Abstract
Purpose
This study aimed to further stratify prognostic factors in patients with stage IV non-small cell lung cancer (NSCLC) by measuring their metabolic tumor volume (MTV) using F-18 fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT).
Materials and Methods
The subjects of this retrospective study were 57 patients with stage IV NSCLC. MTV, total lesion glycolysis (TLG), and maximum standardized uptake value (SUVmax) were measured on F-18 FDG PET/CT in both the primary lung lesion as well as metastatic lesions in torso. Optimal cutoff values of PET parameters were measured by receiver operating characteristic (ROC) curve analysis. Kaplan-Meier survival curves were used for evaluation of progression-free survival (PFS). The univariate and multivariate Cox proportional hazards models were used to select the significant prognostic factors.
Results
Univariate analysis showed that both MTV and TLG of primary lung lesion (MTV-lung and TLG-lung) were significant factors for prediction of PFS (P < 0.001, P = 0.038, respectively). Patients showing lower values of MTV-lung and TLG-lung than the cutoff values had significantly longer mean PFS than those with higher values. Hazard ratios (95 % confidence interval) of MTV-lung and TLG-lung measured by univariate analysis were 6.4 (2.5–16.3) and 2.4 (1.0–5.5), respectively. Multivariate analysis revealed that MTV-lung was the only significant factor for prediction of prognosis. Hazard ratio was 13.5 (1.6–111.1, P = 0.016).
Conclusion
Patients with stage IV NSCLC could be further stratified into subgroups of significantly better and worse prognosis by MTV of primary lung lesion.
Keywords: Metabolic tumor volume, Stage IV non-small cell lung cancer, F-18 FDG PET/CT, Progression-free survival
Introduction
Lung cancer is the leading cause of cancer death in the world, and 80–85 % of lung cancer cases are classified as non-small cell lung cancer (NSCLC) [1]. The outcome for patients with NSCLC remains quite poor despite improvements in radiotherapy and chemotherapy in the past decade [2]. Tumor-node-metastasis (TNM) stage is currently the most important prognostic factor for survival in NSCLC, in which stage IV is the most advanced stage. However, all patients with stage IV NSCLC do not show similar prognosis. The 1-year overall survival rate for patients with tumor nodules in both lungs (M1a) was 45 %, and that for distant extrapulmonary metastases or distant metastases (M1b) was 22 % [3, 4]. We experience different survival among patient with same M1a or M1b stages in practice. Given the consistent improvement in the survival of patients who have been treated with chemotherapy over those receiving supportive care alone, clinicians struggle to stratify these patients into different prognostic groups [5].
Positron emission tomography/computed tomography (PET/CT) using F-18 fluorodeoxyglucose (FDG) has already been successfully applied in oncological practice. Standardized uptake value (SUV)-based PET parameter can predict therapeutic outcome and overall survival in many types of malignancies [6–12]. Recently, metabolic tumor volume (MTV) and total lesion glycolysis (TLG) have been reported as additional diagnostic and prognostic imaging biomarkers for maximum SUV (SUVmax) [13–15]. MTV and TLG are valuable markers in the prediction of prognosis in NSCLC patients, mostly in operable or up to stage III patients [16–18]. Liao et al. [16] reported that whole-body measurement of MTV successfully predicted survival in stage IV NSCLC. Clinical variables including TNM staging system, histological type, performance status, and smoking status are known to predict the prognosis of NSCLC patients [19–21]. This study aimed to further stratify patients with stage IV NSCLC by measuring MTV using F-18 FDG PET/CT and compared it with various clinical factors for prediction of prognosis.
Materials and Methods
Patients
The subjects of this retrospective study were 57 patients (median age, 67 years; range, 32–82 years) with stage IV NSCLC (50 adenocarcinoma, 7 squamous cell carcinoma). They were enrolled from January 2007 to June 2011.
All patients underwent routine staging procedures including history taking, physical examination, blood tests for cells and chemistry panels, CT of the chest and abdomen, brain MRI and FDG PET/CT. Cancer staging was determined by the 7th edition of the American Joint Committee on Cancer (AJCC) Staging Manual. All staging workups were completed before initiation of therapy. Patients were excluded from analysis if any of the following criteria were present: (1) previous diagnosis of other malignant diseases; (2) previous surgical approach for primary lung cancer. Informed consent was waived due to this study’s retrospective design.
