Abstract
Purpose
The aim of this study was to define an optimal CT number range applicable to adipose tissue (AT) measurement in modern PET/CT systems.
Methods
CT number (in Hounsfield units, HU) was measured in three different pure AT compartments in 53 patients. CT number range for AT was determined in three different ways, including pixel histogram analysis, to take the effect of partial volume averaging into account. The effect of changing the CT number range for AT on the total AT volume was investigated.
Results
The lower limits for CT number for pure subcutaneous AT, retroperitoneal AT, and visceral AT were −140, –140, and −130 HU, respectively. The corresponding upper limits were −70, –71, and −52 HU. The CT number range for AT using three methods when considering partial volume averaging was −144 to −141 HU to −30 to −33 HU, showing similar values between the three methods. The optimal CT number range for AT based on these data was −140 to −30 HU. Increases in total AT volume of 7.5 % and 1.8 % were found when the upper or lower limit was extended using 10 HU intervals, respectively, compared with the reference range of −140 to −30 HU.
Conclusion
This study demonstrated that the optimal CT number range of AT that is applicable to modern PET/CT systems can be defined as −140 to −30 HU. The use of this CT number range of AT allowed lean body mass to be determined in whole-body F-18 FDG PET/CT studies.
Keywords: Adipose tissue, Lean body mass, Body composition, PET/CT
Introduction
Standardized uptake value is an important and commonly used index in FDG PET/CT studies [1, 2]. The use of standardized uptake values that are normalized by lean body mass (LBM) is recommended for PET response criteria in solid tumors. However, LBM is usually estimated with a variety of predictive equations. Ideally, the direct determination of individual LBM would be preferable to reduce the variability in LBM when estimated by predictive equations. LBM is defined as either adipose tissue-free body mass or fat-free body mass [3–5].
Computed tomography (CT) can be used to quantify adipose tissue (AT) volume or mass. CT imaging methods for measuring AT have been validated in animals [6], human cadavers [7, 8], and living human studies [9, 10]. CT is now considered the most accurate method for the direct in vivo measurement of AT volume [11, 12]. The first step when measuring AT or adipose-tissue-free mass (i.e., LBM) is to define a CT number range (in Hounsfield units, HU) for AT. Various CT number ranges for AT have been described in the literature, where lower CT number limits for AT have included −250, –200, –190, –150, –140, –130, and −110 HU, while the upper CT number limits have included −70, –50, –40, –30, and −10 HU [8, 9, 13–18].
The most commonly used CT number range is −190 to −30 HU [16]. However, most of these data ranges were obtained using outdated CT scanners, reconstruction algorithm, and CT acquisition parameters. Thus, these ranges will be different from those that are appropriate for the modern multidetector spiral CTs currently used in F-18 FDG PET/CT studies. The CT components of modern PET/CT systems provide high image resolution while reducing the effect of partial volume averaging, an improved reconstruction algorithm that reduces beam hardening and image artifacts due to metallic prosthesis, and a capacity for automated tube current modulation while reducing the effect of photon starvation [19–21]. Therefore, there is a need to define the optimal CT number range for AT that would be applicable to modern PET/CT systems.
The aim of this study was to define the optimal CT number range of AT that would be applicable to modern PET/CT systems, and to determine the LBM in whole-body F-18 FDG PET/CT studies.
Materials and Methods
Whole-body F-18 FDG PET/CT studies (Biograph 16, Siemens, Knoxville, TN, USA) were conducted with 72 patients for routine clinical purposes. The characteristics of patients are shown in Table 1. Institutional review board approval was not required for these routine clinical studies.
Table 1.
Characteristics of patients studied in LBM determination
| Characteristics | Value (range) |
|---|---|
| Men:women | 37:35 |
| Age (years) | 57.9 ± 14.0 (23–81) |
| Weight (kg) | 62.2 ± 13.4 (38.3–93.0) |
| Height (cm) | 162.1 ± 9.6 (140–191) |
| BMI (kg m−2) | 23.5 ± 3.8 (17.1–36.3) |
| LBM (kg) | 43.4 ± 10.0 (26.5–73.4) |
BMI body mass index, LBM lean body mass
Patients were positioned feet first and supine, with their arms laid beside their body, and scans were acquired from head to feet. The CT acquisition parameters were as follows: tube voltage of 120 kVp, tube current of 60 mAs (care dose), tube rotation speed of 0.5 s, table feed of 36 mm s−1, beam collimation of 24 mm for 16 channels, image matrix size of 512 × 512, and a 5-mm thickness slice for a reconstructed image with a 3-mm spacing. Neither intravenous nor oral contrast agents were administered.
