Abstract
Purpose
We investigated whether PET indices measured by 18 F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) can predict prognosis in patients with operable primary breast cancer.
Methods
We reviewed 53 patients with operable primary breast cancer who underwent pretreatment FDG PET/CT. PET indices, maximum standardized uptake value (SUV) and metabolic tumor volume (MTV), were measured in the primary breast tumor (P), metastatic lymph nodes (N) and total tumor (T). The Cox proportional hazards model was used with age, tumor size, clinical lymph node status, method of surgery, presence or absence of neoadjuvant chemotherapy, histological type, histological grade, hormone receptors and HER2 status to predict disease-free survival (DFS) and overall survival (OS).
Results
Median follow-up period was 50 months (range, 17–73 months), during which 17 patients had recurrent disease and nine of whom died. The univariate analysis showed that high SUV of N (NSUV, P = 0.011), MTV of N (NMTV, P = 0.011) and MTV of T (TMTV, P = 0.045) as well as high histological grade (P = 0.008), negative estrogen (P = 0.045) and negative progesterone (P = 0.029) receptor status were associated with shorter DFS. High NSUV (P = 0.035), NMTV (P = 0.035) and TMTV (P = 0.035) as well as high histological grade (P = 0.012) and negative estrogen receptor status (P = 0.009) were associated with shorter OS. NSUV, NMTV and TMTV were found to be significantly associated with high histological grade (P = 0.005). However, those failed to be statistically significant prognostic factors on multivariate analysis.
Conclusions
PET indices seem to be useful in the preoperative evaluation of prognosis in patients with operable primary breast cancer. NSUV, NMTV and TMTV might be considerable factors associated with patient outcome in operable breast cancer.
Keywords: Breast cancer, FDG PET/CT, Maximum standardized uptake value, Metabolic tumor volume, Prognosis
Introduction
Breast cancer is the most common cancer in women, and its incidence is increasing. Prediction of prognosis is important for appropriate therapy [1]. Many factors have been indentified that affect a patient’s prognosis. These include tumor size, histological tumor grade, hormone receptor status, human epidermal growth factor receptor 2 (HER2) overexpression and metastatic lymph node status [2].
Positron emission tomography/computed tomography (PET/CT) using 2-deoxy-2-[18F]fluoro-D-glucose (FDG) has been widely used because of its usefulness in the diagnosis, staging, restaging and post-therapeutic follow-up of breast cancer [3–5]. Moreover, FDG PET/CT has been reported to be valuable in the assessment of prognosis. The standardized uptake value (SUV) represents the degree of FDG uptake, and provides information about prognosis. High SUV of the primary tumor is a good marker for the prediction of disease progression [6]. Recently, metabolic tumor volume (MTV), defined as the volume of tumor tissues with increased FDG uptake, has been investigated. Several recent studies in patients with lung, cervical, ovarian and tonsilar cancers suggest MTV to be a prognostic indicator [7–12]. A few studies have investigated the prognostic value of SUV in breast cancer. However, there was no study that assessed disease-free survival (DFS) and overall survival (OS) using MTV as a prognostic indicator in patients with breast cancer.
Therefore, we aimed to investigate whether the SUV and MTV in primary breast tumor, metastatic lymph nodes and total tumor volume can be used as prognostic indicators of the DFS and OS in patients with operable primary breast cancer.
Materials and Methods
Patients
Fifty-three patients with operable primary breast cancer who underwent pretreatment FDG PET/CT were enrolled from January 2006 to December 2008. Exclusion criteria were male gender, previous or a concurrent contralateral breast cancer and distant metastases. All subjects were surgically treated with either a modified radical mastectomy or wide local tumor resection, with sentinel lymph node biopsy or axillary lymph node dissection, followed by postoperative radiation therapy. Of the enrolled patients, 20 patients who had a primary tumor over 2 cm in size or clinically attached axillary lymph nodes received neoadjuvant chemotherapy with three cycles of Taxene (docetaxel or paclitaxel) and Anthracycline (doxorubicin or epirubicin) before the operation. The need for adjuvant systemic therapy (chemotherapy, endocrine or target therapy) was determined by axillary lymph node status, hormone receptor status, and menopausal status. All patients visited the hospital every 6 months for 5 years, and then once per year. The local Ethics Committee approved this study and all enrolled patients gave written informed consent for the FDG PET/CT study.