PET/CT Imaging
FDG PET/CT studies were performed using a combined PET/CT scanner (Discovery ST System, GE Medical Systems, Milwaukee, WI, USA). All patients fasted for at least 6 h prior to the intravenous administration of FDG. Their blood glucose levels were measured before the injection of FDG; if the level was over 8.3 mmol/l, FDG PET/CT was deferred. Image acquisition for torso PET/CT was obtained from the skull base to the upper thigh. Scanning started at approximately 1 h after the injection of 7.4 MBq FDG per kilogram of body weight. CT images used parameters with a peak voltage of 120 kVp, a tube current automated from 10 to 130 mA, a rotation time of 0.7 s, a field of view of 50 cm, a scan length of 40–50 s and a slice thickness of 3.75 mm. Immediately following the CT acquisition, PET data were acquired in the same anatomical locations with a 15.7 cm axial field of view in the two-dimensional (2-D) mode with a 150 s/bed position. The CT data were used for attenuation correction and the images were reconstructed using a conventional iterative ordered subsets expectation maximization (OSEM) algorithm.
Assessment of MTV, TLG, and SUVmax of Primary Lung Lesion
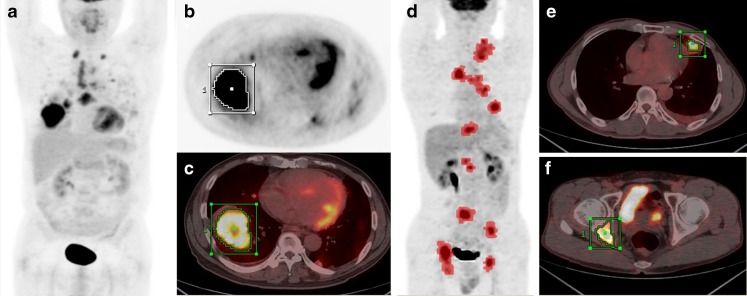
MTV, TLG and SUVmax of primary lung lesion were measured (MTV-lung; TLG-lung; and SUVmax-lung). Image display and analysis were performed using an Advantage Workstation 4.4 (GE Medical Systems, Milwaukee, WI, USA), which provided multiplanar reformatted images. SUVmax based on body weight and MTV were determined by the attenuation-corrected PET data using volume viewer software. Of the various methods described for determining metabolic volume, a fixed threshold of SUV 2.5 was used [18, 22, 23]. The boundaries of tumor were drawn large enough to incorporate each target lesion in the axial, coronal and sagittal FDG PET/CT images. The contour around the target lesions inside the boundaries was automatically produced, and the voxels presenting SUV intensity > 2.5 within the contouring margin were incorporated to define the tumor volumes (Fig. 1a–c). TLG was calculated by multiplying the mean SUV (SUVmean) of the primary tumor by metabolic volume of the tumor [8]. The SUVmax, MTV and TLG of primary lung lesions were recorded as SUVmax-lung, MTV-lung, and TLG-lung, respectively.
Fig. 1.
Measurement of metabolic tumor volume (MTV) of primary lung and metastatic tumor lesions in torso using standardized uptake value (SUV)-based automated contouring program. a–c A 64-year-old male patient with multiple lung metastases (M1a). Volume of interest (VOI) was drawn in the primary tumor. d–f A 51-year-old male patient with multiple osseous metastases (M1b). Pink-dotted lesions on maximum intensity projection image (d) show a tumoral lesion exceeding SUV intensity above 2.5
Assessment of MTV, TLG, and SUVmax of all Tumor Lesions in Torso PET/CT
In addition to the primary lung lesion analysis, MTV, TLG and SUVmax were also measured by torso PET/CT. We drew rectangular shaped volumes of interest (VOIs) fully encasing the primary tumor, regional lymph node and all distant metastases in the axial, coronal and sagittal PET/CT images. The boundaries of voxels presenting SUV intensity were automatically produced with a threshold of SUV exceeding 2.5. Normal organs which showed physiologic FDG uptake (such as the heart, stomach, liver, intestines, kidney, ureter and bladder) were manually subtracted from the VOI. Inflammation or other benign FDG-avid lesions were selected based on histopathological reports, other imaging modalities such as contrast-enhanced CT and response to therapy, and were excluded from the measurement (Fig. 1d–f). Finally, SUVmax, MTV and TLG in torso tumor lesions were recorded as SUVmax-torso, MTV-torso and TLG-torso, respectively.