In the present study, AT was defined as voxels that were identifiable and measurable by CT in the CT number range for AT.
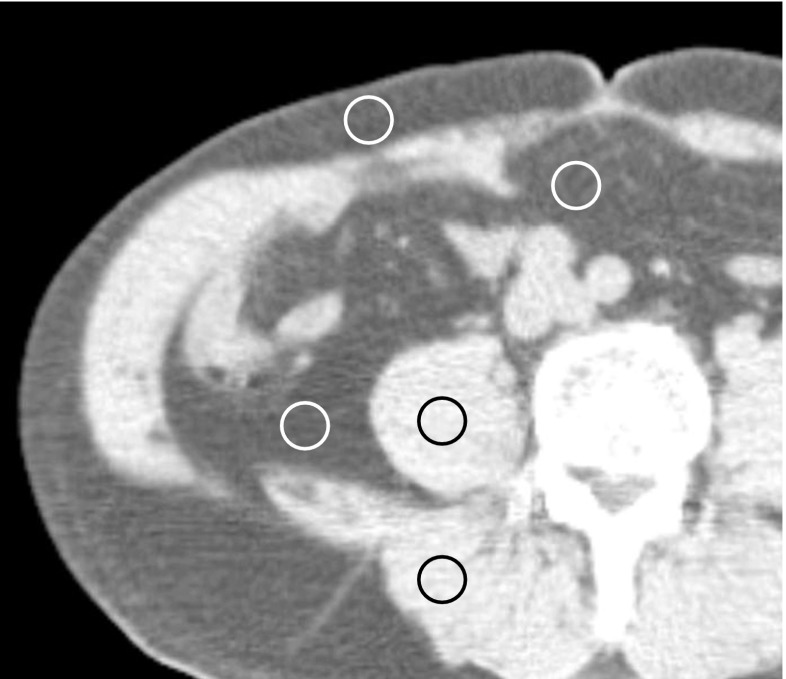
To investigate the CT number distribution for pure AT, a region of interest (ROI, approximately 1 cm2) was drawn in subcutaneous AT (SAT), visceral AT (VAT), and retroperitoneal AT (RAT) on a single image of the abdomen at the level of the umbilicus in 53 patients (Fig. 1). The ROI could not be drawn with some patients due to a lack of AT, most notably in the VAT.
Fig. 1.
ROIs of approximately 1 cm2 were placed over the subcutaneous adipose tissue (AT), visceral AT, and retroperitoneal AT, and then range of CT number for pure AT were measured in three AT compartments. Similarly, ROIs were drawn in the psoas and paraspinal muscles
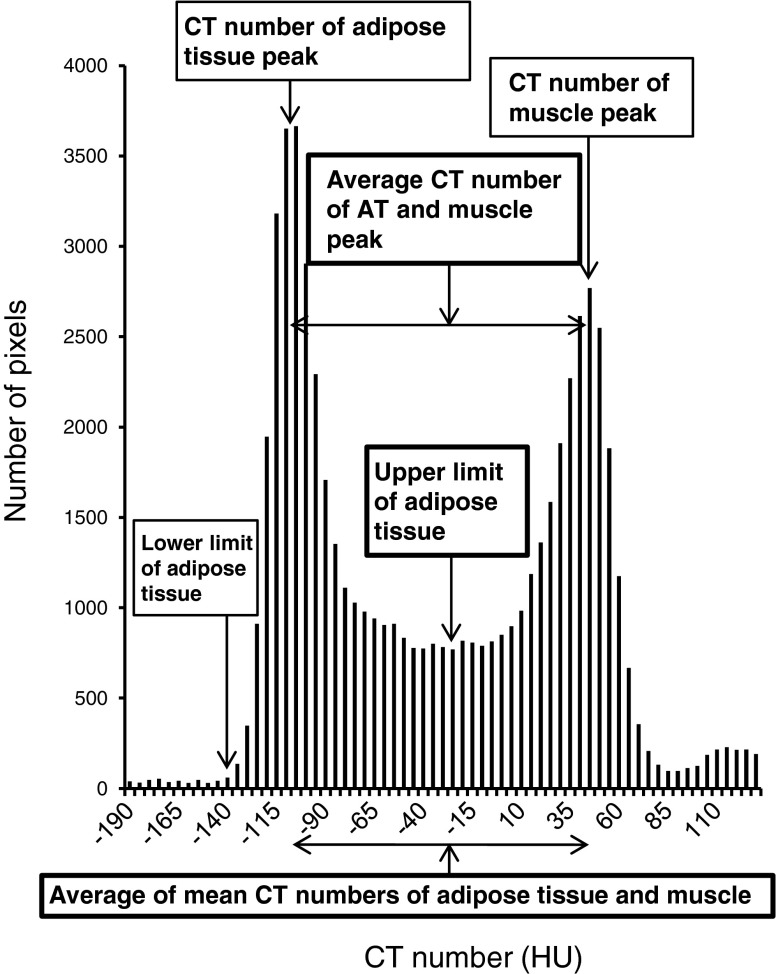
The effect of partial volume averaging was taken into account when determining optimal CT number range for AT. Histograms were constructed of the total pixels contained in a single slice of the abdomen image at the level of the umbilicus (n = 45) and in the chest at the level of pulmonary artery (n = 66). CT number ranges were then determined using the following three different methods as follows (Fig. 2).
The lowest point between two peaks of histogram, representing AT and muscle, was chosen as the upper CT number limit for AT in each patient. The point where the peak of AT approached the horizon was defined as the lower CT number limit for AT.
The midpoint between the two peaks of the histogram, representing AT and muscle, was chosen as the upper CT number limit for AT.
The average of the mean CT numbers of AT and muscle was chosen as the upper CT number limit for AT. The mean CT number for AT and muscle that was used was measured in the corresponding region of the abdomen and chest of the same image slice that the histogram of total pixels was based on (Fig. 1).
Fig. 2.
Optimal range of CT number for adipose tissue obtained by three different methods demonstrates nearly identical upper and lower limits
To evaluate the effect of changing the CT number range for AT on the total AT volume, the relative changes in total AT volume were calculated by varying the CT number range in 10-HU intervals.
