbs on TPBS [6, 7]. However, there is still some controversy regarding the TPBS image criteria for CRPS-1. One controversy involves the role of flow and pool phase images. Several studies have challenged the role of these images [8–10], whereas others reported that they are helpful for diagnosis [11]. Quantitative analysis is another one. It is still unclear whether and how quantitative analysis has incremental value for the diagnosis of CRPS-1, despite several positive results [11–14]. Most of all, the diagnostic value of the so-called “atypical” finding of decreased delayed uptake with or without decreased blood flow/pool needs to be specified. It was reported that finding on TPBS changes with a stage progression of CRPS-1 [15–17] and that delayed uptake can be decreased in CRPS-1 [6, 18, 19], especially in later stages and in children. However, there are scarce data on the diagnostic value of atypical findings.
The diagnostic performance of TPBS has been highly variable, with sensitivity between 14% and 100% and specificity between 50% and 100% in several reports [8–10, 16, 20–23]. With these results, some researchers have argued that TPBS is not useful for diagnosing CRPS-1 [20, 21]. However, appropriate assessment of the diagnostic performance requires appropriate standard diagnostic criteria and refinement of image criteria with regard to these controversial points.
In this study, we analyzed TPBS image pattern in patients with suspected CRPS-1 after trauma or an operation. Afterward, TPBS image criteria for CRPS-1 were modified with image pattern and quantification information, adopting the clinical diagnostic criteria of the Budapest group as the standard diagnosis. The diagnostic performance of TPBS with the optimally modified image criteria was also assessed.
Materials and Methods
Patients
The Institutional Review Board of our hospital approved this retrospective study, and informed consent was waived. Among the patients who were referred to the pain center of our hospital with a clinical impression of CRPS-1 from 2004 to 2009, 140 patients whose TPBS was available for analysis were consecutively enrolled in this study. In general, the patients complained of severe chronic pain in a unilateral extremity after events of specific trauma or an operation without definite organic cause for the pain at the point. The initial diagnostic impression was CRPS-1 in the patients, and all patients were assessed using the clinical diagnostic criteria by the Budapest group for diagnosing CRPS-1 [3]. The time interval between the initiating event and TPBS (TIevent-scan) was determined by review of medical record. Among the 140 patients, 79 (56%) patients met the CRPS-1 criteria (CRPS-1 group) and the other 61 (44%) did not (non-CRPS group; Table 1).
Table 1.
Characteristics of patients and disease
| Characteristics | Total | CPRS-1 | Non-CPRS | P | |
|---|---|---|---|---|---|
| Cases | 140 | 79 | 61 | ||
| Age (years) | 39 ± 15 | 37 ± 15 | 41 ± 15 | 0.158 | |
| Gender | Male | 80 | 44 | 36 | n.s. |
| Female | 60 | 35 | 25 | ||
| Affected limb | Upper | 35 | 20 | 15 | n.s. |
| Lower | 105 | 59 | 46 | ||
| Side | Right | 67 | 37 | 30 | n.s. |
| Left | 73 | 42 | 31 | ||
| TIevent-scan (weeks) | 64 ± 80 | 55 ± 69 | 75 ± 92 | n.s. | |
n.s. not significant, P > 0.05; CRPS-1 complex regional pain syndrome type I, TI event-scan time interval between the initiating event and scan
Three-Phase Bone Scan and Image Analysis
TPBS was performed using large field-of-view gamma cameras (Ecam, Siemens, or Forte, Philips) equipped with low-energy general purpose collimators. For a flow phase image, a dynamic scan (1 frame/s) was performed for 60 s after an intravenous bolus injection of 740 MBq 99mTc-methylene diphosphonate (MDP). Afterward, a static scan was acquired for 3 min for a blood pool phase image. A delayed phase image was acquired about 4 h after the injection.