Statistical Analysis
The threshold value allowing differentiation between the two groups of patients was selected by the receiver operating characteristic (ROC) method. All PET parameters were compared with each of the dichotomized variables by t-test. Progression-free survival (PFS) was measured from the date of FDG PET/CT scan to the date of disease progression/death or last follow-up. Kaplan-Meier estimates and the log rank test were performed to assess the equality of the survival functions across variables in PFS. Cox proportional hazard model was used to develop the univariate and multivariate models describing the association of the independent variables with PFS. Independent variables included age, sex, histological type of lung cancer, M staging of TNM classification, smoking history, Eastern Cooperative Oncology Group (ECOG) performance status, SUVmax-lung, MTV-lung, TLG-lung, SUVmax-torso, MTV-torso, TLG-torso. Estimated hazard ratio (HR) with 95 % confidence interval (CI) was presented for each variable. A value of P less than 0.05 was considered statistically significant. The statistical software package for social science (SPSS, version 19.0.0; SPSS, Chicago, IL, USA) and MedCalc statistical package (version 12.0.0.0, MedCalc Software, Mariakerke, Belgium) were used for statistical testing.
Results
Patient Characteristics
The characteristics of 57 patients are summarized in Table 1. The median age of the patients was 67 years (range, 32–82 years). Most of patients (91 %) received platinum-based combination chemotherapy as a first therapeutic regimen, while five patients were treated by tyrosine kinase inhibitor. Palliative radiotherapy for metastatic lesion was given to 16 patients (28 %). The median follow-up period from the time of FDG PET/CT scan was 414.5 days (range, 19–1,643 days). During the follow-up, disease progression was developed in 30 patients (53 %). Mean PFS was 279 days (range, 19–1,643 days).
Table 1.
Patient characteristics
| Characteristic | Number (%) |
|---|---|
| Total patients | 57 |
| Age (years) | |
| Median age | 67 |
| Range | 32–82 |
| Sex | |
| Male | 27 (47 %) |
| Female | 30 (53 %) |
| Smoker | 27 (47 %) |
| Diabetes | 6 (11 %) |
| Hypertension | 14 (25 %) |
| Histological type | |
| Adenocarcinoma | 50 (88 %) |
| Squamous cell carcinoma | 7 (12 %) |
| M staging | |
| M1a | 20 (35 %) |
| M1b | 37 (65 %) |
| EGFR mutation | |
| Negative | 43 (75 %) |
| Positive | 14 (25 %) |
| ECOG performance status | |
| 0 | 13 (23 %) |
| 1 | 37 (65 %) |
| 2 | 5 (9 %) |
| 3 | 2 (3 %) |
| Initial chemotherapy regimen | |
| Platinum-based combination chemotherapy | 52 (91 %) |
| Tyrosine kinase inhibitor | 5 (9 %) |
| Palliative radiotherapy for metastatic lesion | 16 (28 %) |
EGFR epidermal growth factor receptor, ECOG Eastern Cooperative Oncology Group
Cutoff Values of PET Parameters for PFS
The area under the curve (AUC) of MTV-lung was 0.52, and its cutoff value was 68.26. AUC and cutoff values of TLG-lung and SUVmax-lung were 0.50 and 194.16, 0.51 and 11.20, respectively. AUC and cutoff values of MTV-torso, TLG-torso, and SUVmax-torso were 0.58 and 656.20, 0.59 and 442.20, 0.53 and 11.20, respectively.
Prognosis Stratification by Using SUVmax, MTV and TLG
Comparison of PET variables of primary lung lesion is summarized in Table 2. MTV was lower in younger patients (less than 67 years old), in male patients, and in patients showing higher ECOG performance status. SUVmax was lower in M1a group.
Table 2.