Imaging Acquisitions
FDG PET/CT studies were performed using a combined PET/CT scanner (Discovery ST System; GE Medical Systems, Milwaukee, WI, USA). All patients fasted for at least 6 h prior to the intravenous administration of FDG. Their blood glucose levels were measured before the injection of FDG; if the level was over 8.3 mmol/l, the FDG PET/CT was deferred. Image acquisition for torso scanning was started at approximately 1 h after the injection of 7.4 MBq FDG per kilogram of body weight. CT images were acquired from the skull base to the upper thigh using parameters with a peak voltage of 120 kVp, a tube current automated from 10 to 130 mA, a rotation time of 0.7 s, a field of view of 50 cm, a scan length of 40–50 s, and a slice thickness of 3.75 mm. Immediately following the CT acquisition, the PET data were acquired in the same anatomical locations with 15.7 cm axial field of view acquired in two-dimensional (2-D) mode with 150 s/bed position. The CT data were used for attenuation correction and the images were reconstructed using a conventional iterative ordered subsets expectation maximization (OSEM) algorithm.
Image Analysis
Image display and analysis were performed using an Advantage Workstation 4.4 (GE Medical Systems, Milwaukee, WI, USA), which provided multiplanar reformatted images. Maximum standardized uptake values (SUVmax) based on body weight and MTV were determined by the attenuation-corrected PET data using volume viewer software. Of the various methods described for determining metabolic volumes, a fixed threshold of SUV 2.5, as previously reported, was used [9, 13]. The boundaries of tumor were drawn large enough to incorporate each target lesion in the axial, coronal and sagittal FDG PET/CT images. The contour around the target lesions inside the boundaries was automatically produced, and the voxels presenting SUV intensity > 2.5 within the contouring margin were incorporated to define the tumor volumes. According to the location, PSUV was defined as the SUVmax of primary breast tumor, NSUV as that of metastatic nodes and TSUV as the higher value between PSUV and NSUV. In the same manner, PMTV was defined as the MTV of primary breast tumor, NMTV as that of metastatic lymph nodes and TMTV as the total summed values of PMTV and NMTV.
Pathologic Examination
We analyzed the patients’ pathological data, including histologic type of tumor, histological grade, hormone receptor status, and axillary lymph node status. Histological grade was identified by a modified Scarff-Bloon-Richardson grading system. Lymph nodes were stained with hematoxylin and eosin (H&E) and examined for tumor cell metastasis. Hormone receptor (estrogen [ER] and progesterone [PR] receptors) and HER2 status was determined by immunohistochemical (IHC) analysis using a tissue microarray. The immunohistochemical analyses used an ER antibody (1D5; DAKO, Carpinteria, USA), a PR antibody (PgR636; DAKO), and a HER2 antibody (4B5; DAKO). Hormone receptors were considered positive if expression was ≥ 10 %. The HER2 expression results by IHC analysis were scored as negative, 1+, 2+ or 3+ according to the manufacturer’s recommendations. Cases with an HER2 IHC staining score of more than 2 were tested by HER2 gene amplification using the fluorescence in situ hybridization (FISH) method. Cases with an IHC staining score of 3+ were defined as HER2 positive, or in the case of an IHC staging score of 2+, FISH positive.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 19.0 (SPSS, Chicago, IL). Survival time was derived from the date of FDG PET/CT scan to the date of death/recurrence or last follow-up. Cox regression analysis was used to develop the univariate and multivariate models describing the association of the independent variables with DFS and OS. Independent variables analyzed included age, tumor size, lymph node status on physical examination, surgery, neoadjuvant chemotherapy, histology, histological grade, estrogen and progesterone receptors, HER2 status, PSUV, PMTV, NSUV, NMTV, TSUV and TMTV. OS and PFS curves were produced using Kaplan-Meier methods and survival difference between groups was assessed by the log-rank test. The median value of SUV or MTV was used to define the two groups. A P value less than 0.05 was considered statistically significant. The 95 % confidence interval (CI) was determined for each index.
Results
Patient Characteristics
The patient characteristics are shown in Table 1. The median age of the patients was 52 years (range, 32–83 years), and the median follow-up period from the time of FDG PET/CT scan for all patients was 50 months (range, 17–73 months). The size of the primary tumor was T stage 1 in 15 (28 %), T stage 2 in 30 (57 %) and T stage 3 in 8 (15 %). Axillary lymph node involvement by physical examination was observed in 26 patients (49 %). TNM classification was stage I in 5 (9 %), stage II in 15 (28 %) and stage III in 33 (62 %).