Calculation of the total AT volume was conducted using a built-in software package provided by the manufacturer. Total AT volume throughout the body was calculated automatically by multiplying total number of voxels with −140 to −30 HU by a voxel volume. LBMs were subsequently determined as follows: LBM (kg) = body weight (kg) – total AT volume (l) × AT density of 0.95 (kg l−1) [22].
Statistical Analysis
Statistical analysis was conducted using PASW Statistics 18 (SPSS, Chicago, IL, USA). P < 0.05 was considered statistically significant. Any differences in the comparisons of measured values were tested using a one-way analysis of variance (ANOVA) or a t-test. When any difference was found using ANOVA, post hoc analysis with Duncan’s statistics was conducted.
Results
The lower limits for CT number for SAT and RAT were −140 HU each, and the corresponding upper limits were −70 and −71 HU, respectively. The upper and lower limits for VAT were −130 and −52 HU, respectively, showing significant differences in CT number limits for VAT from the other two AT compartments (Table 2).
Table 2.
Distribution of CT number for pure adipose tissue in three compartments and those for pure muscle compartments
| Compartment | Lower limit (mean ± SD) | Upper limit (mean ± SD) | Mean (mean ± SD) |
|---|---|---|---|
| Subcutaneous AT (n = 53) | −140.09 ± 10.84 | −70.23 ± 11.80 | −105.97 ± 8.67 |
| Visceral AT (n = 41) | −130.41 ± 13.44 | −52.07 ± 17.84 | −94.70 ± 9.43 |
| Retroperitoneal AT (n = 45) | −139.60 ± 10.53 | −70.67 ± 11.86 | −106.53 ± 6.64 |
| Psoas Muscle (n = 49) | 6.65 ± 12.02 | 77.24 ± 10.32 | 41.63 ± 7.38 |
| Paraspinal Muscle (n = 47) | 7.15 ± 12.59 | 76.77 ± 9.41 | 42.83 ± 7.06 |
AT adipose tissue, CT computed tomography
The CT number ranges for AT using three methods when considering partial volume averaging ranged from −144 to −141 HU to −30 to −33 HU, indicating that values were almost similar among the three methods (Table 3). No clinically significant differences in the CT number range for AT was detected between the three methods (Table 3).
