Abstract
Insects use chemosensory cues to feed and mate. In Drosophila, the effect of pheromones has been extensively investigated in adults, but rarely in larvae. The colonization of natural food sources by Drosophila buzzatii and Drosophila simulans species may depend on species-specific chemical cues left in the food by larvae and adults. We identified such chemicals in both species and measured their influence on larval food preference and puparation behaviour. We also tested compounds that varied between these species: (i) two larval volatile compounds: hydroxy-3-butanone-2 and phenol (predominant in D. simulans and D. buzzatii, respectively), and (ii) adult cuticular hydrocarbons (CHs). Drosophila buzzatii larvae were rapidly attracted to non-CH adult conspecific cues, whereas D. simulans larvae were strongly repulsed by CHs of the two species and also by phenol. Larval cues from both species generally reduced larval attraction and pupariation on food, which was generally—but not always—low, and rarely reflected larval response. As these larval and adult pheromones specifically influence larval food search and the choice of a pupariation site, they may greatly affect the dispersion and survival of Drosophila species in nature.
Keywords: group-effect, acetoin, fatty acid, diet, olfaction, taste
1. Introduction
In social insects, larval and pupal communication often relies on sensory modalities involving acoustic, chemical and tactile signals [1–3]. This is also the case in gregarious insect larvae [4–6] whose aggregation behaviour often depends on chemical cues such as cuticular substances and other compounds mixed in faeces [7,8]. In non-social insects, chemical cues emitted by adults can also influence larval behaviour [9,10]. Reciprocally, when larvae develop in the food, they can leave chemical cues affecting adult behaviour including female attraction and oviposition [11,12].
In Drosophila species, there is very little information on the effect that chemical cues—either produced by larvae and/or by adults—induce on larval behaviour. This contrasts with the well-documented effect induced by adult sex pheromones on adult behaviour. Drosophila adult pheromones vary for their volatility. Low-volatility pheromones (cuticular hydrocarbons, CHs) stick on the cuticle and can be deposited on the substrate: they are mostly—but not exclusively—perceived by taste organs [13–16]. Highly volatile compounds, such as cis-vaccenyl acetate and CH503 are detected by olfactory organs [17,18]. These molecules can influence a variety of adult behaviours; namely aggregation, courtship, mating and aggression [19–22]. Pre-imaginal exposure to specific food components mixed, or not, with pheromones can also affect subsequent adult behaviour in Drosophila mojavensis, Drosophila arizonae [23] and Drosophila paulistorum [24], but the identity of compounds involved remains unknown.
A recent study combining Drosophila field and laboratory investigations revealed that species-specific chemicals could potentially influence larval food preference and the choice of pupariation site. When the two species Drosophila buzzatii and Drosophila simulans share the same breeding sites (Opuntia ficus-indica prickly pears), the distribution pattern of their pupae in different parts of the fruit changes compared with fruits hosting either species [25]. The hypothesis that ‘the choice of pupariation site in nature depends on species-specific chemicals’ [26] was supported by laboratory experiments showing individual larval and pupa preference to food processed by the homospecific species [25]. The cues influencing these behaviours could be produced both by larvae and adults, but their chemical identity was not revealed. Here, we identified chemical cues left in the food by larvae and adults of both species, and we measured their effect on larval food preference and pupariation behaviour.
2. Material and methods
(a). Fly stocks
The D. buzzatii and D. simulans strains used (gift of Prof. Raùl Godoy-Herrera, Santiago, University of Chile) were derived from multi-female lines originated from fruits collected in Chile [25] and maintained in our laboratory on a corn flour/yeast/agar food under a 12 L : 12 D cycle at 25°C more than 2 years before testing.
(b). Food types
Two hours before the experiment, the food patches to be tested were impregnated either with plain food (P-food) or with food processed by first instar (L1) to L3 larvae resulting of the mating between numerous adult males and females (L + A-food). Therefore, L + A-food contained larvae (and larval cues) as well as chemical traces left by adults. Adult-labelled food (A-food) consisted of P-food on which 30 pairs of mature virgin flies (7-day-old adults) were kept during 3 h. We also tested P-food mixed with adult CHs (CH-food). In this experiment, P-food patches were impregnated with 5 µl of a hexane-whole extract corresponding to five mature flies (2.5 males + 2.5 females). In other experiments, we added 5 µl of methylene chloride (CH2Cl2) containing either 100 ng hydroxy-3-butanone-2 (H3B2) or 200 ng phenol on patches (already impregnated either with P- or L + A-food). CH-, H3B2- and phenol-food were tested after evaporation of the solvent at room temperature (about 30 s).
(c). Behaviour
We always used early third instar larvae (L3). Larvae to be tested were separated from the media and maintained in distilled water just before the tests. Tests were made using freshly prepared Petri dishes (9.5 cm diameter, 1 cm high) containing a 2% water agar layer (thickness 5 mm). Each Petri dish contained two food patches (Whatman paper grade 42, 1.5 cm diameter, pre-washed in distilled water, in ethanol and dried overnight at 70°C) impregnated with various chemical cues (see above). Food patches—from which excess food was removed with a spatula—were separately pinned down on the agar dish with a thin needle. For each test, two patches, separated of approximately 30 mm, were placed in diametrically opposed zones. A group of 10 larvae were transferred, using a fine brush, at a mid-distance between the patches. Then, the dish was covered with a lid to reduce evaporation of the tested media and the time of observation started. The number of larvae present above and below each patch was noted each min for the first 5 min of the experiment and then every 5 min until 30 min. The 30 min duration was estimated based on preliminary experiments showing no visible change of larval pattern distribution with longer duration. In this report, we show the distribution at all time-points to visualize the dynamic response of larvae, but we only tested four time-points (1, 5, 15 and 30 min) corresponding to representative data points. All observations were performed under white light at 24.5 ± 0.5°C.
Moreover, and for the sake of clarity, all data representing the distribution ‘over’ versus ‘under’ food patches is shown in the electronic supplementary material section. Petri dishes containing assayed larvae were further kept for the next 2 days and the number of pupae above and below each patch was noted.