Table 1.
Patient characteristics
| Variables | No. of patients (n = 53) | % |
|---|---|---|
| Age (years) | ||
| Median (range) | 52 (32–83) | - |
| Tumor size | ||
| T1 | 15 | 28 % |
| T2 | 30 | 57 % |
| T3 | 8 | 15 % |
| Lymph node status (clinical) | ||
| N0 | 27 | 51 % |
| N1-2 | 26 | 49 % |
| Surgery | ||
| Breast conserving surgery | 22 | 42 % |
| Mastectomy | 31 | 58 % |
| Neoadjuvant chemotherapy | ||
| Not done | 32 | 60 % |
| Done | 21 | 40 % |
| Histology | ||
| Invasive ductal carcinoma | 43 | 81 % |
| Invasive lobular carcinoma | 4 | 8 % |
| Others | 6 | 11 % |
| Histological grade | ||
| I | 10 | 19 % |
| II | 22 | 42 % |
| III | 21 | 39 % |
| Estrogen receptor | ||
| Positive | 34 | 64 % |
| Negative | 19 | 36 % |
| Progesterone receptor | ||
| Positive | 32 | 60 % |
| Negative | 21 | 40 % |
| HER2 | ||
| Positive | 21 | 40 % |
| Negative | 32 | 60 % |
| Follow-up period (months) | ||
| Median (range) | 50 (17–73) | - |
During the follow-up, the patient number of locoregional recurrences or distant metastases was 17 (33 %) and that of distant metastases with or without locoregional recurrence was 11 (21 %). The distribution of distant metastases was five (46 %) in lung, three (27 %) in bone and three (27 %) in liver. Nine (17 %) patients died because of breast cancer during the course of this study. Five-year OS and PFS were 79 % and 65 %, respectively.
Survival Analysis
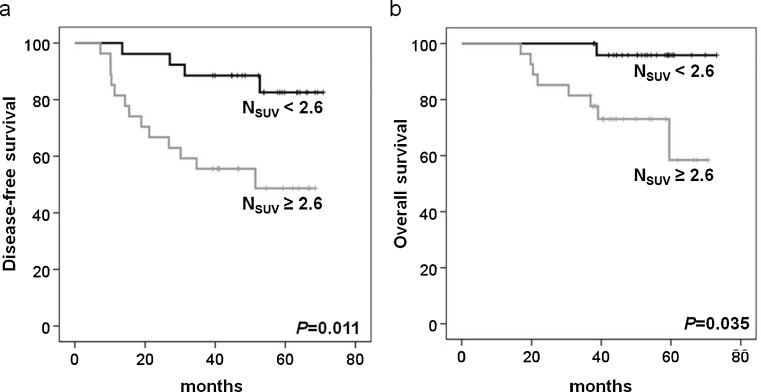
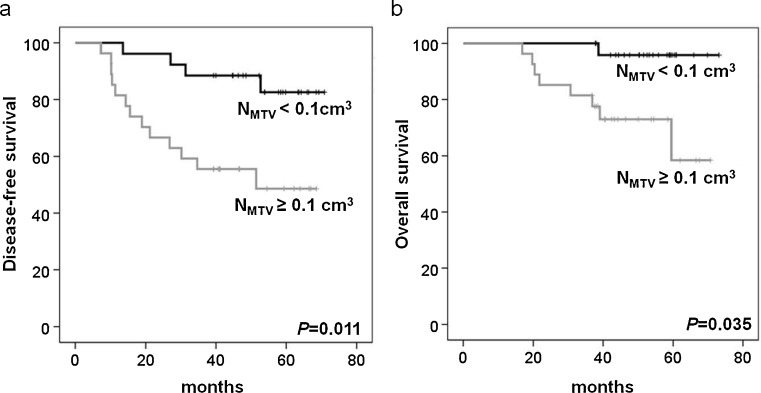
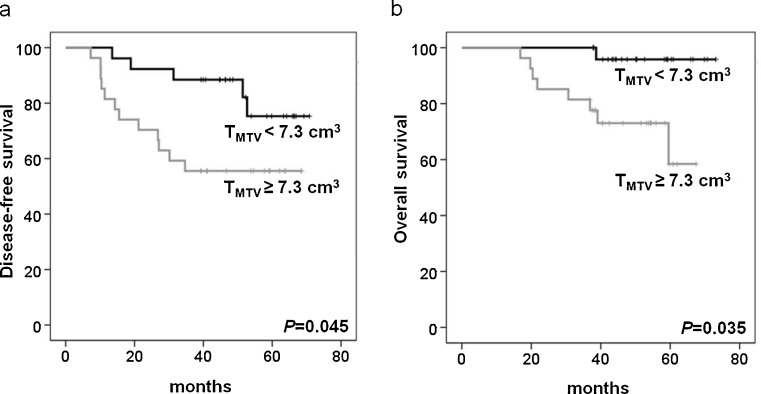
On univariate analysis, higher NSUV, NMTV and TMTV were significant predictors for poorer PFS and OS among the PET variables. Five-year DFS was significantly shorter in higher NSUV (82 % vs 48 %; P = 0.011, Fig. 1a), NMTV (82 % vs 48 %; P = 0.011, Fig. 2a) and TMTV (74 % vs 56 %; P = 0.045, Fig. 3a). Five-year OS was also significantly shorter in higher NSUV (96 % vs 61 %; P = 0.035, Fig. 1b), NMTV (96 % vs 61 %; P = 0.035, Fig. 2b) and TMTV (95 % vs 63 %; P = 0.035, Fig. 3b). Of the clinicopathological variables, histological grade III (P = 0.008), negative estrogen (P = 0.045) and negative progesterone receptor (P = 