Abstract
Individual recognition can be facilitated by creating representations of familiar individuals, whereby information from signals in multiple sensory modalities become linked. Many vertebrate species use auditory–visual matching to recognize familiar conspecifics and heterospecifics, but we currently do not know whether representations of familiar individuals incorporate information from other modalities. Ring-tailed lemurs (Lemur catta) are highly visual, but also communicate via scents and vocalizations. To investigate the role of olfactory signals in multisensory recognition, we tested whether lemurs can recognize familiar individuals through matching scents and vocalizations. We presented lemurs with female scents that were paired with the contact call either of the female whose scent was presented or of another familiar female from the same social group. When the scent and the vocalization came from the same individual versus from different individuals, females showed greater interest in the scents, and males showed greater interest in both the scents and the vocalizations, suggesting that lemurs can recognize familiar females via olfactory–auditory matching. Because identity signals in lemur scents and vocalizations are produced by different effectors and often encountered at different times (uncoupled in space and time), this matching suggests lemurs form multisensory representations through a newly recognized sensory integration underlying individual recognition.
Keywords: cross-modal, multimodal, individual identity, individual recognition, sex differences, Lemur catta
1. Introduction
Signals that incorporate multiple sensory modalities enhance cognitive processes, including learning and decision-making [1]. Despite growing interest in multisensory communication, our understanding of how animals use more than one sensory modality to recognize others is in its infancy. Multisensory individual recognition requires animals to learn and match identity information contained in more than one modality. For example, horses (Equus caballus) [2], crows (Corvus macrorhynchos) [3] and rhesus monkeys (Macaca mulatta) [4,5] can recognize familiar conspecifics by matching the identity information in vocal signals to the identity information in visual signals. Horses [6,7] and rhesus monkeys [5], as well as dogs (Canis familiaris) [8] can also recognize familiar humans through auditory–visual matching. Many species rely on olfactory signals for communication (recently reviewed in [9]) and recognition [10,11], and some species including hamsters require physical contact (and possibly visual information) to integrate multiple scents [12]. However, the role of scents in multisensory individual recognition is not completely understood.
Animals may form cognitive representations of familiar individuals by associating different types of information, including identity information from multiple sensory modalities, which allows them to form multisensory representations [2,5,13]. Identity information from separate modalities can be learned simultaneously, or learned at different times and independently linked to the overall representation of an individual. In auditory–visual recognition, for example, both strategies are likely to influence learning. Vocal and visual signatures can be learned at different times during repeated interactions, but opportunities also exist for their simultaneous learning because visible facial movements are produced during vocalizations, temporally and spatially coupling identifying signals across visual and auditory modalities. While there is ample evidence of face–voice matching in a number of species [14–16], there is a paucity of evidence of multisensory recognition via cues produced by different effectors or encountered at different times, such as scents and vocalizations. Scents can persist in the environment for long periods, potentially providing long-lasting information about a signaller's identity, condition or reproductive status [17]. By contrast, vocalizations provide current but transient information about the signaller. As a result, using information from both signals would be advantageous for keeping track of the current status of conspecifics. However, we currently do not have any empirical evidence that identity signals contained in scents could be matched to those contained in vocalizations.