In control experiments, we tested the response to a pair of patches impregnated with P-food (figure 1a). In other experiments, we either paired one P-food patch with one ‘impregnated-food’ patch or two impregnated-food patches. Control and ‘impregnated-food’ experiments were simultaneously performed. We pooled all control experiments since they did not diverge between experiments (n = 12 per food-type and species) involving L + A-, A- and CH-food.
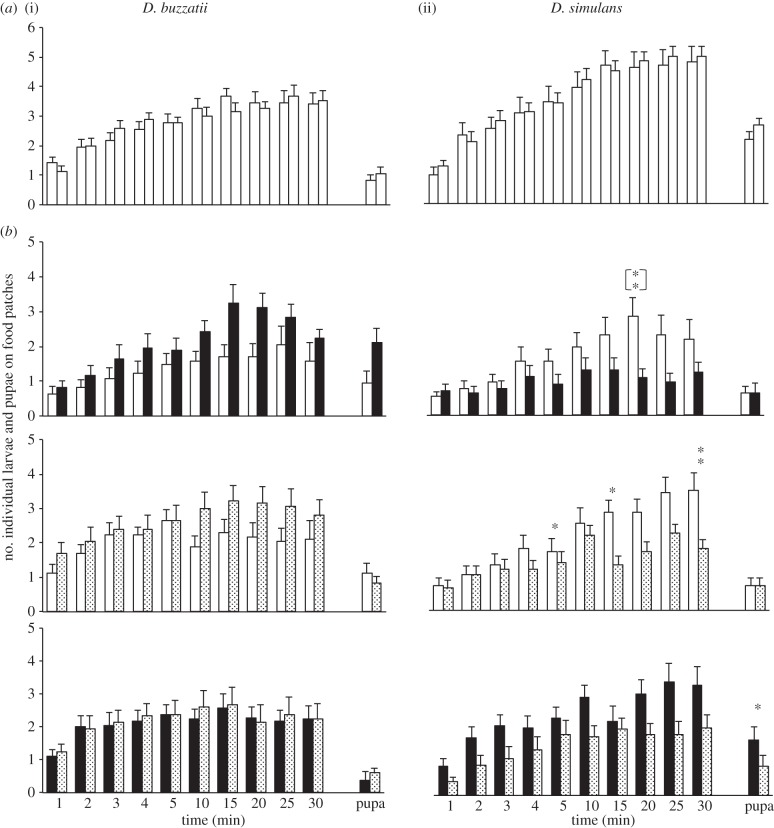
Figure 1.
Larval and pupal distribution on plain food (P-food) and food processed by larvae and adults (L + A-food). Histograms show the mean (±s.e.m.) distribution of early third instar larvae and pupae (bars on the right side on each histogram set) on two food patches. We assayed in a dual-choice test food preference in groups of 10 D. buzzatii (i) and D. simulans (ii) larvae during 30 min. The choice either consisted (a) of a pair of P-food patches (empty bars), or (b) of a P-food patch paired with a patch of L + A-food processed by D. buzzatii (filled bars) or by D. simulans (dotted bars), or with both L + A-food patches paired. The statistical difference for distribution between both food patches was tested using a Wilcoxon test (at 1, 5, 15 and 30 min for larvae): **p < 0.01; *p < 0.05; no sign: p = n.s. A significant difference was also found in D. simulans larvae tested with D. buzzatii L + A-food paired with P-food after 20 min ([**]). n = 36 for P-food, 16–18 for P- with L + A-food and 12 for paired L + A patches.
(d). Chemical analysis
In parallel to the behavioural tests, we analysed the chemical composition of food patches. All patches were impregnated as described above. After removing the excess medium, the patch was incubated and periodically vortexed for 10 min at room temperature in a vial containing 2 ml of solvent and 500 ng n-pentadecane (C15) as internal standard. Then, the patch was removed from the vial using fine forceps. Extracts were stored at −20°C until analysis. Just before analysis, the extract was concentrated under a gentle flow of nitrogen to obtain 50 µl. After preliminary analyses, and to extract most polar and apolar components, we used a mixture of hexane/CH2Cl2 (50/50, v/v) as solvent. The extracts were analysed using a QP2010 Shimadzu GC-MS apparatus in splitless mode equipped with a CP Wax 58 FFAP (polar type, 50 m × 0.25 mm i.d., 0.20 µm film thickness, Agilent). The column was held isothermally at 40°C for 2 min, then programmed at the rate of 3°C min−1 to 240°C. Helium was used as carried gas at a linear velocity of 47 cm s−1. The injector port was set at 280°C. The mass spectrometer was operated at 70 eV, and scanning was performed from 29 to 600 amu at 0.5 scans s−1. The injection split was opened 1 min after the injection. Compounds were identified using their retention time and their fragmentation patterns; diagnostic ions were compared with both the NIST/EPA/NIH library and the mass-spectrum of the synthetic chemical standards (Sigma-Aldrich, St Quentin Fallavier, France) analysed under the same conditions. For quantitative analyses, the response factors of C15 and the major studied compounds were determined at 1, 5, 10, 25 and 50 ng.
(e). Statistics
For each experiment, we assessed the statistical difference for larval (and pupal) distribution between both food patches using a Wilcoxon test (XLStats). For the sake of clarity, differences were mostly tested at 1, 5, 15 and 30 min. We also compared the amount of chemicals and the distribution of larvae at 30 min—and that of pupae—over and/or under food patches using a Kruskal–Wallis test (p < 0.05). For each condition, the distribution of larvae (at 30 min) and pupae was compared between the two sides of the food patch using a Wilcoxon test (at p < 0.05). We also compared the distribution of H3B2 amounts between the two lines using a Mann–Whitney test (p < 0.05).
3. Results
(a). Effect of larval and adult cues mixed in the food (L + A-food)
First, we tested the response of D. buzzatii and D. simulans larvae to standard laboratory food either plain (P-food) or processed both by larvae and adults (L + A-food) of the two species. Then, we compared the chemical composition of these types of food and tested the behavioural effect induced by two larval species-predominant compounds.