0.029) status were significant predictors for PFS, and histological grade III (P = 0.012) and negative estrogen receptor status (P = 0.009) were significant predictors for OS. However, age, tumor size, lymph node status, treatment modality, histology, HER2, PSUV, PMTV and TSUV did not influence PFS and OS. The multivariate analysis showed that higher NSUV, NMTV, TMTV, histological grade and negative hormone receptor status were not significant prognostic factors (Tables 2 and 3).
Fig. 1.
Kaplan-Meier analyses of disease-free (a) and overall survival (b) according to NSUV
Fig. 2.
Kaplan-Meier analyses of disease-free (a) and overall survival (b) according to NMTV
Fig. 3.
Kaplan-Meier analyses of disease-free (a) and overall survival (b) according to TMTV
Table 2.
Univariate and multivariate analysis for disease-free survival
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95 % CI) | P | HR (95 % CI) | P | |
| Age (years) | ||||
| ≥ 52 | 0.929 (0.358–2.410) | 0.880 | ||
| Tumor size | ||||
| T3 | 0.831 (0.190–3.634) | 0.805 | ||
| Lymph node status (clinical) | ||||
| Positive | 2.165 (0.797–5.878) | 0.130 | ||
| Surgery | ||||
| Mastectomy | 1.585 (0.585–4.296) | 0.365 | ||
| Neoadjuvant chemotherapy | ||||
| Done | 2.128 (0.818–5.537) | 0.122 | ||
| Histology | ||||
| Invasive ductal carcinoma | 0.941 (0.270–3.277) | 0.924 | ||
| Histological grade | ||||
| III | 3.852 (1.420–10.448) | 0.008 | 2.404 (0.740–7.813) | 0.145 |
| Estrogen receptor | ||||
| Positive | 0.376 (0.145–0.978) | 0.045 | 0.689 (0.080–5.926) | 0.734 |
| Progesterone receptor | ||||
| Positive | 0.339 (0.129–0.895) | 0.029 | 2.470 (0.320–19.075) | 0.386 |
| HER2 | ||||
| Positive | 1.897 (0.730–4.929) | 0.189 | ||
| PSUV | ||||
| ≥7.3 | 1.463 (0.556–3.853) | 0.441 | ||
| PMTV (cm3) | ||||
| ≥11.1 | 2.082 (0.769–5.638) | 0.149 | ||
| NSUV | ||||
| ≥2.6 | 4.275 (1.387–13.177) | 0.011 | 3.082 (0.967–9.816) | 0.057 |
| NMTV (cm3) | ||||
| ≥0.1 | 4.275 (1.387–13.177) | 0.011 | 3.082 (0.967–9.816) | 0.057 |
| TSUV | ||||
| ≥7.3 | 1.463 (0.556–3.853) | 0.441 | ||
| TMTV (cm3) | ||||
| ≥13.8 | 2.912 (1.024–8.279) | 0.045 | 2.013 (0.663–6.108) | 0.217 |
Table 3.