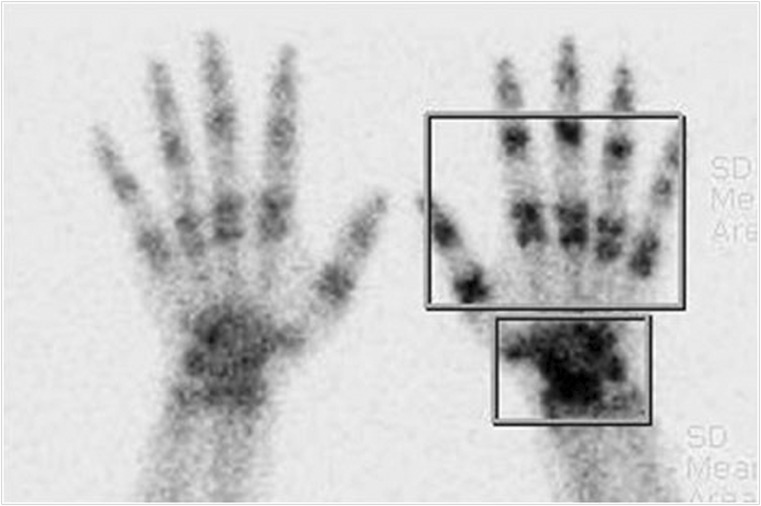
Three experienced nuclear medicine physicians analyzed TPBS images visually and quantitatively. Image analyses were performed for the two phase sets of flow/pool (F/P) and delayed uptake (DU) phases, in which the flow and pool phases were regarded as a single vascular phase set. Increase or decrease in F/P and DU was assessed for pattern analysis in case of periarticular distribution. Focal increase or decrease was not used in the pattern analysis because they suggested focal pathologic lesions rather than CRPS-1. For the quantitative analysis, regions of interest (ROIs) were drawn on the DU phase images for carpal (or tarsal for foot) and phalangeal periarticular areas (Fig. 1), and an ROI of same size and symmetric shape was drawn in contralateral side. Focal increase or decrease in carpal (or tarsal for foot) without abnormality in phalangeal periarticular area was excluded because of other possible pathology rather than CRPS-1 per se [27]. Afterward, a lesion-to-contralateral ratio (LCR) was calculated from the ratio of total radioactivity counts of ROIs (affected side/contralateral side).
Fig. 1.
ROIs for quantitative analysis. ROIs were drawn for carpal (or tarsal for foot) and phalangeal areas
Modification of Image Criteria and Statistical Analysis
Combined F/P and DU image findings were classified into nine pattern groups (Table 2) and the CRPS-1 positive-rate, which is similar to the positive predictive value (PPV), in each pattern group was calculated to correlate specific image findings with the diagnosis. Differences in CRPS-1 positive-rates according to TIevent-scan were also assessed for each pattern group. The diagnostic significance of the quantitative data and an optimal cutoff value were determined using receiver operating characteristics (ROC) curve and area under the curve (AUC) analysis. Finally, TPBS image criteria were modified to provide optimal diagnostic performance.
Table 2.
Image pattern groups and proportions of CRPS-1 or non-CRPS in each group
| Pattern group | Patients (n) | |||
|---|---|---|---|---|
| Delayed uptake | Flow/pool | Total | CRPS-1 | Non-CRPS |
| Inc. | Inc. | 42 | 29 (69%) | 13 (31%) |
| Sym. | 12 | 9 (75%) | 3 (25%) | |
| Dec. | 10 | 9 (90%) | 1 (10%) | |
| Sum | 64 | 47 (73%) | 17 (27%) | |
| Sym. | Inc. | 0 | - | - |
| Sym. | 39 | 11 (28%) | 28 (72%) | |
| Dec. | 13 | 3 (23%) | 10 (77%) | |
| Sum | 52 | 14 (27%) | 38 (73%) | |
| Dec. | Inc. | 0 | - | - |
| Sym. | 0 | - | - | |
| Dec. | 24 | 18 (75%) | 6 (25%) | |
| Sum | 24 | 18 (75%) | 6 (25%) | |
| Total | 140 | 79 (56%) | 61 (44%) | |
Inc. increased, Sym. symmetric, Dec. decreased; CRPS-1 complex regional pain syndrome type I
Continuous variables were tested using Student’s t-tests, and proportions were compared using chi-square tests. Data are expressed as mean ± standard deviation, and P values more than 0.05 were considered non-significant (n.s.). A commercial software package (MedCalc 11.1.1.0; MedCalc Software, Mariakerke, Belgium) was used for the statistical analysis.
Results
Patient Characteristics
The patient characteristics are summarized in Table 1. There was no significant difference in age, gender, pain site, and TIevent-scan between the CRPS-1 and non-CRPS groups.