Table 3.
Optimal range of CT number for adipose tissue by three different methods
| Abdomen (n = 45) | Chest (n = 66) | |
|---|---|---|
| mean ± SD | mean ± SD | |
| Lower limit of AT | −144.1 ± 7.7 | −141.1 ± 7.3 |
| Upper limit of AT | −30.6 ± 6.2 | −32.8 ± 7.5 |
| Mean CT no. of AT | −105.5 ± 8.4 | −104.3 ± 9.5 |
| Mean CT no. of muscle | 44.0 ± 5.0 | 44.3 ± 5.4 |
| Average of mean CT no. of AT and muscle | −30.8 ± 4.6 | −30.0 ± 4.8 |
| CT no. of AT peak | −102.6 ± 8.6 | −101.1 ± 15.9 |
| CT no. of muscle peak | 40.7 ± 7.4 | 41.1 ± 5.9 |
| Average CT no. of AT and muscle peak | −30.9 ± 4.7 | −30.0 ± 7.8 |
AT adipose tissue, CT computed tomography
Based on these data, the optimal CT number range for AT was defined as −140 to −30 HU.
Increases in total AT volume of 7.5 % and 1.8 % were found when extending the upper or lower limit by 10 HU, respectively, when compared with the reference value range of −140 to −30 HU (Table 4).
Table 4.
Relative Changes of Total AT Volume (%) by Varying the CT Number Ranges for AT with 10-HU Intervals, Compared with the Reference Value of −140 to −30 HU (n = 72)
| −50 | −40 | −30 | −20 | −10 | |
|---|---|---|---|---|---|
| −130 | 82.9 % | 89.9 % | 95.7 % | 103.3 % | 110.7 % |
| −140 | 87.2 % | 94.2 % | 100.0 % | 107.5 % | 115.0 % |
| −150 | 89.0 % | 96.0 % | 101.8 % | 109.3 % | 116.8 % |
| −160 | 90.6 % | 97.6 % | 103.4 % | 111.0 % | 118.4 % |
| −170 | 91.3 % | 98.3 % | 104.1 % | 111.7 % | 119.1 % |
AT adipose tissue, CT computed tomography, HU Hounsfield unit
Using this CT number range, LBMs were successfully determined by CT in all 72 patients and the LBM was 43.4 ± 10.0 kg (mean ± SD) (Table 1).
Discussion
We initially investigated the CT number distribution for pure AT to determine whether any difference might exist between the three different AT compartments. Significant differences in the CT number distribution for AT were found between VAT and the other two AT compartments (i.e., SAT and RAT) (Table 2). This difference in the CT number distribution for VAT compared with the other two AT compartments could be attributable to the effect of partial volume averaging, which is partly due to blood vessels within pixels, and differences in biological properties between AT compartment, including differences in the fat fraction of AT, blood flow, and glucose metabolism [14, 23, 24]. This observation agreed with a previous report [25].
The effect of partial volume averaging could be evaluated by constructing pixel histograms for mixtures of tissues, such as AT and soft tissues, and this effect was taken into account when determining the optimal CT number range for AT, particularly for the upper limit. Histograms of the total pixels contained in a single image slice of the abdomen (n = 45) and the chest (n = 66), were constructed as previously described [16, 26]. Statistically significant differences in both the upper and lower limits were detected between the abdomen and chest images (P = 0.039). However, the differences were less than 3 HU and they were considered to be clinically insignificant because the calibration of CT number measurements for water is typically 0 ± 4 HU (mean ± SD).