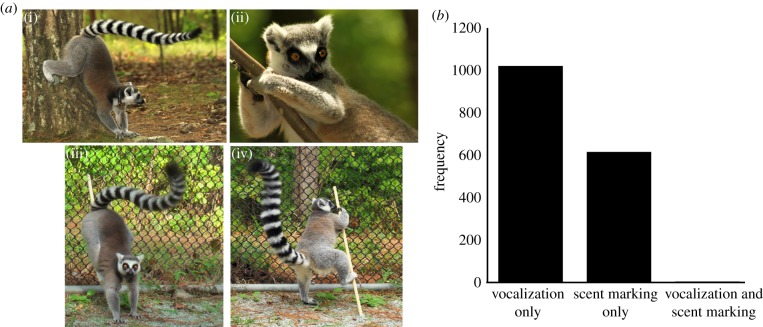
Here, we investigate whether the ring-tailed lemur, a highly social species [18], which uses both vocalizations and scents for communication [19,20], is able to match the identity information contained in these two signals. Both male and female lemurs scent mark to deposit odours from their genital glands; males also wrist mark to deposit antebrachial gland scents that are sometimes mixed with brachial gland secretions (figure 1a) [21]. Scents play a major role in advertising reproductive status [22], intrasexual competition, [22] intragroup communication [21,23] and territoriality [24], and carry identifying signatures used for individual and kin recognition [22,25–28]. Lemurs also produce a rich array of vocalizations [18,29]. Their contact calls (cohesion calls [30]) have multiple functions: they are frequently given as antiphonal calls in response to others, and serve as long-range communication signals to locate group members during movement [31]. Similar to scents, contact calls contain individual signatures [30,32]. Together, scents and vocalizations facilitate group cohesion, coordination and movement [23]. However, scent marking and contact calling are uncoupled in time; these two behaviours occur together very rarely (figure 1b).
Figure 1.
Ring-tailed lemur scent marking behaviour. (a) Genital marking (i) and wrist marking (ii) in natural habitat enclosures. Genital marking (iii) and wrist marking (iv) during experiments. (b) Over 12 months of daily observations in five groups (total of approx. 70 individuals, across two lemur populations), 1019 contact calls and 614 scent markings were observed. Lemurs marked and vocalized simultaneously in only three instances. (Online version in colour.)
To determine whether lemurs can recognize familiar individuals through olfactory–auditory matching, we presented them with scents and vocalizations of females from their social group. When lemurs are isolated from group members during foraging or group movement, they frequently hear others' calls from a distance and move towards the source. Along the way, they may encounter the calling individual or that individual's scent mark. We mimicked this situation by temporarily separating individuals from their group and presenting them with the playback of a contact call immediately before they encountered a scent mark. In counter-balanced trials, we coupled the scent of a familiar female either with her vocalization (matched condition) or with the vocalization of another familiar female (mismatched condition). In control trials, we presented the scent by itself (no-vocalization condition). If lemurs are able to match the identifying information in scents and vocalizations, then they should respond to the matched condition differently than they do to the mismatched condition, providing evidence that they form multisensory representations which link together identity signals in auditory and olfactory modalities.
2. Material and methods
(a). Sample collection
We worked with two lemur groups in ‘natural habitat enclosures’ (NHE) at the Duke Lemur Center (Duke University, NC, USA; NHE2: four males, four females; NHE4: three males, four females; electronic supplementary material, table S1). These groups semi free-range in forested enclosures up to 7 hectares in size, separated by chain-link fencing, and have visual, acoustic and olfactory contact. Lemurs also have frequent access to several indoor and outdoor pens. We used one of these outdoor pens (3.05 m (l) × 2.4 m (h) × 2.4 m (w)) as the testing arena.
Because both sexes respond strongly to female scents [21,22], we used only female scents and vocalizations. To collect scents, we gently restrained females and rubbed a cotton swab (pre-washed with methanol and pentane) against their labial folds [33]. In each group, each week between June and August 2010, we collected four swabs per individual from the dominant female and two adult subordinate females. The samples were stored in precleaned gas chromatography vials in a −80°C freezer until they were used during testing in July–August 2011. Between June and August 2010, we also collected vocalizations by recording the spontaneously produced contact calls using an HD camcorder (Canon Vixia HF-100, equipped with external directional microphone Sennheiser ME66; 40 Hz–20 kHz, ±2.5 dB). Calls were normalized to 100% of peak amplitude in Adobe Audition CS4 (Adobe Systems Incorporated, San Jose, CA, USA, v. 4.0). All lemurs were over 1 year old at the time of sample collection and testing. Based on social network data collected by one of us (I.G.K.), with the exception of two changes in one group (the oldest female died, her daughter was removed from the group because of intragroup aggression), the group structure between 2010 and 2011 remained similar.