(i). Effect of L + A food on larval behaviour
In the control experiment (P- versus P-food), a total of 70% D. buzzatii larvae gathered on the two P-food patches without showing preference (figure 1a(i)). In tests involving L + A-food, larvae showed no significant preference (figure 1b). In the ‘D. buzzatii- versus D. simulans-food’ test, only 45% larvae migrated to the food patches.
In the control test and after 30 min, more than 80% D. simulans larvae gathered without preference on the P-food patches (figure 1a(ii)). They avoided both L + A-food when paired with P-food. Heterospecific food induced a transient effect (at 20 min: p = 0.006), whereas homospecific food induced a long-lasting avoidance effect (from 5 to 30 min: p = 0.039 and p = 0.003, respectively; figure 1b). Drosophila simulans larvae showed no preference in the ‘D. buzzatii-versus D. simulans-food’ test, but their pupae preferred heterospecific food (p < 0.05).
In summary, D. buzzatii larvae showed no significant preference to food patches, whereas D. simulans larvae avoided L + A-food in a variable manner.
(ii). Compounds in L + A-food
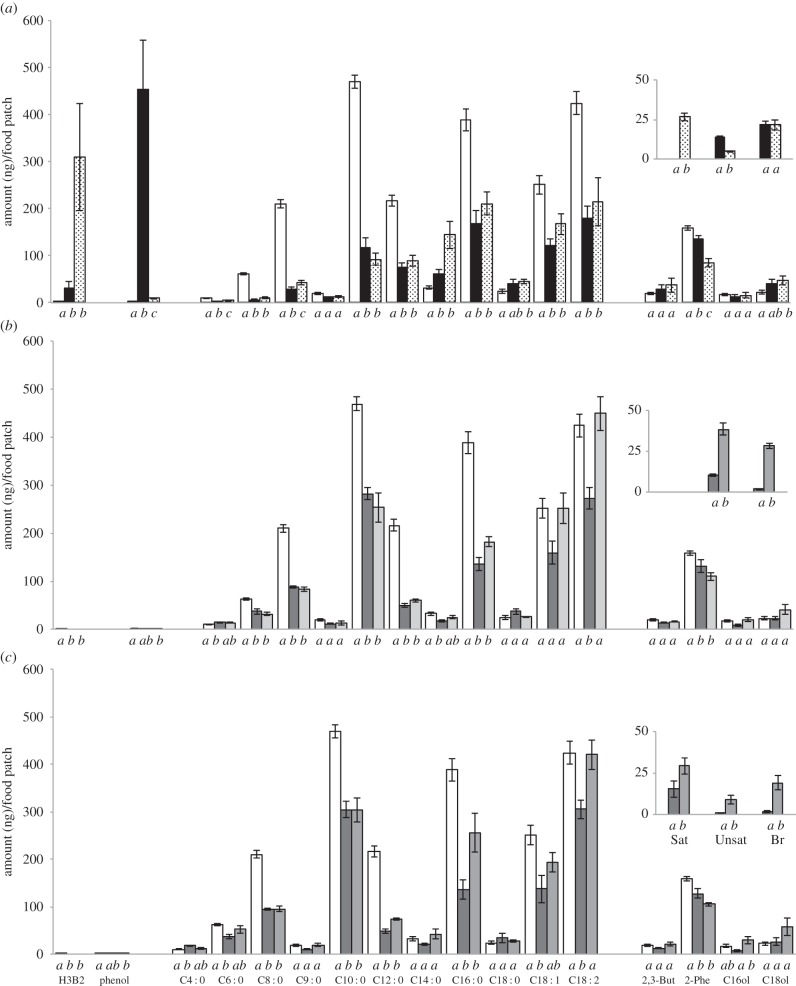
The comparison of the chemical composition of P-food with both L + A-food reveals three major differences (figure 2a; electronic supplementary material, table S1): (i) two very volatile compounds, ‘phenol’ and ‘hydroxy-3-butanone-2’ (H3B2; acetoin), were found in high levels in D. buzzatii- and D. simulans-food, respectively, but not in P-food; (ii) the amount of most saturated and unsaturated fatty-acids (FAs; C4 : 0 to C16 : 0; C18 : 1 and C18 : 2, respectively) decreased in L + A-food, compared with P-food, except C14 : 0 which increased in both L + A-food; and (iii) substantial amounts of species-specific adult CHs were detected in both L + A-food, suggesting contamination by adults. Note that saturated linear CHs (Sat; alkanes) were only detected in D. simulans. Few other quantitatively minor compounds slightly varied: for example, 2-phenylethanol (2-Phe) decreased in D. simulans—but not D. buzzatii—L + A-food compared to P-food.
Figure 2.
Chemical composition of different types of food patches processed by D. buzzatii and D. simulans. Chemical analysis was carried out on the extracts of food patches impregnated with the different types of food in parallel to those assayed in behaviour: P-food (open bars, all series), L + A-food processed by D. buzzatii and D. simulans ((a); filled and dotted bars, respectively), A- and CH-food (dark grey and light grey bars, respectively) processed by D. buzzatii (b) and D. simulans adults (c). Histograms represent the mean (±s.e.m.) amount, in nanograms per food patch, for each compound (indicated under the bottom graph) and for CHs (shown in the inset on the right of each graph: Sat, Unsat and Br correspond to the sum of alkanes, alkenes and ramified CHs, respectively). For abbreviation of compounds, please refer to the electronic supplementary material, table S1. For each graph, the quantitative variation of each compound was tested using a Kruskal–Wallis test (different letters under histogram bars indicate significant difference at p < 0.05; n = 20).
(iii). Behavioural effect of pure H3B2 and phenol mixed with P- or L + A-food
We tested the behavioural effect of phenol and H3B2, two volatile compounds abundant in D. buzzatii and D. simulans L + A-food, respectively. To assess their possible interaction with other components of the processed food, each compound was either added to P- or to each L + A-food (electronic supplementary material, figure S1). The response of D. buzzatii larvae was not altered (left panels), but D. simulans larvae were repulsed by phenol either added to P- or D. buzzatii-food (at 15 min: p = 0.045 and p = 0.013, respectively).