The data from abdominal image, and from chest image containing large amount of air and beating heart are almost similar, indicating consistency of the results. Extending the lower limit from −140 HU to −170 HU resulted in only a 4.1 % increase in the total AT volume, whereas extending the upper limit from −30 HU to −20 HU resulted in a 7.5 % increase in the total AT volume (Table 4). Narrowing the upper limit from −30 HU by 10-HU intervals resulted in 5.8 % and 7.0 % decreases in total AT volume, respectively. This observation is in agreement with previous reports [8, 26]. Thus, defining the upper CT number limit for AT was critical to the determination of AT volume. However, in the present study, an upper limit of −30 HU was consistently produced by the three methods, so this limit is considered to be reasonable and robust. Air does not normally contact directly with human adipose tissues. The skin, pleura and gastrointestinal wall normally interpose between air and adipose tissue. The skin thickness (epidermis and dermis) of head and trunk is typically 2.0 mm for adult male and 1.6 mm for adult female, which are greater than or equivalent to the typical pixel size of modern spiral CT [27]. Thus the majority of the pixels having a CT number of, for example, −190 to −141 HU, may be considered to be the pixels containing mixtures of air and soft tissues such as the skin. In practical determination of the lower limit, the majority of pixels with −190 to −141 HU were between skin and air or between the intestinal wall and the luminal gas of the intestine, while only two pixels appeared within the SAT of the representative patients. This observation may happen more likely when respiratory movement of abdominal and chest wall increases, and long scanning time or large voxel size is applied as well.
Theoretically, X-ray tube voltage by different scanner type may affect the CT number for particular tissues owing to different linear attenuation coefficients, but tube current does not affect CT number for particular tissue, although low-dose scanning used in the current study increases image noise as described in a previous phantom study [25]. The clinical significance of using slightly different tube voltage in measuring total AT volume is questionable. Five different CT scanners by different manufacturers, with possibly different tube voltages, have been used to evaluate CT number for AT, and to measure AT volume in four subjects [18]. Each subject underwent CT examination using five different CT scanners. In this study, the investigators have reported that CT number for AT varies between scanner types, but calculated total AT volume of each subject was almost identical among scanners.
Based on our data, –140 to −30 HU was considered the optimal CT number range for AT, although either −150 HU or −130 HU could produce acceptably small errors as alternative lower limits for AT.
Previous investigators have used various combinations of lower and upper CT number limits for AT, including, –190 to −30, –150 to −50, –140 to −40, –130 to −30, –130 to −10 and −110 to −70 HU [8, 9, 13–18]. Probably the most commonly used range is −190 to −30 HU, which was proposed by Kvist et al. [16]. CT number for AT is affected by factors such as partial volume averaging, beam hardening, photon starvation, image artifacts due to the presence of metallic materials in or on the patient, and patient movement. Kvist et al. [16] deeply considered the effects of some of these factors, particularly image artifacts due to metallic prosthetics and beam hardening, when determining a CT number range for AT and this led them to define the lower CT number limit for AT as −190 HU. However, beam hardening can be minimized using filtration, calibration correction, and beam hardening correction software in modern PET/CT systems [19]. Automated tube current modulation (care dose) or adaptive filtration software are now used in some scanners to reduce photon starvation effects [21]. Streaking artifacts caused by metallic prosthetics can be greatly reduced using an improved reconstruction algorithm or special software corrections [20]. Modern PET/CT systems provide high image resolution with thin slice thicknesses and fast scanning times, thereby reducing the effect of partial volume averaging and motion artifacts [19]. These artifacts may still be observed in images, but any effects of these artifacts on the determination of AT volume are considered limited compared with the large number of errors obtained with other body fat measurement methods that lie in the range of 3–15 % [16]. Wang et al. [28] compared 16 currently used methods for total body fat measurement in 23 healthy subjects using a six-compartment criterion model based on in vivo neutron activation analysis. They found that the mean percentage difference (difference relative to the mean) ranged from −12 to 23 %.
Some investigators have proposed flexible CT number ranges for AT using the mean CT number ± 2 SD, or the minimum/maximum CT numbers for each patient [18, 25, 29]. However, the CT number distributions for VAT differ from other AT compartment and the mean CT number ± 2 SD or the minimum/maximum CT numbers would have to be determined for each patient, which makes the application of such a method highly inconvenient in clinical settings. In addition, using mean CT number ± 2 SD, they did not take into account the partial volume averaging between AT and soft tissues such as muscle, resulting in underestimation of AT volume. In this regard, a fixed CT number range is preferred to the flexible CT number ranges.