Univariate and multivariate analysis for overall survival
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95 % CI) | P | HR (95 % CI) | P | |
| Age (years) | ||||
| ≥52 | 1.998 (0.498–8.008) | 0.329 | ||
| Tumor size | ||||
| T3 | 1.890 (0.391–9.133) | 0.429 | ||
| Lymph node status (clinical) | ||||
| Positive | 2.507(0.616–10.208) | 0.199 | ||
| Surgery | ||||
| Mastectomy | 2.980(0.607–14.617) | 0.178 | ||
| Neoadjuvant chemotherapy | ||||
| Done | 2.249 (0.600–8.423) | 0.229 | ||
| Histology | ||||
| Invasive ductal carcinoma | 1.420 (0.292–6.895) | 0.664 | ||
| Histological grade | ||||
| III | 14.380 (1.797–115.064) | 0.012 | 3.598 (0.389–33.264) | 0.259 |
| Estrogen receptor | ||||
| Positive | 0.064 (0.008–0.510) | 0.009 | 7.571 (0.849–67.544) | 0.070 |
| Progesterone receptor | ||||
| Positive | 0.008 (0.000–2.432) | 0.098 | ||
| HER2 | ||||
| Positive | 1.329 (0.350–5.047) | 0.676 | ||
| PSUV | ||||
| ≥7.3 | 1.261 (0.338–4.709) | 0.730 | ||
| PMTV (cm3) | ||||
| ≥11.1 | 4.112 (0.852–19.846) | 0.078 | ||
| NSUV | ||||
| ≥2.6 | 9.419 (1.176–75.459) | 0.035 | 5.238 (0.633–43.378) | 0.125 |
| NMTV (cm3) | ||||
| ≥ 0.1 | 9.419 (1.176–75.459) | 0.035 | 5.238 (0.633–43.378) | 0.125 |
| TSUV | ||||
| ≥7.3 | 1.261 (0.338–4.709) | 0.730 | ||
| TMTV (cm3) | ||||
| ≥13.8 | 9.419 (1.176–75.459) | 0.035 | 5.238 (0.633–43.378) | 0.125 |
Relationship between PET indices and Clinicopathological indices
NSUV, NMTV and TMTV were found to be significantly associated with high histological grade (P = 0.005) but not with tumor size, lymph node status, histology, estrogen receptor, progesterone receptor and HER2 status (Table 4).
Table 4.
Relationship between PET indices and clinicopathological indices
| Variables | NSUV | P | NMTV | P | TMTV | P | |||
|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | ||||
| Tumor size | 0.250 | 0.250 | 0.250 | ||||||
| T1-2 | 24 | 21 | 24 | 21 | 24 | 21 | |||
| T3 | 2 | 6 | 2 | 6 | 2 | 6 | |||
| Lymph node status (clinical) | 0.173 | 0.173 | 0.056 | ||||||
| Negative | 16 | 11 | 16 | 11 | 17 | 10 | |||
| Positive | 10 | 16 | 10 | 16 | 9 | 17 | |||
| Histology | 0.728 | 0.728 | 0.728 | ||||||
| Invasive ductal carcinoma | 21 | 22 | 21 | 22 | 21 | 22 | |||
| Others | 6 | 4 | 6 | 4 | 6 | 4 | |||
| Histological grade | 0.005 | 0.005 | 0.005 | ||||||
| 1-2 | 21 | 11 | 21 | 11 | 21 | 11 | |||
| 3 | 5 | 16 | 5 | 16 | 5 | 16 | |||
| Estrogen receptor | 0.254 | 0.254 | 0.569 | ||||||
| Negative | 7 | 12 | 7 | 12 | 7 | 12 | |||
| Positive | 19 | 15 | 19 | 15 | 19 | 15 | |||
| Progesterone receptor | 0.093 | 0.093 | 0.264 | ||||||
| Negative | 7 | 14 | 7 | 14 | 8 | 13 | |||
| Positive | 19 | 13 | 19 | 13 | 18 | 14 | |||
| HER2 | 0.093 | 0.093 | 0.264 | ||||||
| Negative | 19 | 13 | 19 | 13 | 18 | 14 | |||
| Positive | 7 | 14 | 7 | 14 | 8 | 13 | |||
Discussion
In the present study, we demonstrate that PET indices are useful in the preoperative evaluation of prognosis in patients with operable primary breast cancer, along with well-known clinicopathologic indices. Among the PET indices, high NSUV, NMTV and TMTV were able to predict poorer outcomes on univariate analysis. However, these failed to be statistically significant prognostic factors on multivariate analysis.