(b). Effect of adult chemical cues labelling the food (A- and CH-food)
The data obtained with L + A-food suggest that either larval, adult or both types of chemical cues can influence larval behaviour and pupariation. Therefore, and to directly measure the effect of adult chemicals left in the food, we performed two other experiments. First, P-food was labelled during 3 h by freely walking groups of mature virgin flies of both sexes (A-food). Second, species-specific CHs extracted from five mature flies (both sexes mixed) were added into P-food (CH-food). We determined the chemical composition of A- and CH-food and measured the larval preference and pupariation behaviour induced by each food type, in the two species.
(i). Compounds in A- and CH-food
The examination of A- and CH-food revealed three major features (figure 2b,c; electronic supplementary material, table S1): (i) neither phenol nor H3B2 were present in A- and CH-food supporting the larval origin of these compounds. Similarly to L + A-food, both A- and CH-food contained; (ii) species-specific CHs (such as Sat in D. simulans); and (iii) generally, less saturated FAs, compared with P-food. However, the level of C14 : 0 and of two unsaturated FAs (C18 : 1 and C18 : 2) was not, or much less, affected compared with L + A-food.
(ii). Larval behaviour to A- and CH-food
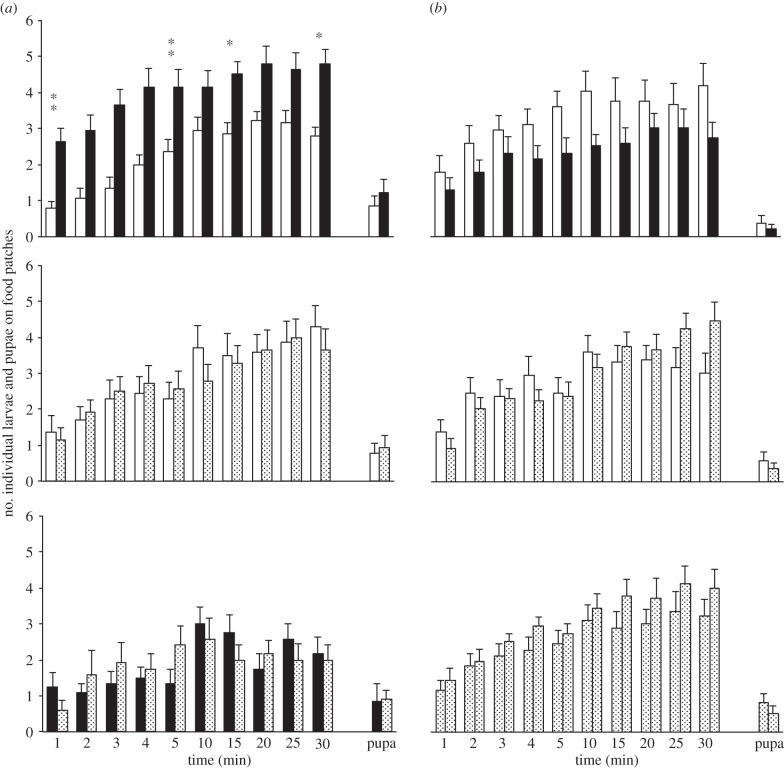
In the ‘P- versus A-food’ tests, D. buzzatii larvae migrated very quickly on homospecific A-food (after 1 min: p = 0.005; figure 3a). This preference involved 70–80% larvae and lasted 30 min (p = 0.043). However, overall larval response decreased with the two A-food patches paired (less than 50%). By contrast, CHs added in P-food induced no significant effect (figure 3b).
Figure 3.
Drosophila buzzatii larval and pupal distribution on plain food (P-food, open bars) and food labelled with adult chemical cues (A-food) or with adult CHs (CH-food). Drosophila buzzatii larvae and pupae were tested with A-food (a) and with CH-food (b) processed by D. buzzatii (filled bars) and/or by D. simulans species (dotted bars). For A- and CH-food, the two species-processed foods were either paired with P-food or simultaneously tested (bottom histograms). For more information, see figure 1. n = 14–21.
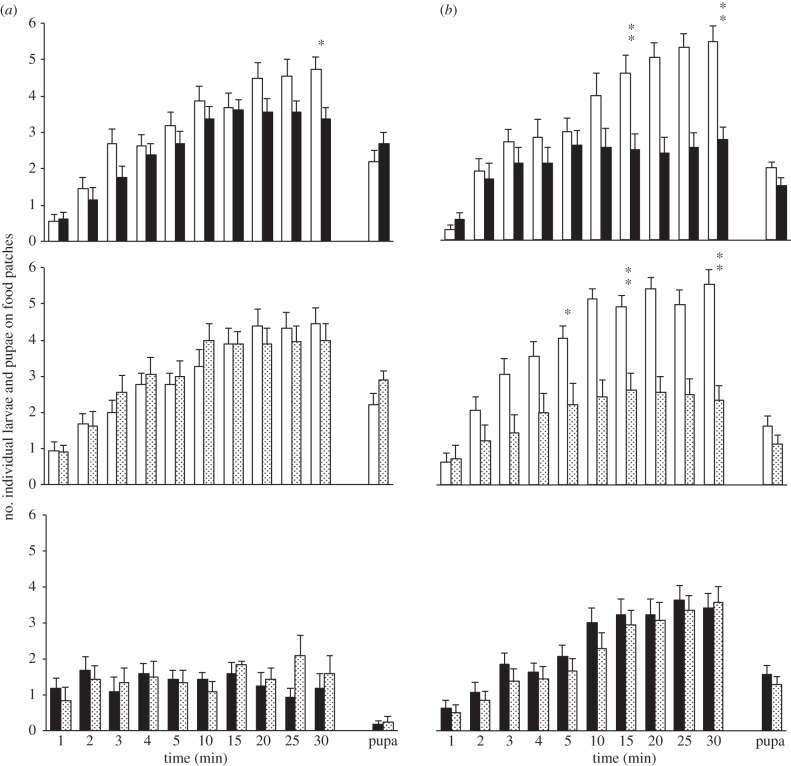
In the ‘P- versus A-food’ tests, 70% D. simulans larvae migrated to food patches and were slightly repulsed by D. buzzatii A-food (at 30 min; p = 0.02; figure 4a). Strikingly, very few larvae (less than 30%) responded when the A-food processed by the two species were paired. In the ‘P- versus CH-food’ tests, D. simulans larvae showed a very strong repulsion against either CH-food, this maybe explaining the high attraction to P-food (figure 4b).