Conclusion
The present study found that optimal CT number range for AT that would be applicable to modern PET/CT systems can be defined as −140 to −30 HU. The use of this optimal CT number range for AT allowed LBMs to be determined in whole-body F-18 FDG PET/CT studies.
Acknowledgment
This study was supported by Wonkwang University in 2010.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Kim D-W, Kim C, Park S-A, Jung S-A. Experience of dual time point brain F-18 FDG PET/CT Imaging in patients with infectious disease. Nucl Med Mol Imaging. 2010;44(2):137–142. doi: 10.1007/s13139-010-0026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J, Kim H, Kim J, Moon D, Kim Y, Kim D, et al. 18F-FDG PET/CT is useful for pretreatment assessment of the histopathologic type of thymic epithelial tumors. Nucl Med Mol Imaging. 2010;44(3):177–184. doi: 10.1007/s13139-010-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellisari A, Roche AF. Anthropometry and ultrasound. In: Heymsfield SB, Lohman TG, Wang Z, Going SB, editors. Human body composition. Champaign: Human Kinetics; 2005. pp. 109–127. [Google Scholar]
- 4.Chowdhury B, Sjostrom L, Alpsten M, Kostanty J, Kvist H, Lofgren R. A multicompartment body composition technique based on computerized tomography. Int J Obes Relat Metab Disord. 1994;18(4):219–234. [PubMed] [Google Scholar]
- 5.Wang ZM, Pierson RN, Jr, Heymsfield SB. The five-level model: a new approach to organizing body-composition research. Am J Clin Nutr. 1992;56(1):19–28. doi: 10.1093/ajcn/56.1.19. [DOI] [PubMed] [Google Scholar]
- 6.Ross R, Leger L, Guardo R, De Guise J, Pike BG. Adipose tissue volume measured by magnetic resonance imaging and computerized tomography in rats. J Appl Physiol (Bethesda, Md : 1985) 1991;70(5):2164–2172. doi: 10.1152/jappl.1991.70.5.2164. [DOI] [PubMed] [Google Scholar]
- 7.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (Bethesda, Md : 1985) 1998;85(1):115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 8.Rossner S, Bo WJ, Hiltbrandt E, Hinson W, Karstaedt N, Santago P, et al. Adipose tissue determinations in cadavers—a comparison between cross-sectional planimetry and computed tomography. Int J Obes. 1990;14(10):893–902. [PubMed] [Google Scholar]
- 9.Seidell JC, Bakker CJ, van der Kooy K. Imaging techniques for measuring adipose-tissue distribution–a comparison between computed tomography and 1.5-T magnetic resonance. Am J Clin Nutr. 1990;51(6):953–957. doi: 10.1093/ajcn/51.6.953. [DOI] [PubMed] [Google Scholar]
- 10.Sjostrom L, Kvist H, Cederblad A, Tylen U. Determination of total adipose tissue and body fat in women by computed tomography, 40K, and tritium. Am J Physiol. 1986;250(6 Pt 1):E736–E745. doi: 10.1152/ajpendo.1986.250.6.E736. [DOI] [PubMed] [Google Scholar]
- 11.Mattsson S, Thomas BJ. Development of methods for body composition studies. Phys Med Biol. 2006;51(13):R203–R228. doi: 10.1088/0031-9155/51/13/R13. [DOI] [PubMed] [Google Scholar]
- 12.Ross R. Advances in the application of imaging methods in applied and clinical physiology. Acta Diabetol. 2003;40(Suppl 1):S45–S50. doi: 10.1007/s00592-003-0025-y. [DOI] [PubMed] [Google Scholar]
- 13.Busetto L, Baggio MB, Zurlo F, Carraro R, Digito M, Enzi G. Assessment of abdominal fat distribution in obese patients: anthropometry versus computerized tomography. Int J Obes Relat Metab Disord. 1992;16(10):731–736. [PubMed] [Google Scholar]