Image Pattern of CRPS-1
In all cases, the blood flow and pool phase image patterns were in agreement; increased or decreased radioactivity when compared with contralateral side. Among the nine patterns derived from the combined F/P and DU image findings, three patterns (symmetric DU with increased F/P, decreased DU with increased F/P, and decreased DU with symmetric F/P) were not observed in either the CRPS-1 or non-CRPS groups (Table 2). In general, periarticular DU was the determinant factor, so the CRPS-1 positive-rate in the increased DU group was 73%, which was significantly higher than that in the symmetric DU group (27%; P < 0.0005). In particular, the CRPS-1 positive-rate tended to be higher in the “mismatched” groups of increased DU with symmetric F/P (75%) or decreased F/P (90%) groups than that in the increased DU with increased F/P group (69%), although these differences were not statistically significant. Decreased DU was such a significant diagnostic finding that the CRPS-1 positive-rate was 75% in the decreased DU group, which was significantly higher than in the symmetric DU group (P < 0.0005).
When both increased and decreased periarticular DU were used as the CRPS-1 diagnostic criteria, the sensitivity and specificity were 82% (65/79) and 62% (38/61), respectively.
Time Intervals Between Initiating Event and TPBS
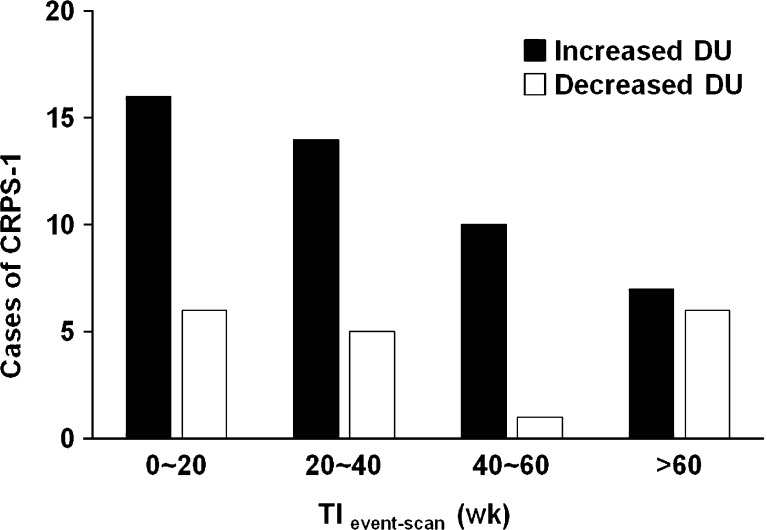
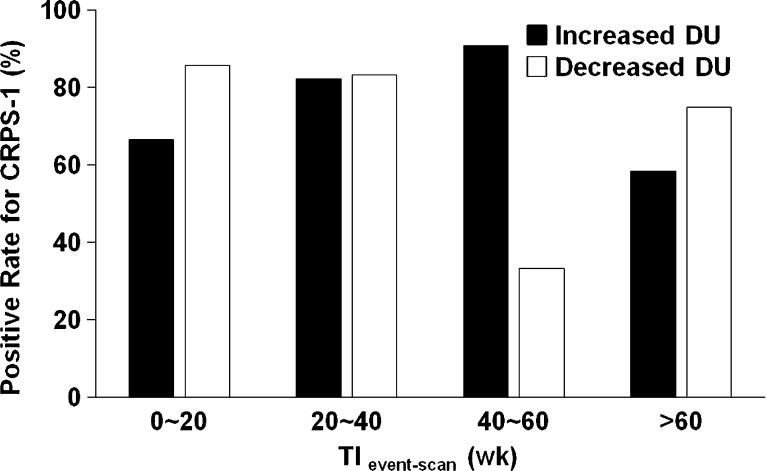
TIevent-scan was shorter in the increased DU group than in the decreased DU group (39 ± 40 week vs 80 ± 114 week, P = 0.034) in CRPS-1, and CRPS-1 cases with increased DU were more frequently observed when TIevent-scan was shorter (Fig. 2). However, there was no specific tendency in the decreased DU group (Fig. 2). There was no remarkable difference in CRPS-1 positive-rate by TIevent-scan for any pattern group (Fig. 3). Although an abnormally low positive-rate was observed in the decreased DU group in which TIevent-scan was 40–60 weeks, the data were distorted because of the small number of cases (n = 3), and the difference was not statistically significant.
Fig. 2.