Figure 4.
Drosophila simulans larval and pupal distribution on plain food (P-food, open bars) and food labelled with adult chemical cues (A-food) or with adult CHs (CH-food). Drosophila simulans larvae and pupae were tested with A-food (a) or with CH-food (b) processed by D. buzzatii (filled bars) and/or by D. simulans species (dotted bars). For A- and for CH-food, the two species-processed foods were either paired with P-food or simultaneously tested (bottom histograms). For more information, see figure 1. n = 14–20.
In summary, D. buzzatii larvae were attracted by homospecific ‘non-CH’ adult cues, whereas D. simulans larvae were strongly repulsed by CHs of both species. The simultaneous presentation of both A-food patches strongly inhibited larval attraction and pupariation on food patches (especially for D. simulans; electronic supplementary material, figure S2). The overall comparison of the effects induced by L + A-, A- and CH-food suggests that larvae use adult cues to discriminate food sources, whereas larval cues tend to reduce the attractive and/or arrestant effect of food patches (electronic supplementary material, figure S2). Drosophila simulans showed a very contrasted tropism relatively to food patch side: after 30 min, in most cases, larvae stayed under the food patch and pupae over the patch. Drosophila buzzatii showed a less contrasted response: larvae preferentially migrated under the food patch in fewer experiments, whereas pupae were rarely found on food patches except with D. buzzatii L + A-food mixed with both H3B2 and phenol.
4. Discussion
Our data indicate that chemical cues produced by D. buzzatii and D. simulans adults—and to a lesser extent by larvae—can influence larval orientation to food sources. Both species released different amounts of chemical cues in the food, and their behaviour was somewhat differently affected by these cues. Drosophila buzzatii larvae were variably—sometimes very rapidly—attracted to adult homospecific cues, whereas D. simulans larvae were repulsed by most homo- and heterospecific cues and more specially by adult CHs. If these two phylogenetically distant species (their divergence occurred about 60 Ma [27] can occasionally share the same food source (Opuntia ficus-indica fruits), their global diet markedly diverges: the cactophilic D. buzzatii species feed on a limited type of resources [28], whereas D. simulans has a generalist diet [29]. As we used a single strain per species, we cannot totally rule out the possibility that the observed differences are not interspecific but intraspecific.
Do group tests better reflect the natural situation than individual tests [25,26]? In our hand, groups of larvae showed no preference to L + A-food processed by either species (figure 1, two bottom histograms) differently to individual larvae which showed homospecific preference [25]. This discrepancy could be explained if, in groups, pioneer larvae mark food patches with some chemical cues affecting the response of followers. This hypothesis is supported by the lower number of larvae migrating on food patches involving L + A-food (electronic supplementary material, figure S2). The aversive effect of larval cues could also explain the decreased number of pupae on food patches in group tests (less than 15%) compared with individual tests (30%) [25].
As we had not direct means to measure the behavioural effect induced by the complete set of larval cues, we estimate larval cues effect based on the comparison between L + A-food versus either A- or CH-food. This comparison suggests that the repulsive effect induced by L + A-food in D. simulans larvae was caused by food contamination by adult CHs. We have also assessed the direct effect of two predominant larval compounds, H3B2 and phenol, mixed in P- or in L + A-food (electronic supplementary material, figure S1). Phenol (concurrently tested with H3B2) inhibited food attraction of D. simulans larvae after 10 min, whereas H3B2 induced no effect. This fits with the fact that phenol, but not H3B2, showed a quantitative species- or population-specific difference. The absence of interspecific effect for H3B2 can be explained by the absence of significant interspecific difference probably due to its large intra-population or -specific quantitative variation in D. simulans (electronic supplementary material, figure S3). Surprisingly, the simultaneous presence of both compounds mixed in D. buzzatii L + A-food strongly enhanced D. buzzatii pupariation behaviour (electronic supplementary material, figure S2). As this effect did not occur with any other food condition, this exceptional phenotype may result from the interaction between phenol, H3B2 and other components of the D. buzzatii L + A-food.
Phenol and H3B2 were already known to affect the behaviour of adult insects. For example, phenol was shown to act as a sex pheromone in the grass grub beetle [30] and to attract cockchafer males [31], repulse blowflies [32] and stimulate mosquito oviposition [33]. H3B2 stimulates adult scarab beetles and cockroaches [34–36] and can enhance—in combination with acetic acid and ethanol—attraction in Drosophila suzukii flies [37].
Drosophila adult cues induced a marked species- or population-specific effect on larvae. Drosophila buzzatii larvae were rapidly attracted (in less than 1 min) to non-CH homotypic adult compounds, whereas D. simulans larvae were strongly repulsed against adult CHs of both species. Drosophila simulans aversion may be induced by adult—particularly branched —CHs which were found in higher amounts in both CH- and L + A-food than in A-food (figure 2; electronic supplementary material, table S1). Our data are coherent with the natural distribution pattern of pupae in fruits where most D. buzzatii pupae are found in the top part of the fruit and most D. simulans pupae in the middle and bottom parts [25]. We hypothesize that chemical cues left by adults of the two species—when walking, mating and laying eggs on top of the fruits—can attract more D. buzzatii and repulse more D. simulans larvae.