Several studies suggested that a high SUV of primary tumor is associated with a worse prognosis in patients with breast cancer. According to Song et al. [6], the SUVmax of the primary tumor could be a useful marker to predict prognosis in patients with invasive ductal carcinoma of the breast. Other studies suggested that a high FDG uptake by breast tumor was correlated with poor prognostic factors such as histological grade and type, tumor size, invasiveness, hormonal receptor negativity and triple negativity [14–20]. This study differs from these prior investigations. The SUVmax of the primary tumor as well as the SUVmax of the metastatic lymph nodes were evaluated in this study. In addition, our patients’ outcomes were directly assessed by clinical follow-up, not by correlation with known clinical and biological prognostic parameters. DFS and OS analyses were done during the longer follow-up.
MTV, which is defined as the volume of tumor tissues with increased FDG uptake, is a recently investigated index in FDG PET. There are some studies that show MTV is a better prognostic indicator than clinical outcomes in ovarian, cervical, tonsilar and lung malignancies [7, 9, 10, 13]. However, there was no study that assessed DFS and OS using MTV as a pretreatment prognostic indicator in patients with breast cancer. Furthermore, previous investigators did not evaluate the MTV of the metastatic lymph nodes or total tumor volume; they evaluated the MTV of the primary tumor alone.
This study shows that high NSUV, NMTV and TMTV can predict significantly poorer outcomes in patients with operable primary breast cancer on univariate analysis, but PSUV, PMTV and TSUV cannot. The reason why both PSUV and PMTV are not prognostic factors of poorer outcomes may relate to the histopathological diagnosis which was made before the FDG PET/CT scan. In this study, all subjects had needle or excisional biopsies, and the mean time from the procedure to the FDG PET/CT scan was 10.9 ± 6.3 days. Preceding procedures can affect the PSUV of the primary tumor because of their inflammatory reaction. The removal of tumor tissue can also lead to underestimation of MTV of the real primary tumor. Consequently, PSUV and PMTV failed to show the statistical significance in this study. This result for PSUV is in good agreement with a previous study [21]. In contrast to PSUV and PMTV, both NSUV and NMTV are significant prognostic factors. Many studies of breast cancer have suggested that axillary lymph node metastasis is strongly related to poor prognosis [22, 23], even on evaluation by using FDG PET/CT scan [24]. We found that NMTV is also a prognostic factor in breast cancer as well as NSUV. In the measurement of MTV of axillary lymph nodes, we set a fixed SUV cutoff value of 2.5 in the same manner as with measurement of MTV of primary tumor. Metastatic lymph nodes were defined as the lymph nodes with SUVmax ≥ 2.3, which was the proven threshold value in previous study [24]. Although metastatic lymph node volumes between 2.3 and 2.5 of SUVmax were not included in measurement of NMTV, there was highly significant association between NSUV and NMTV (chi-square test, P < 0.001). Thus, we expect that NMTV also could predict poor outcome in this study. TSUV could not predict prognosis, while TMTV could predict it well. TSUV reflects PSUV rather than NSUV, because most of the subjects had a higher PSUV than NSUV except for five cases. However, TMTV can reflect the systemic tumor burden, as it incorporates the volumes of the primary tumor and metastatic lymph nodes. Because we enrolled operable breast cancer patients without distant metastasis in this study, TMTV indicates whole-body metabolic tumor volume in this study. Recent studies showed that whole-body metabolic tumor volume is a strong prognostic factor in lung cancers [8, 12]. Similar to other studies, this study showed that high TMTV was a prognostic factor of poorer outcomes in breast cancer without distant metastasis.
However, this study failed to show any significance of PET indices for the outcome by the Cox multivariate survival analysis. The cause of such a discrepancy might be due to a small sample size and the correlation between PET indices and histologic grade. In a sub-study, exclusive of histological grade, NSUV and NMTV were significant prognostic factors for disease progression (P = 0.026). Further study is needed to determine the usefulness of NSUV and NMTV as prognostic factors in patients with breast cancer.