(b). Testing
To test multisensory recognition, a signal in one modality (i.e. a vocalization) can be presented in conjunction with multiple signals from the second modality (i.e. pictures or scents) within the same trial to determine which signal will elicit a response [5,7]. Alternatively, only one signal from each modality may be used in each trial to compare an individual's responses across multiple trials [2,3,8]. We used the latter approach and presented lemurs with one vocalization and one scent in a given trial. We chose this option to avoid exposing the test lemurs to multiple scents at a given time, and to avoid habituation, which was especially important because we ran our experiments outside of the breeding season which happens during late autumn in the Northern Hemisphere. Although ring-tailed lemurs are most interested in conspecific scents during the breeding season, they are attentive to scents year round [22]. We selected one time of the year because the chemical composition of females' scents show seasonal variation [33] and the non-breeding season, specifically, because captive female lemurs often receive hormonal contraception during the breeding season, which alters their scents and olfactory signatures [34].
On testing days, we moved the whole group to an indoor pen far from the testing arena to minimize olfactory and vocal interference. We presented the scents on fresh wooden dowels; in each trial, we used two dowels (2.5 cm diameter, 60 cm length, separated by 60 cm, tied to pen fencing directly opposite from the entrance door to minimize side biases) and applied the scent to one (stimulus) dowel while using the other as a control to measure baseline responses. Most lemurs were familiar with dowels (electronic supplementary material, table S1) [28]. We hid a mobile speaker (Anchor Audio AN-30 Portable 30 W Speaker, 100 Hz–15 kHz, ±3 dB) in the vegetation outside the pen and centred it between the dowels. Vocalizations were adjusted to 70 decibels at a 5 m distance from the speaker, approximately the distance we expected the lemurs to be from the speaker during playbacks. We played the vocalization within 10 s of lemurs' entry into the arena before they contacted the dowels, and videotaped responses for 10 min, which allowed us to obtain data from individuals slower to approach the dowels.
Each lemur was tested with scents from two females belonging to their social group; one from the dominant female of the group and another from a subordinate adult female from the same group (electronic supplementary material, table S1). The dominant females were tested with scents from two subordinate females. Each lemur encountered each scent in three conditions (resulting in six trials per lemur): (i) scent and vocalization from the same female (matched); (ii) scent from one female and vocalization from another (mismatched); and (iii) scent-only (no-vocalization). In a given day, only one group was tested and each lemur received only one condition. Each group had at least 4 days between different trials, which were randomized with respect to the relative locations of the dowels and the order of matched and mismatched conditions.
(c). Data analysis
We measured interest in scents and vocalizations through frame-by-frame scoring of videos (30 frames s–1), conducted by an individual blind to experimental conditions. Individual recognition can be tested by analysing responses to signals that carry identifying signatures [13]. Response measures used in our study included duration of dowel sniffing (nose being within 20 cm of the dowel), duration and frequency of dowel marking (genital marking by females, genital or wrist marking by males) and duration of looking towards the speaker. Because of response differences between the sexes, we used different dependent measures for each sex. For example, males can continue to sniff the dowels while marking with their wrists (electronic supplementary material, video S1; figure 1a), making it difficult to determine whether they are sniffing the female scent or their own marks. Conversely, because not all females who sniffed the dowels marked them, our sample size for female marking was small (three females marked in four trials for matched, five trials for mismatched, four trials for no-vocalization). Therefore, we focused on duration and frequency of marking in males, and duration of sniffing in females. We could easily separate sniffing and marking in females, as females only perform genital marking, which requires turning away from the scent and performing a hand stand (figure 1a).