Cases of CRPS-1 in each pattern group according to the time interval between the initiating event and scan (TIevent-scan). CRPS-1 cases were more frequently observed when TIevent-scan was shorter in the increased delayed uptake (DU) group, whereas there was no specific tendency in the decreased DU group
Fig. 3.
Positive-rates of CRPS-1 according to the time interval between the initiating event and scan (TIevent-scan) for each pattern group. There was no significant difference in positive-rates by TIevent-scan
Quantitative Analysis and Modified Diagnostic Criteria
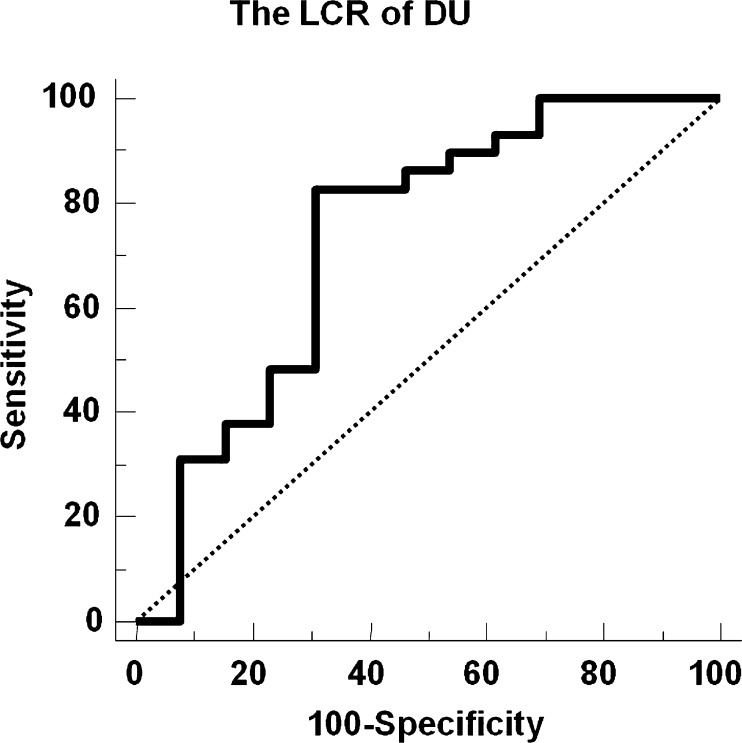
To enhance the diagnostic performance of TPBS, the LCR of DU was tested as one of image criteria in the increased and decreased DU pattern groups. The LCR of DU had significant diagnostic power (AUC = 0.732 on ROC analysis; Fig. 4) only in the increased DU with increased F/P group. In this group, the LCR of DU showed a sensitivity of 83% and a specificity of 69% with the cutoff value of 1.43. Specificity was significantly improved (vs 0%; P = 0.001) without a significant impairment in sensitivity (vs 100%; P = n.s.). However, the LCR had no diagnostic significance in the decreased DU group.
Fig. 4.
ROC curve analysis of quantitative data in the subgroup having increased DU with increased F/P. With an optimal cutoff LCR of 1.43, the area under the curve was 0.732. The sensitivity and specificity with the optimal cutoff were 83% and 69%, respectively
From these results, a set of modified TPBS image criteria for CRPS-1 were created: (1) decreased DU with decreased F/P; (2) increased DU with symmetric or decreased F/P; (3) increased DU with increased F/P, in which the LCR of DU is greater than 1.43. Given these criteria, the sensitivity and specificity of TPBS for diagnosing CRPS-1 were 80% and 72%, respectively.
Discussion
In this study, TPBS image patterns were analyzed with regard to CRPS-1. Periarticular DU was the most significant finding for diagnosing CRPS-1, and F/P provided additional information, especially when F/P was decreased or symmetric despite an increased DU. Interestingly, so-called “atypical” finding of decreased DU was also a significant factor and had a similar CRPS-1 positive-rate to that of increased DU. In addition, quantitative analysis provided an incremental diagnostic value in the subgroup having increased DU with increased F/P, resulting in improved specificity without impairment of sensitivity.