The present study had several limitations. First, a small number of patients were included retrospectively. However, we enrolled only patients with operable breast cancer to standardize treatment modalities. Second, MTV can be affected by the partial volume effect, the time between tracer injection and imaging, and plasma glucose levels; also, the methods of measurement of MTV are under controversy. Further prospective studies on the accurate measurement of MTV involving a larger number of patients will be needed to confirm the prediction of prognosis in patients with breast cancer.
In conclusion, PET indices seem to be useful in the preoperative evaluation of prognosis in patients with operable primary breast cancer. NSUV, NMTV and TMTV might be considerable factors associated with patient outcome in operable breast cancer. PET indices are expected to enable better follow-up of patients with operable breast cancer and aid in the making of appropriate treatment decisions for these patients.
Acknowledgments
Conflicts of interest
None.
References
- 1.Yoo K, Shin H, Park S, Yoon H, Shin A, Kang D, et al. Is Breast Cancer Incidence Rate Further Increasing in Korea? Korean J Epidemiol. 2001;23(2):1–7. [Google Scholar]
- 2.Megale Costa LJ, Soares HP, Gaspar HA, Trujillo LG, Santi PX, Pereira RS. Ratio between positive lymph nodes and total dissected axillaries lymph nodes as an independent prognostic factor for disease-free survival in patients with breast cancer. Am J Clin Oncol. 2004;27(3):304–306. doi: 10.1097/01.COC.0000071941.70772.DC. [DOI] [PubMed] [Google Scholar]
- 3.Uematsu T, Kasami M, Yuen S. Comparison of FDG PET and MRI for evaluating the tumor extent of breast cancer and the impact of FDG PET on the systemic staging and prognosis of patients who are candidates for breast-conserving therapy. Breast Cancer. 2009;16(2):97–104. doi: 10.1007/s12282-008-0065-9. [DOI] [PubMed] [Google Scholar]
- 4.Emmering J, Krak NC, Van der Hoeven JJ, Spreeuwenberg MD, Twisk JW, Meijer S, et al. Preoperative [18 F] FDG-PET after chemotherapy in locally advanced breast cancer: prognostic value as compared with histopathology. Ann Oncol. 2008;19(9):1573–1577. doi: 10.1093/annonc/mdn185. [DOI] [PubMed] [Google Scholar]
- 5.Cermik TF, Mavi A, Basu S, Alavi A. Impact of FDG PET on the preoperative staging of newly diagnosed breast cancer. Eur J Nucl Med Mol Imaging. 2008;35(3):475–483. doi: 10.1007/s00259-007-0580-5. [DOI] [PubMed] [Google Scholar]
- 6.Song B-I, Hong CM, Lee HJ, Kang S, Jeong SY, Kim HW, et al. Prognostic value of primary tumor uptake on F-18 FDG PET/CT in patients with invasive ductal breast cancer. Nucl Med Mol Imaging. 2011;45(2):117–124. doi: 10.1007/s13139-011-0081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung HH, Kwon HW, Kang KW, Park NH, Song YS, Chung JK, et al. Prognostic value of preoperative metabolic tumor volume and total lesion glycolysis in patients with epithelial ovarian cancer. Ann Surg Oncol. 2011. doi:10.1245/s10434-011-2153-x. [DOI] [PubMed]
- 8.Oh JR, Seo JH, Chong A, Min JJ, Song HC, Kim YC, et al. Whole-body metabolic tumour volume of 18F-FDG PET/CT improves the prediction of prognosis in small cell lung cancer. Eur J Nucl Med Mol Imaging. 2012. doi:10.1007/s00259-011-2059-7. [DOI] [PubMed]
- 9.Chung HH, Kim JW, Han KH, Eo JS, Kang KW, Park NH, et al. Prognostic value of metabolic tumor volume measured by FDG-PET/CT in patients with cervical cancer. Gynecol Oncol. 2011;120(2):270–274. doi: 10.1016/j.ygyno.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Moon SH, Choi JY, Lee HJ, Son YI, Baek CH, Ahn YC et al. Prognostic value of 18F-FDG PET/CT in patients with squamous cell carcinoma of the tonsil: Comparisons of volume-based metabolic parameters. Head Neck. 2012; doi:10.1002/hed.22904. [DOI] [PubMed]