Our data also indicate that larval preference can be influenced both by olfactory cues (attracting larvae in less than 1 min) and gustatory cues (arresting larvae between 5 and 30 min). The sequential perception of olfactory and gustatory cues is also necessary for adult discrimination of Drosophila sex pheromones [38–40]. We cannot currently explain why the simultaneous presentation of A-food patches of the two species dramatically reduced the general attractivity of food, particularly in D. simulans larvae. This may either be the result of (i) a quantitative effect, e.g. the summation of compounds shared by both species, or (ii) a qualitative effect resulting in an interference between species-specific compounds. This shows that the experiments performed with each type of processed food do not allow us to predict larval response in tests combining different types of processed food. As A- and CH-food were labelled by mixed virgin adults of both sexes, it could be worth testing the effect of adult sex-specific cues, and those resulting of their sexual interaction. As CHs are not sexually dimorphic (in both species), potential candidates would be male internally produced pheromones influencing adult behaviour such as (Z)-10-heptadecen-2-one, 2-tridecanone, 2-pentadecanone and 2-heptadecanone in D. buzzatii [41] and cis-vaccenyl acetate in D. simulans [42,43]. The impact of these compounds on larval and pupariation behaviour currently remains unknown.
In any population or species, individuals search for the best food source to feed, reproduce and leave progeny. Insect ability to show species-specific response and adaptation to environmental cues may reduce interspecific competition and population overlap [44–46]. Our data suggest that chemical cues left both by Drosophila larvae and adults influence species-specific strategy for larval food search and choice of a pupariation site.
Larval food preference may depend on exposure during early larval development to conspecific pheromones associated with food [25,47]. Early developmental exposure to food cues may also affect adult response to these cues [48,49]. Memory persistence through the complete metamorphosis remains an enigma in holometabolous insects (such as Drosophila), because a large part of the nervous system is reorganized during this process. Studies involving mixed Drosophila culture (of two strains, sub-species or species) showed that pre-imaginal exposure to homo- versus heterospecific (or homo- versus heterotypic) chemical cues affect adult sexual behaviour and mate discrimination [24,50,51]. However, the identity of these cues currently remains unknown and neither H3B2 nor phenol seems sufficient to induce food-choice conditioning in either Drosophila species. The associative process may involve combination of these molecules with other compounds of the ‘bouquet’. If a precise chemosensory ‘memory’ is crucial for insects living on a specific host–plant and in parasite–parasitoid association [12,52], the strict association with a specific host may also involve a mutualistic interaction with microorganisms and yeast [11]. This may facilitate the metabolization of the nutriment available in this food source [53] and the production of specific food-derived components with pheromonal properties [11,54]. This mutualistic interaction may vary between Drosophila species [55], explaining the divergence of compounds released by D. buzzatii and D. simulans species.
In summary, our data reveal that the migration of Drosophila larvae to food sources depends on adult—and to a lesser extent larval—species- or population-specific chemical cues left in food. These putative pheromones may guide the dispersion of insects in nature, this shaping their adaptation to novel food sources.
Acknowledgements
Nelly Debrosse, Serge Loquin and José Solonot are thanked for their technical help, and Claude Everaerts for help with the statistics.
Data accessibility
Electronic supplementary material is available at http://dx.doi.org/10.1098/rspb.2014.0043.
Funding statement
This work was partly funded supported by the Centre National de la Recherche Scientifique (INSB), the Burgundy Regional Council (PARI) and ECOS-Sud.
References
- 1.Wyatt TD. 2003. Pheromones and animal behavior: communication by smell and taste. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Casacci LP, Thomas JA, Sala M, Treanor D, Bonelli S, Balletto E, Schönrogge K. 2013. Ant pupae employ acoustics to communicate social status in their colony's hierarchy. Curr. Biol. 23, 323–327. ( 10.1016/j.cub.2013.01.010) [DOI] [PubMed] [Google Scholar]
- 3.Penick CA, Trobaugh B, Brent CS, Liebig J. 2013. Head-butting as an early Indicator of reproductive disinhibition in the termite Zootermopsis nevadensis. J. Insect Behav. 26, 23–34. ( 10.1007/s10905-012-9332-x) [DOI] [Google Scholar]
- 4.Yack JE, Smith ML, Weatherhead PJ. 2001. Caterpillar talk: acoustically mediated territoriality in larval Lepidoptera. Proc. Natl Acad. Sci. USA 98, 11 371–11 375. ( 10.1073/pnas.191378898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa JT, Fitzgerald TD, Pescador-Rubio A, Mays J, Janzen DH. 2004. Social behavior of larvae of the neotropical processionary weevil Phelypera distigma (Boheman) (Coleoptera: Curculionidae: Hyperinae). Ethology 110, 515–530. ( 10.1111/j.1439-0310.2004.00999.x) [DOI] [Google Scholar]
- 6.Flowers RW, Costa JT. 2005. Larval communication and group foraging dynamics in the red-headed pine sawfly, Neodiprion lecontei (Fitch) (Hymenoptera: Symphyta: Diprionidae). Ann. Entomol. Soc. Am. 96, 336–343. ( 10.1603/0013-8746(2003)096[0336:LCAGFD]2.0.CO;2) [DOI] [Google Scholar]