TPBS is one of widely used imaging studies in CRPS-1. Although osteoporosis on simple X-ray or marrow edema and soft tissue swelling on MRI are supportive findings for CRPS-1, they are not as sensitive or specific as TPBS [16]. However, there are wide variations in the diagnostic sensitivity and specificity of TPBS [8–10, 16, 20–23], and some authors argued that TPBS cannot provide useful information for making a clinical diagnosis of CRPS-1 [20, 21]. One of the potential causes of these variations is the changes in clinical diagnostic criteria of CRPS-1. A CRPS-1 diagnosis is based on clinical criteria composed of symptoms and signs, which have been changed by researcher consensus. The change in the diagnostic criteria reportedly resulted in considerable variation in the diagnostic performance of the clinical criteria themselves with sensitivity of 70-98% and specificity of 36-96% [24]. Therefore, the diagnostic performance of TPBS for CRPS-1 should be reevaluated and updated with the change in the clinical diagnostic criteria. This study adopted the most recent consensus clinical diagnostic criteria.
Another cause of the variation is the dynamic nature of CRPS-1. It was suggested that CRPS-1 has clinical stages [25] and TPBS findings change according to clinical stages [15–17]. It was reported that F/P normalizes or even decreases, and afterward, DU normalizes as the disease progresses [15]. Therefore, it has been suggested that image analysis or the indication for TPBS be modified accordingly [15, 17, 20]. However, in the present study, there was no significant influence of TIevent-scan on CRPS-1 positive-rates by image pattern group, and it was difficult to set a specific time point to discriminate each stage in the general patient population. It seems that changes of F/P and DU are so dynamic to set a specific time point. Therefore, only TPBS image findings may contribute the information for a diagnosis, while TIevent-scan can not. In such cases, F/P images may give additional information. In this study, somewhat paradoxical mismatches of increased DU with symmetric or decreased F/P strongly supported a diagnosis of CRPS-1. This is reasonable, because those findings are often unexpected in other conditions that cause increased DU.
Another intriguing point is that decreased DU with decreased F/P was a significant finding for diagnosing CRPS-1. In this pattern group, the CRPS-1 positive-rate was 75%, which was no less than that in the group with “typical” finding (increased DU and increased F/P; 73%). Decreased DU has been described as an atypical but supportive of CRPS-1 [6, 18], and the mechanism was speculated to be vasoconstriction and atrophy [26]. However, as the findings may also result from disuse atrophy or relative overuse of the contralateral extremity, other pathologic conditions may show similar findings. As our hospital is a tertiary referral hospital, most of the patients who are referred to the pain center are strongly suspected of having CRPS-1. Thus, the patients in this study were a kind of selected group, which might have been the cause for the unexpectedly high positive-rate of decreased DU. Although further study is required in different patient groups, decreased DU is suggested to be a strong supportive finding for CRPS-1.
Quantification may provide incremental value for the diagnostic performance of TPBS. Several authors have adopted quantitative analysis of TPBS for the diagnosis [11], severity and prognosis assessment [12, 13], and therapeutic monitoring [14] of CRPS-1. Because TPBS findings show dynamic changes with stage progression, it is uncertain whether a quantified value would be a marker for disease severity. However, in the selected subgroup having increased DU with increased F/P, it may function as a significant CRPS-1 image marker, as it did in this study. It is speculated that CRPS-1 causes a more definite increase in periarticular DU and that the stage conversion of TPBS occurs swiftly despite the variable duration of each phase.
Many questions remain about the pathogenesis, diagnosis, and treatment of CRPS-1, which affects patients’ quality of life so profoundly. As adequate diagnosis is the basis for the studies, clinicians have tried to set optimal diagnostic criteria. In addition to clinical diagnostic criteria, objective methods like TPBS, sympathetic skin response and thermography can be used for diagnosis of CRPS-1. Most of them are supplementary and complementary for adequate diagnosis. Among them, TPBS is a valuable imaging study for diagnosing CRPS-1, even given the most recent clinical diagnostic criteria as was in this study. Moreover, TPBS can provide pathophysiological information on stage progression of CRPS-1. The results of this study suggest that TPBS may be used more effectively for diagnosing CRPS-1.
In this study, TPBS images of CRPS-1-suspected patients were analyzed using the clinical diagnostic criteria by the Budapest group. DU was the most significant image finding for diagnosing CRPS-1, and F/P provided additional information. In addition to increased periarticular DU, decreased DU was a supportive finding for CRPS-1 in this study. In the subgroup having increased DU with increased F/P, quantitative analysis provided incremental information for diagnosing CRPS-1, with an LCR cutoff value of 1.43. Sensitivity and specificity were 80% and 72%, respectively, using the optimally modified TPBS image criteria. Therefore, TPBS with optimally modified image criteria is an effective imaging study for CRPS-1 even with the most recent consensus clinical diagnostic criteria.