Lemurs visited both dowels multiple times during a trial. We ran all analyses on data normalized by calculating the percentage of time that each lemur spent investigating the stimulus dowel relative to the control dowel. We analysed sex differences with ANOVA. We and completed all other analyses with Wilcoxon tests on matched pairs, which allowed us to compare each individual's response across different experimental conditions. We also report effect sizes (Cohen's d) for all significant paired comparisons. Because each lemur encountered only one experimental condition in a given trial, normalizing for responses across dowels allowed us to account for individual variation, which included daily differences in motivation and interest in the experiment, regardless of which scent or condition was presented.
3. Results
Consistent with previous studies [21,22], males and females differed in their responses towards scents (figure 1a; electronic supplementary material, videos S1, S2). Out of the seven males and eight females we tested, all males contacted the dowels, but only six marked, whereas six out of eight females contacted the dowels, but only three marked. Two females (aged 4 and 6) did not contact the dowels, staying instead on the branches, suggesting that they may not have been fully habituated to being separated from their group. We thus excluded their trials from the analyses. In comparison with females, across all experimental conditions, males contacted the dowels faster after entering the experimental arena (ANOVA; F1,76 = 9.50, p = 0.003; mean ± s.d. = males: 30.14 ± 39.6 s; females: 71.72 ± 75.9 s), marked the dowels more frequently (F1,48 = 14.551, p < 0.001; males: 12.25 ± 10.4 marks; females: 1.15 ± 0.5 marks) and for longer periods (F1,48 = 7.32, p = 0.009; males: 22.01 ± 3.3 s; females: 4.63 ± 5.5 s). The latency of first dowel contact was not influenced by the experimental condition in either sex (males: F2,41 = 0.021; p = 0.979; females: F2,34 = 0.032, p = 0.967).
Both sexes attended to the stimulus dowel more in the matched condition than in the mismatched condition. Males spent more time marking the stimulus dowel after hearing a matching vocalization than a mismatching vocalization (S = −22, p = 0.053, n = 12 total trials; two scents for each of the six marking males, Cohen's d = 0.583; 76.54 ± 22.6% for matched and 63.10 ± 23.5% for mismatched; figure 2a). Males also marked the stimulus dowel more frequently in the matched trials (S = −21.5, p = 0.054, n = 12, Cohen's d = 0.514; 75.08 ± 22.3% for matched and 64.29 ± 19.5% for mismatched; figure 2b). There was no statistical difference in each individual's responses across the matched and the no-vocalization conditions (S = −13.5, p = 0.129). However, an analysis of the means (electronic supplementary material, table S2) revealed that, in general, males marked the stimulus dowel more frequently (F1,20 = 5.432, p = 0.031; 75.08 + 22.3 for matched and 52.48 + 21.5 for no-vocalization) and for longer periods (F1,20 = 7.811, p = 0.012; 76.53 + 22.6 for matched and 49.3 + 21.4 for no-vocalization) in the matched condition than in the no-vocalization condition. None of the males' responses were influenced by which group member the vocalization (marking duration: F7,23 = 1.209, p = 0.35; marking frequency: F7,23 = 1.15, p = 0.38), or the scent came from (marking duration: F4,32 = 0.758, p = 0.561; marking frequency: F4,32 = 1.41, p = 0.256).
Figure 2.