- 7.Pires HHR, Lorenzo MG, Diotaiuti L, Lazzari CR, Lorenzo Figueiras AN. 2002. Aggregation behaviour in Panstrongylus megistus and Triatoma infestans: inter and intraspecific responses. Acta Tropica 81, 47–52. ( 10.1016/S0001-706X(01)00185-1) [DOI] [PubMed] [Google Scholar]
- 8.Vitta ACR, Figueiras AN, Lazzari CR, Diotaluti L, Lorenzo MG. 2002. Aggregation mediated by feces and footprints in Triatoma pseudomaula (Heteroptera : Reduviidae), a Chagas disease vector. Mem. Inst. Oswaldo Cruz 97, 865–867. ( 10.1590/S0074-02762002000600020) [DOI] [PubMed] [Google Scholar]
- 9.Smiseth PT, Andrews C, Brown E, Prentice PM. 2010. Chemical stimuli from parents trigger larval begging in burying beetles. Behav. Ecol. 21, 526–531. ( 10.1093/beheco/arq019) [DOI] [Google Scholar]
- 10.Poivet E, Rharrabe K, Monsempes C, Glaser N, Rochat D, Renou M, Marion-Poll F, Jacquin-Joly E. 2012. The use of the sex pheromone as an evolutionary solution to food source selection in caterpillars. Nat. Commun. 3, 1047 ( 10.1038/ncomms2050) [DOI] [PubMed] [Google Scholar]
- 11.Witzgall P, et al. 2012. “This is not an apple”–yeast mutualism in codling moth. J. Chem. Ecol. 38, 949–957. ( 10.1007/s10886-012-0158-y) [DOI] [PubMed] [Google Scholar]
- 12.Stuhl C, Sivinski J, Teal P, Praranhos B, Aluja M. 2011. A compound produced by frugivorous Tephritidae (Diptera) larvae promotes oviposition behavior by the biological control agent Diachasmimorpha longicaudata (Hymenoptera: Braconidae). Environ. Entomol. 40, 727–736. ( 10.1603/EN10198) [DOI] [PubMed] [Google Scholar]
- 13.Ferveur JF. 2005. Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav. Genet. 35, 279–295. ( 10.1007/s10519-005-3220-5) [DOI] [PubMed] [Google Scholar]
- 14.Lacaille F, et al. 2007. An inhibitory sex pheromone tastes bitter for Drosophila males. PLoS ONE 2, e661 ( 10.1371/journal.pone.0000661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everaerts C, Farine JP, Cobb M, Ferveur JF. 2010. Drosophila cuticular hydrocarbons revisited: mating status alters cuticular profiles. PLoS ONE 3, e9607 ( 10.1371/journal.pone.0009607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farine JP, Ferveur JF, Everaerts C. 2012. Volatile Drosophila cuticular pheromones are affected by social but not sexual experience. PLoS ONE 7, e40396 ( 10.1371/journal.pone.0040396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butterworth FM. 1969. Lipids of Drosophila, a newly detected lipid in the male. Science 163, 1356–1357. ( 10.1126/science.163.3873.1356) [DOI] [PubMed] [Google Scholar]
- 18.Yew JY, Dreisewerd K, Luftmann H, Müthing J, Pohlentz G, Kravitz EA. 2009. A new male sex pheromone and novel cuticular cues for chemical communication in Drosophila. Curr. Biol. 19, 1–10. ( 10.1016/j.cub.2009.06.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jallon JM. 1984. A few chemical words exchanged by Drosophila during courtship and mating. Behav. Genet. 14, 441–478. ( 10.1007/BF01065444) [DOI] [PubMed] [Google Scholar]
- 20.Bartelt RJ, Schaner AM, Jackson LL. 1985. cis-Vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J. Chem. Ecol. 11, 1747–1756. ( 10.1007/BF01012124) [DOI] [PubMed] [Google Scholar]
- 21.Kurtovic A, Widmer A, Dickson BJ. 2007. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446, 542–546. ( 10.1038/nature05672) [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Anderson DJ. 2010. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature 463, 227–231. ( 10.1038/nature08678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stennett MD, Edges WJ. 1997. Premating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. III. Epicuticular hydrocarbon variation is determined by use of different host plants in Drosophila mojavensis and Drosophila arizonae. J. Chem. Ecol. 23, 2803–2824. ( 10.1023/A:1022519228346) [DOI] [Google Scholar]
- 24.Kim YK, Phillips DR, Chao T, Ehrmann L. 2004. Developmental isolation and subsequent adult behavior of Drosophila paulistorum. VI. Quantitative variation in cuticular hydrocarbons. Behav. Genet. 34, 385–394. ( 10.1023/B:BEGE.0000023644.87050.1a) [DOI] [PubMed] [Google Scholar]
- 25.Beltrami M, Medina-Munoz MC, Del Pino F, Ferveur JF, Godoy-Herrera R. 2012. Chemical cues influence pupation behavior of Drosophila simulans and Drosophila buzzatii in nature and in the laboratory. PLoS ONE 7, e39393 ( 10.1371/journal.pone.0039393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beltrami M, Medina-Munoz MC, Arce D, Godoy-Herrera R. 2010. Drosophila pupation behavior in the wild. Evol. Ecol. 24, 347–358. ( 10.1007/s10682-009-9310-8) [DOI] [Google Scholar]
- 27.Tamura K, Subramanian S, Kumar S. 2004. Temporal patterns of fruit fly (Drosophila) evolution revealed by mutation clocks. Mol. Biol. Evol. 21, 36–44. ( 10.1093/molbev/msg236) [DOI] [PubMed] [Google Scholar]
- 28.De Oliveira CC, Manfrin MH, Sene FM, Jackson LL, Etges WJ. 2011. Variations on a theme: diversification of cuticular hydrocarbons in a clade of cactophilic Drosophila. BMC Evol. Biol. 11, 1–19. ( 10.1186/1471-2148-11-179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashburner M. 1989. Drosophila: a laboratory handbook. New York, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 30.Henzell RF, Lowe MD. 1970. Sex attractant of the grass grub beetle. Science 168, 1005–1006. ( 10.1126/science.168.3934.1005) [DOI] [PubMed] [Google Scholar]