Comparison of SUV-based PET parameters of primary lung lesion and clinical variables
| SUVmax-lung | P | MTV-lung | P | TLG-lung | P | |
|---|---|---|---|---|---|---|
| Age | ||||||
| < 67 | 10.4 ± 4.3 | 0.054 | 35.8 ± 35.9 | 0.049 | 174.6 ± 210.2 | 0.081 |
| ≥ 67 | 12.9 ± 5.2 | 67.4 ± 74.3 | 364.7 ± 500.0 | |||
| Sex | ||||||
| Male | 10.7 ± 4.3 | 0.128 | 35.6 ± 45.4 | 0.035 | 181.2 ± 256.1 | 0.060 |
| Female | 12.7 ± 5.4 | 68.8 ± 69.0 | 392.6 ± 510.7 | |||
| Histological type | ||||||
| Squamous cell carcinoma | 14.8 ±7.4 | 0.245 | 65.0 ± 51.4 | 0.521 | 383.1 ± 326.6 | 0.486 |
| Adenocarcinoma | 11.2 ± 4.3 | 49.4 ± 61.0 | 267.1 ± 418.8 | |||
| M staging | ||||||
| M1a | 9.7 ± 3.2 | 0.012 | 35.9 ± 38.1 | 0.151 | 177.8 ± 216.1 | 0.161 |
| M1b | 12.7 ± 5.3 | 59.7 ± 67.6 | 337.3 ± 474.3 | |||
| Smoking | ||||||
| Positive | 12.5 ± 5.4 | 0.193 | 67.5 ± 70.0 | 0.057 | 386.6 ± 514.5 | 0.064 |
| Negative | 10.8 ± 4.2 | 36.8 ± 44.9 | 186.6 ± 253.7 | |||
| ECOG Performance status | ||||||
| 0 or 1 | 11.7 ± 5.0 | 0.604 | 56.1 ± 62.2 | < 0.001 | 310.1 ± 427.9 | 0.157 |
| 2 to 3 | 10.7 ± 4.2 | 17.4 ± 10.2 | 76.1 ± 40.7 | |||
ECOG Eastern Cooperative Oncology Group, SUVmax-lung maximum standardized uptake value of primary lung lesion, MTV-lung metabolic tumor volume of primary lung lesion, TLG-lung total lesion glycolysis of primary lung lesion
Comparison of PET variables of tumor in the torso region is summarized in Table 3. Values of SUVmax-torso, MTV-torso and TLG-torso were significantly higher in patients with M1b stage than in those with M1a stage.
Table 3.
Comparison of SUV-based PET parameters of torso lesions and clinical variables
| SUVmax-torso | P | MTV-torso | P | TLG-torso | P | |
|---|---|---|---|---|---|---|
| Age | ||||||
| < 67 | 12.2 ± 4.6 | 0.187 | 296.7 ± 284.0 | 0.904 | 975.4 ± 884.7 | 0.925 |
| ≥ 67 | 14.0 ± 5.5 | 285.1 ± 402.6 | 950.2 ± 1072.2 | |||
| Sex | ||||||
| Male | 12.8 ± 5.4 | 0.646 | 216.3 ± 245.8 | 0.096 | 702.2 ± 756.3 | 0.040 |
| Female | 13.4 ± 5.1 | 372.3 ± 432.5 | 1249.0 ± 1136.8 | |||
| Histological type | ||||||
| Squamous cell carcinoma | 15.1 ± 7.1 | 0.272 | 229.2 ± 292.0 | 0.630 | 836.3 ±819.9 | 0.724 |
| Adenocarcinoma | 12.8 ± 4.9 | 298.7 ± 362.0 | 978.7 ±1012.9 | |||
| M staging | ||||||
| M1a | 10.9 ± 4.3 | 0.014 | 147.5 ± 172.5 | 0.006 | 488.1 ± 573.7 | 0.001 |
| M1b | 14.2 ± 5.4 | 367.3 ± 400.3 | 1217.0 ± 1070.9 | |||
| Smoking | ||||||
| Positive | 13.1 ± 5.3 | 0.923 | 362.8 ± 420.0 | 0.142 | 1202.6 ± 1118.6 | 0.079 |
| Negative | 13.0 ± 5.3 | 224.9 ± 269.5 | 744.0 ± 807.6 | |||
| ECOG Performance status | ||||||
| 0 or 1 | 13.2 ± 5.3 | 0.630 | 289.1 ± 362.4 | 0.952 | 956.9 ± 1001.0 | 0.930 |
| 2 to 3 | 12.2 ± 4.7 | 297.9 ± 296.4 | 992.1 ± 942.0 | |||
SUVmax-torso maximum standardized uptake value of torso tumor lesion, MTV-torso metabolic tumor volume of torso tumor lesion, TLG-torso total lesion glycolysis of torso tumor lesion
Results of univariate and multivariate analyses of clinical and PET variables for PFS are shown in Table 4. Univariate analysis showed that MTV-lung and TLG-lung were significant factors for prediction of PFS. However, all torso PET parameters did not show any statistical significance. Patients showing lower values of MTV-lung and TLG-lung than the cutoff values had significantly longer mean PFS than those with higher values (329.8 ± 367.2 vs 107.7 ± 57.7 days, respectively, P < 0.001; 374.0 ± 395.8 vs 128.2 ± 95.5 days, respectively, P = 0.038). There was a good correlation among three PET parameters of primary tumor (SUVmax-lung and MTV-lung, r = 0.601, P < 0.001; SUVmax-lung and TLG-lung, r = 0.658, P <0.001; and MTV-lung and TLG-lung, r = 0.974, P <0.001). Multivariate analysis revealed that MTV-lung was the only significant factor for prediction of prognosis (HR and 95 % CI = 13.487 and 1.637–111.101, respectively).