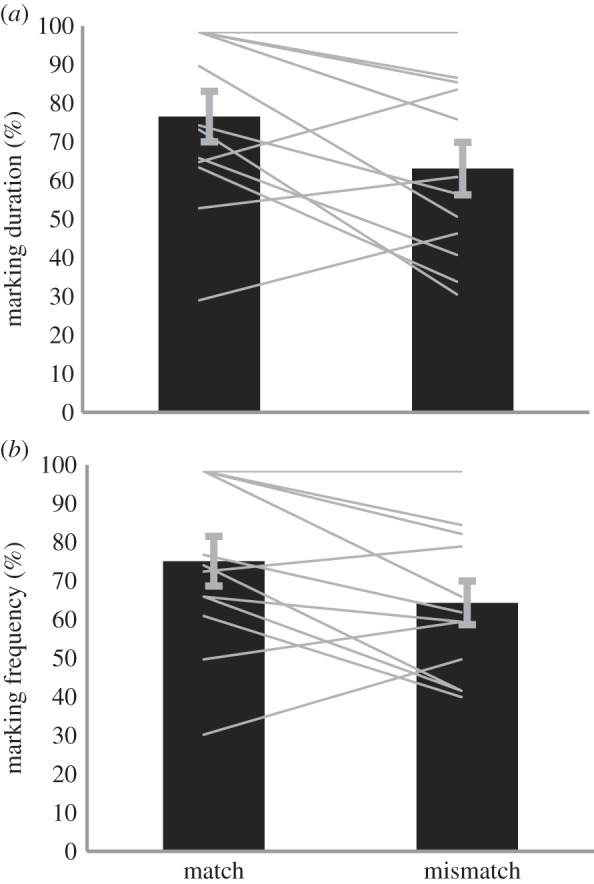
Male attention to scents: relative (a) duration and (b) frequency of marking. We set the total time spent around dowels at 100% and report the relative time spent for the stimulus dowel. Bars show mean ± s.e.m., lines show individuals' responses. Males marked the dowels for longer periods and more frequently when the vocalization matched instead of mismatched the scent on the stimulus dowel (electronic supplementary material, video S1).
Females spent more time sniffing the stimulus dowel after hearing the matching vocalization than the mismatching vocalization (S = −26, p = 0.019, n = 12 total trials; two scents for each six females, Cohen's d = 1.096; 78.62 ± 11.9% for matched and 54.07 ± 29.0% for mismatched; figure 3). Duration of sniffing the stimulus dowel was not statistically different between the matched and the no-vocalization conditions (S = −3, p = 0.69) and was not influenced by which lemur the scent came from (F5,29 = 2.34, p = 0.06). However, the identity of the vocalizing females did affect duration of sniffing the stimulus dowel (F7,22 = 4.17, p = 0.01). Further analysis revealed that reduced responses after hearing the playback of the youngest female drove this finding.
Figure 3.
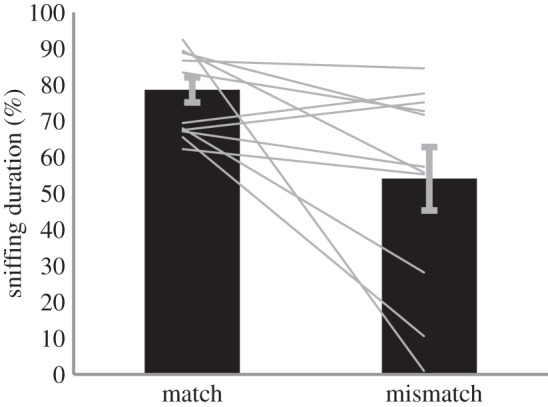
Female attention to scents: relative duration of sniffing dowels. We set the total time spent around dowels at 100% and report the relative time spent for the stimulus dowel. Bars show mean ± s.e.m., lines show individuals' responses. Females spent significantly more time attending to the stimulus dowel in the matched condition than in the mismatched condition (electronic supplementary material, video S2).
When analysing attention to the vocalizations, we only used trials in which the lemurs looked towards the speaker in both the matched and the mismatched conditions. We measured attention to vocalizations by the duration of the lemurs' head-turn towards the direction of the speaker within 10 s of playback, but only before they contacted the dowels. Males spent significantly more time looking towards the speaker in the matched condition (S = −33.5, p = 0.017, n = 12 total trials; Cohen's d = 1.135, 2.41 ± 1.4 s for matched and 1.01 ± 1.1 s for mismatched; figure 4). Female attention to vocalizations, however, did not differ based on the experimental condition (S = −2.00, p = 0.909, n = 13 trials, match: 3.98 ± 3.9 s, mismatch: 3.16 ± 2.3 s; figure 4).
Figure 4.