- 14.Christen T, Sheikine Y, Rocha VZ, Hurwitz S, Goldfine AB, Di Carli M, et al. Increased glucose uptake in visceral versus subcutaneous adipose tissue revealed by PET imaging. JACC Cardiovasc Imaging. 2010;3(8):843–851. doi: 10.1016/j.jcmg.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grauer WO, Moss AA, Cann CE, Goldberg HI. Quantification of body fat distribution in the abdomen using computed tomography. Am J Clin Nutr. 1984;39(4):631–637. doi: 10.1093/ajcn/39.4.631. [DOI] [PubMed] [Google Scholar]
- 16.Kvist H, Sjostrom L, Tylen U. Adipose tissue volume determinations in women by computed tomography: technical considerations. Int J Obes. 1986;10(1):53–67. [PubMed] [Google Scholar]
- 17.Liem ET, De Lucia Rolfe E, L’Abee C, Sauer PJ, Ong KK, Stolk RP. Measuring abdominal adiposity in 6 to 7-year-old children. Eur J Clin Nutr. 2009;63(7):835–841. doi: 10.1038/ejcn.2008.57. [DOI] [PubMed] [Google Scholar]
- 18.Yoshizumi T, Nakamura T, Yamane M, Islam AH, Menju M, Yamasaki K, et al. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211(1):283–286. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- 19.Barrett JF, Keat N. Artifacts in CT: recognition and avoidance. Radiographics. 2004;24(6):1679–1691. doi: 10.1148/rg.246045065. [DOI] [PubMed] [Google Scholar]
- 20.Mahnken AH, Raupach R, Wildberger JE, Jung B, Heussen N, Flohr TG, et al. A new algorithm for metal artifact reduction in computed tomography: in vitro and in vivo evaluation after total hip replacement. Investig Radiol. 2003;38(12):769–775. doi: 10.1097/01.rli.0000086495.96457.54. [DOI] [PubMed] [Google Scholar]
- 21.Yazdi M, Beaulieu L. Artifacts in spiral X-ray CT scanners: problems and solutions international. J Biol Life Sci. 2008;4(3):135–139. [Google Scholar]
- 22.Martin AD, Daniel MZ, Drinkwater DT, Clarys JP. Adipose tissue density, estimated adipose lipid fraction and whole body adiposity in male cadavers. Int J Obes Relat Metab Disord. 1994;18(2):79–83. [PubMed] [Google Scholar]
- 23.Schoen RE, Evans RW, Sankey SS, Weissfeld JL, Kuller L. Does visceral adipose tissue differ from subcutaneous adipose tissue in fatty acid content? Int J Obes Relat Metab Disord. 1996;20(4):346–352. [PubMed] [Google Scholar]
- 24.Viljanen AP, Lautamaki R, Jarvisalo M, Parkkola R, Huupponen R, Lehtimaki T, et al. Effects of weight loss on visceral and abdominal subcutaneous adipose tissue blood-flow and insulin-mediated glucose uptake in healthy obese subjects. Ann Med. 2009;41(2):152–160. doi: 10.1080/07853890802446754. [DOI] [PubMed] [Google Scholar]
- 25.Rogalla P, Meiri N, Hoksch B, Boeing H, Hamm B. Low-dose spiral computed tomography for measuring abdominal fat volume and distribution in a clinical setting. Eur J Clin Nutr. 1998;52(8):597–602. doi: 10.1038/sj.ejcn.1600612. [DOI] [PubMed] [Google Scholar]
- 26.van der Kooy K, Seidell JC. Techniques for the measurement of visceral fat: a practical guide. Int J Obes Relat Metab Disord. 1993;17(4):187–196. [PubMed] [Google Scholar]
- 27.Basic anatomical and physiological data for use in radiological protection: reference values. A report of age- and gender-related differences in the anatomical and physiological characteristics of reference individuals. ICRP Publication 89. Ann ICRP. 2002;32(3–4):5–265. [PubMed]
- 28.Wang ZM, Deurenberg P, Guo SS, Pietrobelli A, Wang J, Pierson RN, Jr, et al. Six-compartment body composition model: inter-method comparisons of total body fat measurement. Int J Obes Relat Metab Disord. 1998;22(4):329–337. doi: 10.1038/sj.ijo.0800590. [DOI] [PubMed] [Google Scholar]
- 29.Demura S, Sato S. Prediction of visceral fat area in Japanese adults: proposal of prediction method applicable in a field setting. Eur J Clin Nutr. 2007;61(6):727–735. doi: 10.1038/sj.ejcn.1602576. [DOI] [PubMed] [Google Scholar]