Acknowledgments
Conflict of Interest
None.
Contributor Information
Hyun Woo Kwon, Phone: +82-2-20722920, FAX: +82-2-7669083, Email: hnwoo00@gmail.com.
Jin Chul Paeng, Phone: +82-2-20723793, FAX: +82-2-7669083, Email: paengjc@snu.ac.kr.
Francis Sahngun Nahm, Phone: +82-31-7877499, FAX: +82-2-7669083, Email: hiitsme@hanmail.net.
References
- 1.Harden RN, Bruehl SP. Diagnosis of complex regional pain syndrome: signs, symptoms, and new empirically derived diagnostic criteria. Clin J Pain. 2006;22:415–419. doi: 10.1097/01.ajp.0000194279.36261.3e. [DOI] [PubMed] [Google Scholar]
- 2.Reinders MF, Geertzen JH, Dijkstra PU. Complex regional pain syndrome type I: use of the International Association for the Study of Pain diagnostic criteria defined in 1994. Clin J Pain. 2002;18:207–215. doi: 10.1097/00002508-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007;8:326–331. doi: 10.1111/j.1526-4637.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 4.Stanton-Hicks M, Janig W, Hassenbusch S, Haddox JD, Boas R, Wilson P. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain. 1995;63:127–133. doi: 10.1016/0304-3959(95)00110-E. [DOI] [PubMed] [Google Scholar]
- 5.Harvey RL, Roth EJ, Yu D. Rehabilitation in stroke syndromes. In: Braddom RL, editor. Physical medicine and rehabilitation. China: Elsevier; 2007. pp. 1175–1212. [Google Scholar]
- 6.Fournier RS, Holder LE. Reflex sympathetic dystrophy: diagnostic controversies. Semin Nucl Med. 1998;28:116–123. doi: 10.1016/S0001-2998(98)80022-6. [DOI] [PubMed] [Google Scholar]
- 7.Intenzo CM, Kim SM, Capuzzi DM. The role of nuclear medicine in the evaluation of complex regional pain syndrome type I. Clin Nucl Med. 2005;30:400–407. doi: 10.1097/01.rlu.0000162605.14734.11. [DOI] [PubMed] [Google Scholar]
- 8.Davidoff G, Werner R, Cremer S, Jackson MD, Ventocilla C, Wolf L. Predictive value of the three-phase technetium bone scan in diagnosis of reflex sympathetic dystrophy syndrome. Arch Phys Med Rehabil. 1989;70:135–137. [PubMed] [Google Scholar]
- 9.Werner R, Davidoff G, Jackson MD, Cremer S, Ventocilla C, Wolf L. Factors affecting the sensitivity and specificity of the three-phase technetium bone scan in the diagnosis of reflex sympathetic dystrophy syndrome in the upper extremity. J Hand Surg Am. 1989;14:520–523. doi: 10.1016/S0363-5023(89)80016-4. [DOI] [PubMed] [Google Scholar]
- 10.O’Donoghue JP, Powe JE, Mattar AG, Hurwitz GA, Laurin NR. Three-phase bone scintigraphy. Asymmetric patterns in the upper extremities of asymptomatic normals and reflex sympathetic dystrophy patients. Clin Nucl Med. 1993;18:829–836. doi: 10.1097/00003072-199310000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Park SG, Hyun JK, Lee SJ, Jeon JY. Quantitative evaluation of very acute stage of complex regional pain syndrome after stroke using three-phase bone scintigraphy. Nucl Med Commun. 2007;28:766–770. doi: 10.1097/MNM.0b013e32828e513f. [DOI] [PubMed] [Google Scholar]
- 12.Atkins RM, Tindale W, Bickerstaff D, Kanis JA. Quantitative bone scintigraphy in reflex sympathetic dystrophy. Br J Rheumatol. 1993;32:41–45. doi: 10.1093/rheumatology/32.1.41. [DOI] [PubMed] [Google Scholar]