- 11.Liao S, Penney BC, Zhang H, Suzuki K, Pu Y. Prognostic value of the quantitative metabolic volumetric measurement on 18F-FDG PET/CT in Stage IV nonsurgical small-cell lung cancer. Acad Radiol. 2012;19(1):69–77. doi: 10.1016/j.acra.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Huang W, Zhou T, Ma L, Sun H, Gong H, Wang J, et al. Standard uptake value and metabolic tumor volume of 18F-FDG PET/CT predict short-term outcome early in the course of chemoradiotherapy in advanced non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2011;38(9):1628–1635. doi: 10.1007/s00259-011-1838-5. [DOI] [PubMed] [Google Scholar]
- 13.Kim K, Kim SJ, Kim IJ, Seong Kim Y, Pak K, Kim H. Prognostic value of volumetric parameters measured by F-18 FDG PET/CT in surgically resected non-small-cell lung cancer. Nucl Med Commun. 2012. doi:10.1097/MNM.0b013e328351d4f5. [DOI] [PubMed]
- 14.Kim BS, Sung SH. Usefulness of 18F-FDG uptake with clinicopathologic and immunohistochemical prognostic factors in breast cancer. Ann Nucl Med. 2012;26(2):175–183. doi: 10.1007/s12149-011-0556-1. [DOI] [PubMed] [Google Scholar]
- 15.Sanli Y, Kuyumcu S, Ozkan ZG, Isik G, Karanlik H, Guzelbey B et al. Increased FDG uptake in breast cancer is associated with prognostic factors. Ann Nucl Med. 2012;26(4):345-50. [DOI] [PubMed]
- 16.Groheux D, Giacchetti S, Moretti JL, Porcher R, Espie M, Lehmann-Che J, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38(3):426–435. doi: 10.1007/s00259-010-1640-9. [DOI] [PubMed] [Google Scholar]
- 17.Osborne JR, Port E, Gonen M, Doane A, Yeung H, Gerald W, et al. 18F-FDG PET of locally invasive breast cancer and association of estrogen receptor status with standardized uptake value: microarray and immunohistochemical analysis. J Nucl Med. 2010;51(4):543–550. doi: 10.2967/jnumed.108.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mavi A, Cermik TF, Urhan M, Puskulcu H, Basu S, Yu JQ, et al. The effects of estrogen, progesterone, and C-erbB-2 receptor states on 18F-FDG uptake of primary breast cancer lesions. J Nucl Med. 2007;48(8):1266–1272. doi: 10.2967/jnumed.106.037440. [DOI] [PubMed] [Google Scholar]
- 19.Heudel P, Cimarelli S, Montella A, Bouteille C, Mognetti T. Value of PET-FDG in primary breast cancer based on histopathological and immunohistochemical prognostic factors. Int J Clin Oncol. 2010;15(6):588–593. doi: 10.1007/s10147-010-0120-3. [DOI] [PubMed] [Google Scholar]
- 20.Buck A, Schirrmeister H, Kuhn T, Shen C, Kalker T, Kotzerke J, et al. FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging. 2002;29(10):1317–1323. doi: 10.1007/s00259-002-0880-8. [DOI] [PubMed] [Google Scholar]
- 21.Inoue T, Yutani K, Taguchi T, Tamaki Y, Shiba E, Noguchi S. Preoperative evaluation of prognosis in breast cancer patients by [18 F]2-Deoxy-2-fluoro-D-glucose-positron emission tomography. J Cancer Res Clin Oncol. 2004;130(5):273–278. doi: 10.1007/s00432-003-0536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersson Y, Frisell J, Sylvan M, de Boniface J, Bergkvist L. Breast cancer survival in relation to the metastatic tumor burden in axillary lymph nodes. J Clin Oncol. 2010;28(17):2868–2873. doi: 10.1200/JCO.2009.24.5001. [DOI] [PubMed] [Google Scholar]
- 23.Jatoi I, Hilsenbeck SG, Clark GM, Osborne CK. Significance of axillary lymph node metastasis in primary breast cancer. J Clin Oncol. 1999;17(8):2334–2340. doi: 10.1200/JCO.1999.17.8.2334. [DOI] [PubMed] [Google Scholar]
- 24.Chung A, Liou D, Karlan S, Waxman A, Fujimoto K, Hagiike M, et al. Preoperative FDG-PET for axillary metastases in patients with breast cancer. Arch Surg. 2006;141(8):783–788. doi: 10.1001/archsurg.141.8.783. [DOI] [PubMed] [Google Scholar]