Table 4.
Univariate and multivariate analyses for progression-free survival
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95 % CI) | P | HR (95 % CI) | P | |
| Age (years) | ||||
| < 67 | 1.088 (0.526–2.252) | 0.820 | ||
| ≥ 67 | ||||
| Sex | ||||
| Male | 1.287 (0.593–2.796) | 0.523 | ||
| Female | ||||
| Histological type | ||||
| Squamous cell | 1.507 (0.452–5.022) | 0.504 | ||
| Carcinoma | ||||
| Adenocarcinoma | ||||
| M staging | ||||
| M1a | 1.726 (0.783–3.807) | 0.176 | ||
| M1b | ||||
| Smoking | ||||
| Positive | 1.464 (0.670–3.198) | 0.339 | ||
| Negative | ||||
| ECOG performance status | ||||
| 0 or 1 | 2.451 (0.577–10.411) | 0.224 | ||
| 2 to 3 | ||||
| SUVmax-lung | ||||
| ≤ 11.20 | 1.416 (0.626–3.199) | 0.403 | ||
| > 11.20 | ||||
| MTV-lung (cm3) | ||||
| ≤ 68.26 | 6.377 (2.500–16.266) | < 0.001 | 13.487 (1.637–111.101) | 0.016 |
| > 68.26 | ||||
| TLG-lung | ||||
| ≤ 194.16 | 2.395 (1.049–5.472) | 0.038 | 2.304 (0.303–17.506) | 0.420 |
| > 194.16 | ||||
| SUVmax-torso | ||||
| ≤ 11.20 | 1.253 (0.595–2.641) | 0.552 | ||
| > 11.20 | ||||
| MTV-torso (cm3) | 1.569 (0.208–11.832) | 0.662 | ||
| ≤ 656.20 | ||||
| > 656.20 | ||||
| TLG-torso | 1.816 (0.826–3.992) | 0.138 | ||
| ≤ 442.20 | ||||
| > 442.20 | ||||
HR hazard ratio, CI confidence interval, ECOG Eastern Cooperative Oncology Group, SUVmax maximum standardized uptake value, MTV metabolic tumor volume, TLG total lesion glycolysis
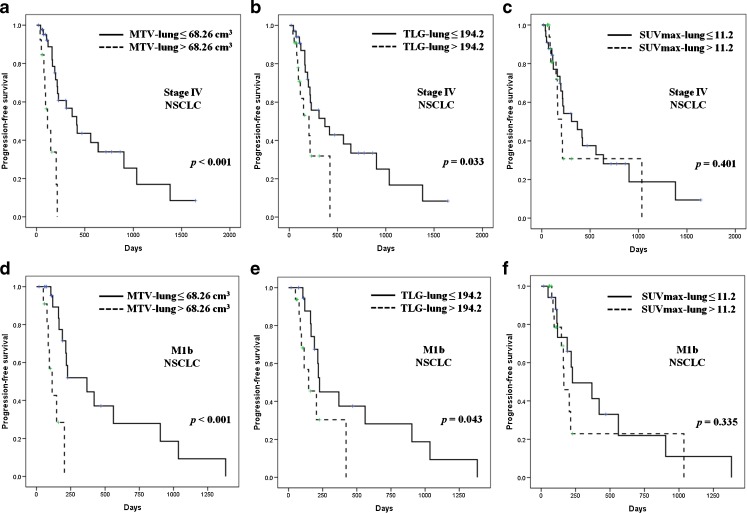
The Kaplan-Meier survival graphs showed a statistically significant difference in PFS between the groups categorized by MTV-lung (P < 0.001) and TLG-lung (P = 0.033), but SUVmax-lung (P = 0.401) did not (Fig. 2a–c). In subgroup analysis, MTV-lung (P < 0.001) and TLG-lung (P = 0.043) were showed statistical differences in only M1b patients, but SUVmax-lung (P = 0.335) did not. (Fig. 2d–f).