- 31.Ruther J, Reinecke A, Tolasch T, Hilker M. 2002. Phenol: another cockchafer attractant shared by Melolontha hippocastani Fabr. and M. melolontha L. Z. Naturforsch. C 57, 910–913. [DOI] [PubMed] [Google Scholar]
- 32.Shamsi SP, Khan NH, Rizvi SSA. 1990. Repellency effects of phenol, phenyl phenol, biphenyl and acetone on Chrysomyia rufifacies (Macq.) and Lucilia cuprina (Wied.). Indian J. Entomol. 52, 669–674. [Google Scholar]
- 33.Du YJ, Millar JG. 1999. Electroantennogram and oviposition bioassay responses of Culex quinquefasciatus and Culex tarsalis (Diptera: Culicidae) to chemicals in odors from Bermuda grass infusions. J. Med. Entomol. 36, 158–166. [DOI] [PubMed] [Google Scholar]
- 34.Tolasch T, Solter S, Tóth M, Ruther J, Francke W. 2003. (R)-Acetoin-female sex pheromone of the summer chafer Amphimallon solstitiale (L.). J. Chem. Ecol. 29, 1045–1050. ( 10.1023/A:1022992516854) [DOI] [PubMed] [Google Scholar]
- 35.Tegoni M, Campanacci V, Cambillau C. 2004. Structural aspects of sexual attraction and chemical communication in insects. Trends Biochem. Sci. 29, 257–264. ( 10.1016/j.tibs.2004.03.003) [DOI] [PubMed] [Google Scholar]
- 36.Farine JP, Sirugue D, Abed-Vieillard D, Everaerts C, Le Quéré JL, Bonnard O, Brossut R. 2007. The male abdominal glands of Leucophaea maderae: chemical identification of the volatile secretion and sex pheromone function. J. Chem. Ecol. 33, 405–415. ( 10.1007/s10886-006-9224-7) [DOI] [PubMed] [Google Scholar]
- 37.Cha DH, Adams T, Rogg H, Landoldt PJ. 2012. Identification and field evaluation of fermentation volatiles from wine and vinegar that mediate attraction of spotted wing Drosophila, Drosophila suzukii. J. Chem. Ecol. 38, 1419–1431. (doi:101603/EN12233) [DOI] [PubMed] [Google Scholar]
- 38.Everaerts C, Lacaille F, Ferveur JF. 2010. Is mate choice in Drosophila males guided by olfactory or gustatory pheromones? Anim. Behav. 79, 1135–1146. ( 10.1016/j.anbehav.2010.02.013) [DOI] [Google Scholar]
- 39.Hoare DJ, Humble J, Jin D, Gilding N, Petersen R, Cobb M, McCrohan C. 2011. Modeling peripheral olfactory coding in Drosophila larvae. PLoS ONE 6, e22996 ( 10.1371/journal.pone.0022996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khurana S, Siddiqi O. 2013. Olfactory responses of Drosophila larvae. Chem. Senses 38, 315–323. ( 10.1093/chemse/bjs144) [DOI] [PubMed] [Google Scholar]
- 41.Schaner AM, Jackson LL. 1992. (Z)-10-heptadecen-2-one and other 2-ketones in the aggregation pheromone blend of Drosophila martensis, D. buzzatii and D. serido. J. Chem. Ecol. 18, 53–64. ( 10.1007/BF00997164) [DOI] [PubMed] [Google Scholar]
- 42.Schaner AM, Bartelt RJ, Jackson LL. 1987. (Z)-11-octadecenyl acetate, an aggregation pheromone of Drosophila simulans. J. Chem. Ecol. 13, 1777–1786. ( 10.1007/BF00980218) [DOI] [PubMed] [Google Scholar]
- 43.Wertheim B, Allemand R, Vet LEM, Dicke M. 2006. Effects of aggregation pheromone on individual behaviour and food web interactions: a field study on Drosophila. Ecol. Entomol. 31, 216–226. ( 10.1111/j.1365-2311.2006.00757.x) [DOI] [Google Scholar]
- 44.Shorrocks B, Rosewell J, Edwards K, Atkinson W. 1984. Interspecific competition is not a major organizing force in many insect communities. Nature 310, 310–312. ( 10.1038/310310a0) [DOI] [Google Scholar]
- 45.Begon M, Townsend CR, Harper JM. 2009. Ecology: from individuals to ecosystems. Oxford, UK: Blackwell Publishing. [Google Scholar]
- 46.Kaplan F, et al. 2013. Interspecific nematode signals regulate dispersal behavior. PLoS ONE 7, e38735 ( 10.1371/journal.pone.0038735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durisko Z, Dukas R. 2013. Attraction to and learning from social cues in fruitfly larvae. Proc. R. Soc. B 280, 1767–1773. ( 10.1098/rspb.2013.1398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barron AB, Corbet SA. 1999. Preimaginal conditioning in Drosophila revisited. Anim. Behav. 58, 621–628. ( 10.1006/anbe.1999.1169) [DOI] [PubMed] [Google Scholar]
- 49.Gandolfi M, Mattiacci L, Dorn S. 2003. Preimaginal learning determines adult response to chemical stimuli in parasitic wasp. Proc. R. Soc. Lond. B 270, 2623–2629. ( 10.1098/rspb.2003.2541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hay DA. 1972. Recognition by Drosophila melanogaster of individuals from other strains of cultures: support for the role of olfactory cues in selective mating? Evolution 26, 171–176. ( 10.2307/2407028) [DOI] [PubMed] [Google Scholar]
- 51.Eoff M. 1973. The influence of being cultured together in hybridization between Drosophila melanogaster and Drosophila simulans. Am. Nat. 107, 247–255. ( 10.1086/282828) [DOI] [Google Scholar]
- 52.Hedlund K, Vet LEM, Dicke M. 1996. Generalist and specialist parasitoid strategies of using odours of adult drosophilid flies when searching for larval hosts. Oikos 77, 390–398. ( 10.2307/3545929) [DOI] [Google Scholar]
- 53.Matzkin LM. 2005. Activity variation in alcohol dehydrogenase paralogs is associated with adaptation to cactus host use in cactophilic Drosophila. Mol. Ecol. 14, 2223–2231. ( 10.1111/j.1365-294X.2005.02532.x) [DOI] [PubMed] [Google Scholar]
- 54.Watts T, Haselkorn TS, Moran NA, Markow TA. 2011. Variable incidence of Spiroplasma infections in natural populations of Drosophila species. PLoS ONE 4, e5703 ( 10.1371/journal.pone.0005703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E. 2010. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc. Natl Acad. Sci. USA 107, 20 051–20 056. ( 10.1073/pnas.1009906107) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Electronic supplementary material is available at http://dx.doi.org/10.1098/rspb.2014.0043.