- 13.Zyluk A, Birkenfeld B. Quantitative evaluation of three-phase bone scintigraphy before and after the treatment of post-traumatic reflex sympathetic dystrophy. Nucl Med Commun. 1999;20:327–333. doi: 10.1097/00006231-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Ozturk E, Mohur H, Arslan N, Entok E, Tan K, Ozguven MA. Quantitative three-phase bone scintigraphy in the evaluation of intravenous regional blockade treatment in patients with stage-I reflex sympathetic dystrophy of upper extremity. Ann Nucl Med. 2004;18:653–658. doi: 10.1007/BF02985958. [DOI] [PubMed] [Google Scholar]
- 15.Demangeat JL, Constantinesco A, Brunot B, Foucher G, Farcot JM. Three-phase bone scanning in reflex sympathetic dystrophy of the hand. J Nucl Med. 1988;29:26–32. [PubMed] [Google Scholar]
- 16.Todorovic-Tirnanic M, Obradovic V, Han R, Goldner B, Stankovic D, Sekulic D, et al. Diagnostic approach to reflex sympathetic dystrophy after fracture: radiography or bone scintigraphy? Eur J Nucl Med. 1995;22:1187–1193. doi: 10.1007/BF00800604. [DOI] [PubMed] [Google Scholar]
- 17.Park SA, Yang CY, Kim CG, Shin YI, Oh GJ, Lee M. Patterns of three-phase bone scintigraphy according to the time course of complex regional pain syndrome type I after a stroke or traumatic brain injury. Clin Nucl Med. 2009;34:773–776. doi: 10.1097/RLU.0b013e3181b7d980. [DOI] [PubMed] [Google Scholar]
- 18.Kozin F, Soin JS, Ryan LM, Carrera GF, Wortmann RL. Bone scintigraphy in the reflex sympathetic dystrophy syndrome. Radiology. 1981;138:437–443. doi: 10.1148/radiology.138.2.7455127. [DOI] [PubMed] [Google Scholar]
- 19.Turpin S, Taillefer R, Lambert R, Leveille J. “Cold” reflex sympathetic dystrophy in an adult. Clin Nucl Med. 1996;21:94–97. doi: 10.1097/00003072-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Lee GW, Weeks PM. The role of bone scintigraphy in diagnosing reflex sympathetic dystrophy. J Hand Surg Am. 1995;20:458–463. doi: 10.1016/S0363-5023(05)80107-8. [DOI] [PubMed] [Google Scholar]
- 21.Schurmann M, Zaspel J, Lohr P, Wizgall I, Tutic M, Manthey N, et al. Imaging in early posttraumatic complex regional pain syndrome: a comparison of diagnostic methods. Clin J Pain. 2007;23:449–457. doi: 10.1097/AJP.0b013e31805c9e66. [DOI] [PubMed] [Google Scholar]
- 22.Holder LE, Mackinnon SE. Reflex sympathetic dystrophy in the hands: clinical and scintigraphic criteria. Radiology. 1984;152:517–522. doi: 10.1148/radiology.152.2.6739825. [DOI] [PubMed] [Google Scholar]
- 23.Holder LE, Cole LA, Myerson MS. Reflex sympathetic dystrophy in the foot: clinical and scintigraphic criteria. Radiology. 1992;184:531–535. doi: 10.1148/radiology.184.2.1620860. [DOI] [PubMed] [Google Scholar]
- 24.Bruehl S, Harden RN, Galer BS, Saltz S, Bertram M, Backonja M, et al. External validation of IASP diagnostic criteria for Complex Regional Pain Syndrome and proposed research diagnostic criteria. International Association for the Study of Pain. Pain. 1999;81:147–154. doi: 10.1016/S0304-3959(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 25.Kurvers HA. Reflex sympathetic dystrophy: facts and hypotheses. Vasc Med. 1998;3:207–214. doi: 10.1177/1358836X9800300305. [DOI] [PubMed] [Google Scholar]
- 26.Wasner G, Schattschneider J, Heckmann K, Maier C, Baron R. Vascular abnormalities in reflex sympathetic dystrophy (CRPS I): mechanisms and diagnostic value. Brain. 2001;124:587–599. doi: 10.1093/brain/124.3.587. [DOI] [PubMed] [Google Scholar]
- 27.Wuppenhorst N, Maier C, Frettloh J, Pennekamp W, Nicolas V. Sensitivity and specificity of 3-phase bone scintigraphy in the diagnosis of complex regional pain syndrome of the upper extremity. Clin J Pain. 2010;26:182–189. doi: 10.1097/AJP.0b013e3181c20207. [DOI] [PubMed] [Google Scholar]