Fig. 2.
Prognosis stratification by metabolic tumor volume in stage IV non-small cell lung cancer (NSCLC) patients with the Kaplan-Meier survival graphs. (a) MTV-lung and (b) TLG-lung successfully distinguished patients with longer prognostic-free survival (PFS). In a subgroup of M1b, (d) MTV-lung and (e) TLG-lung also could distinguish patients with longer PFS. However, SUVmax-lung could not stratify prognosis (c, f). MTV-lung metabolic tumor volume of primary lung lesion, TLG-lung total lesion glycolysis of primary lung lesion, SUVmax-lung maximum standardized uptake value of primary lung lesion
Discussion
In this study, MTV-lung and TLG-lung can differentiate stage IV NSCLC patients into two subgroups of longer and shorter PFS. Moreover, these two parameters can divide PFS even in M1b patients. Multivariate analysis showed that MTV-lung was the only significant predictive factor in the risk stratification of this study group. TLG was dependent on MTV because it was calculated by multiplying the SUVmean by MTV.
Prognostic values of MTV and TLG were previously reported in NSCLC patients, mostly only in operable or with stage I-III patients [16–18]. To the best of our knowledge, this is the second report regarding prognostic value of MTV in stage IV NSCLC patients. Liao et al. [24] was the first to report the values of MTV and TLG in stage IV NSCLC. They showed that pretreatment MTV and TLG in primary tumor might be better prognostic measures than SUVmax and SUVmean measurements. Our findings are consistent with that of Liao et al. [24]. In addition, we analyzed the stratification power of MTV-lung and TLG-lung in M1b patients. Both of them successfully classified patients with better prognosis. Interestingly, clinical parameters which were routinely used in clinical practice such as age, sex, histology of lung cancer, TNM classification, smoking history, ECOG performance status and SUVmax also did not show any statistical significance.
In spite of a good correlation among three PET parameters of primary tumor, SUVmax-lung did not predict PFS. Although SUVmax is convenient to measure and widely used, it has some limitations. SUVmax is a single-pixel value representing the most intense F-18 FDG uptake in the tumor and may not be an adequate surrogate marker to represent tumor biology [13]. In addition, SUVmax variability increases as the lesion matrix size and patient size increase [25]. There is also a statistical bias of SUVmax in that large lesions are more intense because of more available counts [26]. Our results are consistent with those of previous reports that have reported that SUVmax-lung is not an independent prognostic factor [27–29].
Liao et al. [24] reported that whole-body MTV had a prognostic value in stage IV NSCLC, while MTV-torso failed to stratify prognosis in this study. The standard imaging protocol for NSCLC in Korea is torso imaging. We measured MTV from torso images instead of instead of the whole body. Sixteen of 57 patients (28.1 %) had brain metastases at the time of diagnosis. This might be one reason for the different results from the whole-body MTV results of Liao et al.’s study. However, there was no statistical difference in PFS according to the presence or absence of brain metastases (mean PFS 218.4 ± 233.9 days, 302.8 ± 368.5 days, respectively P = 0.738) in these stage IV patients. Also, there was no statistical difference of disease progression frequency in the two groups (no brain metastasis group, 21/41; brain metastasis group, 7/16; P = 0.776). Therefore presence of brain metastasis might not contribute to the disease progression.
We evaluated these PET parameters in M1a staging group, which did not show any statistical significance. The small number of enrolled patients (20 patients of M1a stage in present study) might be influence of statistic value. Further study with large eligible patients will be needed.
The present study had several limitations. First, a small number of patients were included retrospectively. Second, MTV can be affected by the partial volume effect, the time between tracer injection and imaging, and plasma glucose levels. The methods of measuring MTV are still controversial. Further prospective studies on the accurate measurement of MTV involving a larger number of patients are needed to confirm the prediction of prognosis in patients with NSCLC. Third, we used PFS as clinical end point, instead of overall survival. Since the chemotherapeutic regimen was changed after disease progression, we were not able to standardize all chemotherapeutic regimens. Therefore, we did not correlate prognostic factors with overall survival in this study. Forth, MTV was measured using torso instead of whole-body images. But, MTV from torso scan could give more useful information in routine clinical setting, because torso scan in PET/CT is the usual protocol for NSCLC.
In conclusion, MTV of primary lung lesion measured by FDG PET/CT could further stratify patients with stage IV NSCLC into two groups of different prognoses, while new classification of M1a and M1b could not. This observation has a clinical implication in planning palliative treatment decisions for stage IV NSCLC patients.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Huang W, Zhou T, Ma L, Sun H, Gong H, Wang J, et al. Standard uptake value and metabolic tumor volume of F-18 FDG PET/CT predict short-term outcome early in the course of chemoradiotherapy in advanced non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2011;38:1628–35. doi: 10.1007/s00259-011-1838-5. [DOI] [PubMed] [Google Scholar]
- 2.Feng M, Kong FM, Gross M, Fernando S, Hayman JA, Ten Haken RK. Using fluorodeoxyglucose positron emission tomography to assess tumor volume during radiotherapy for non-small-cell lung cancer and its potential impact on adaptive dose escalation and normal tissue sparing. Int J Radiat Oncol Biol Phys. 2009;73:1228–34. doi: 10.1016/j.ijrobp.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.William WN, Jr, Lin HY, Lee JJ, Lippman SM, Roth JA, Kim ES. Revisiting stage IIIB and IV non-small cell lung cancer: analysis of the surveillance, epidemiology, and end results data. Chest. 2009;136:701–9. doi: 10.1378/chest.08-2968. [DOI] [PubMed] [Google Scholar]
- 4.Postmus PE, Brambilla E, Chansky K, Crowley J, Goldstraw P, Patz EF, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the M descriptors in the forthcoming (seventh) edition of the TNM classification of lung cancer. J Thorac Oncol. 2007;2:686–93. doi: 10.1097/JTO.0b013e31811f4703. [DOI] [PubMed] [Google Scholar]
- 5.Socinski MA, Morris DE, Masters GA, Lilenbaum R. Chemotherapeutic management of stage IV non-small cell lung cancer. Chest. 2003;123:226S–43S. doi: 10.1378/chest.123.1_suppl.226S. [DOI] [PubMed] [Google Scholar]
- 6.Roedl JB, Colen RR, Holalkere NS, Fischman AJ, Choi NC, Blake MA. Adenocarcinomas of the esophagus: response to chemoradiotherapy is associated with decrease of metabolic tumor volume as measured on PET-CT. Comparison to histopathologic and clinical response evaluation. Radiother Oncol. 2008;89:278–86. doi: 10.1016/j.radonc.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 7.La TH, Filion EJ, Turnbull BB, Chu JN, Lee P, Nguyen K, et al. Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;74:1335–41. doi: 10.1016/j.ijrobp.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung HH, Kwon HW, Kang KW, Park NH, Song YS, Chung JK, et al. Prognostic value of preoperative metabolic tumor volume and total lesion glycolysis in patients with epithelial ovarian cancer. Ann Surg Oncol. 2012;19:1966–72. doi: 10.1245/s10434-011-2153-x. [DOI] [PubMed] [Google Scholar]
- 9.Gulec SA, Suthar RR, Barot TC, Pennington K. The prognostic value of functional tumor volume and total lesion glycolysis in patients with colorectal cancer liver metastases undergoing Y-90 selective internal radiation therapy plus chemotherapy. Eur J Nucl Med Mol Imaging. 2011;38:1289–95. doi: 10.1007/s00259-011-1758-4. [DOI] [PubMed] [Google Scholar]
- 10.Liu FY, Chao A, Lai CH, Chou HH, Yen TC. Metabolic tumor volume by F-18 FDG PET/CT is prognostic for stage IVB endometrial carcinoma. Gynecol Oncol. 2012;125:566–71. doi: 10.1016/j.ygyno.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Song MK, Chung JS, Shin HJ, Lee SM, Lee SE, Lee HS, et al. Clinical significance of metabolic tumor volume by PET/CT in stages II and III of diffuse large B cell lymphoma without extranodal site involvement. Ann Hematol. 2012;91:697–703. doi: 10.1007/s00277-011-1357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh JR, Seo JH, Chong A, Min JJ, Song HC, Kim YC, et al. Whole-body metabolic tumour volume of F-18 FDG PET/CT improves the prediction of prognosis in small cell lung cancer. Eur J Nucl Med Mol Imaging. 2012;39:925–35. doi: 10.1007/s00259-011-2059-7. [DOI] [PubMed] [Google Scholar]
- 13.Dibble EH. Lara Alvarez AC, Truong MT, Mercier G, Cook EF, Subramaniam RM. F-18 FDG metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: adding value to clinical staging. J Nucl Med. 2012;53:709–15. doi: 10.2967/jnumed.111.099531. [DOI] [PubMed] [Google Scholar]
- 14.Kim BS, Kim IJ, Kim SJ, Nam HY, Pak KJ, Kim KY, et al. The prognostic value of the metabolic tumor volume in FIGO stage IA to IIB cervical cancer for tumor recurrence: Measured by F-18 FDG PET/CT. Nucl Med Mol Imaging. 2011;45:36–42. doi: 10.1007/s13139-010-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi KH, Yoo IR, Han EJ. kim YS, Kim GW, Na SJ, et al. Prognostic value of metabolic tumor volume measured by F-18 FDG PET/CT in locally advanced head and neck squamous cell carcinomas treated by surgery. Nucl Med Mol Imaging. 2011;45:43–51. doi: 10.1007/s13139-010-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee P, Bazan JG, Lavori PW, Weerasuriya DK, Quon A, Le QT, et al. Metabolic tumor volume is an independent prognostic factor in patients treated definitively for non-small-cell lung cancer. Clin Lung Cancer. 2012;13:52–8. doi: 10.1016/j.cllc.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng X, Sun X, Mu D, Xing L, Ma L, Zhang B, et al. Noninvasive evaluation of microscopic tumor extensions using standardized uptake value and metabolic tumor volume in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;82:960–6. doi: 10.1016/j.ijrobp.2010.10.064. [DOI] [PubMed] [Google Scholar]
- 18.Yan H, Wang R, Zhao F, Zhu K, Jiang S, Zhao W, et al. Measurement of tumor volume by PET to evaluate prognosis in patients with advanced non-small cell lung cancer treated by non-surgical therapy. Acta Radiol. 2011;52:646–50. doi: 10.1258/ar.2011.100462. [DOI] [PubMed] [Google Scholar]
- 19.Basaki K, Abe Y, Aoki M, Kondo H, Hatayama Y, Nakaji S. Prognostic factors for survival in stage III non-small-cell lung cancer treated with definitive radiation therapy: impact of tumor volume. Int J Radiat Oncol Biol Phys. 2006;64:449–54. doi: 10.1016/j.ijrobp.2005.07.967. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi T, Takada M, Kubo A, Matsumura A, Fukai S, Tamura A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol. 2010;5:620–30. doi: 10.1097/JTO.0b013e3181d2dcd9. [DOI] [PubMed] [Google Scholar]
- 21.UyBico SJ, Wu CC, Suh RD, Le NH, Brown K, Krishnam MS. Lung cancer staging essentials: the new TNM staging system and potential imaging pitfalls. Radiographics. 2010;30:1163–81. doi: 10.1148/rg.305095166. [DOI] [PubMed] [Google Scholar]
- 22.Seol YM, Kwon BR, Song MK, Choi YJ, Shin HJ, Chung JS, et al. Measurement of tumor volume by PET to evaluate prognosis in patients with head and neck cancer treated by chemo-radiation therapy. Acta Oncol. 2010;49:201–8. doi: 10.3109/02841860903440270. [DOI] [PubMed] [Google Scholar]
- 23.Chung HH, Kim JW, Han KH, Eo JS, Kang KW, Park NH, et al. Prognostic value of metabolic tumor volume measured by FDG-PET/CT in patients with cervical cancer. Gynecol Oncol. 2011;120:270–4. doi: 10.1016/j.ygyno.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Liao S, Penney BC, Zhang H, Suzuki K, Pu Y. Prognostic value of the quantitative metabolic volumetric measurement on F-18 FDG PET/CT in Stage IV nonsurgical small-cell lung cancer. Acad Radiol. 2012;19:69–77. doi: 10.1016/j.acra.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med. 2004;45:1519–27. [PubMed] [Google Scholar]
- 26.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, et al. Prognostic value of metabolic tumor volume measured by F-18 fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol. 2010;17:115–22. doi: 10.1245/s10434-009-0719-7. [DOI] [PubMed] [Google Scholar]
- 28.Song MK, Chung JS, Shin HJ, Moon JH, Lee JO, Lee HS, et al. Prognostic value of metabolic tumor volume on PET/CT in primary gastrointestinal diffuse large B cell lymphoma. Cancer Sci. 2012;103:477–82. doi: 10.1111/j.1349-7006.2011.02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang C, Murphy JD, Khong B, La TH, Kong C, Fischbein NJ, et al. Validation that metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;83:1514–20. doi: 10.1016/j.ijrobp.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]