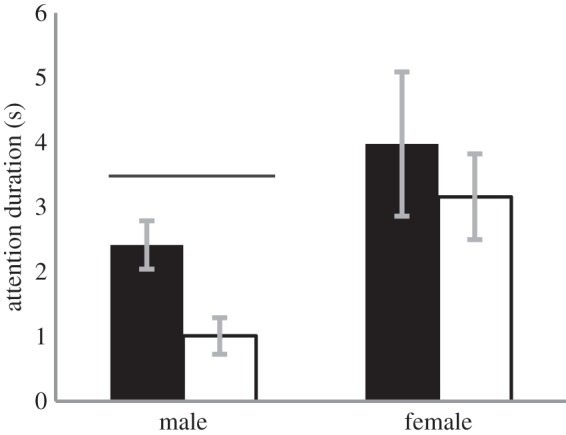
Attention to vocalizations: duration of looking towards the speaker. Males, but not females, looked towards the speaker for significantly longer periods when the vocalization matched the scent. Black bars denote match, white bars, mismatch.
4. Discussion
We provide evidence of individual recognition through olfactory–auditory matching in lemurs. Lemurs attended to the sensory signals for longer periods when the scent and the vocalization came from the same individual, instead of from different individuals, suggesting that they were able to match the identity information contained in scents and vocalizations of familiar females. Males marked the scents more frequently and for longer periods, and females sniffed the scents for longer periods, when both the scent and the vocalization came from the same female. Males also attended to the vocalization of a female for longer periods if her scent instead of another female's scent was present.
Lemurs were more attentive to the scents during the matched trials in which both the olfactory and the auditory information came from the same individual. When a scent mark, which can persist in the environment for long periods, is paired with a vocalization, which is a transient cue indicating that the signaller is in vicinity, the vocalization may provide a context and a time-stamp to the scent mark. In the matched trials, both the scents and the vocalizations provide congruent identity information, which may enhance the message that the scent owner is nearby. As scents play a major role in mate attraction, intrasexual competition and kin recognition [25,35], a female's scent may become more salient and elicit greater attention when it is presented with the congruent sound of her vocalization. Humans also find scents more pleasant when they are presented with congruent sounds (e.g. food odours with the sound of eating; drink odours with the sound of drinking) than when they are presented with incongruent sounds [36]. Increased attention to congruent stimuli is also seen across auditory–visual recognition; horses [7] and rhesus monkeys [5] attend to identity stimuli for longer periods when both visual and auditory cues come from the same individual instead of from different individuals.
To achieve multisensory recognition, animals need to learn identifying information from multiple modalities and access information about one modality when the other is encountered. This would only be possible when information from different modalities is associated with each other, most probably as a result of forming an overall representation of familiar individuals [2,5]. However, we know surprisingly little about the cognitive mechanisms through which identifying signatures from multiple sensory modalities are learned and combined. Forming overall representations of others may allow animals to associate information that is learned simultaneously or at different times. For example, auditory and visual signals are frequently coupled when an individual vocalizes. Similarly, olfactory and visual signals are temporally coupled in several species, including lemurs, when animals produce specific body postures while scent marking [18,21,26,37–41]. Such temporal and direct links between multisensory signals may provide opportunities for the identifying information to be simultaneously learned (during one or more encounters between individuals) in addition to being learned at different times (through repeated encounters). However, the probability of simultaneous learning decreases when the signals of interest are rarely encountered at the same time; this may be the case with lemur scents and sounds.
Lemurs can learn others' scents either by observing a scent marking individual before investigating the mark, and/or by detecting an individual's scent directly on that individual. Vocalizations and scents would show temporal contingency, and thus be learned simultaneously, either if lemurs produce contact calls while scent marking, or if conspecifics detecting a lemur's scent (either through investigation of a scent mark or through direct detection on that individual) hear only the vocalization of the lemur whose scent they detect. However, scent marking does not typically occur concurrently with vocalizations in lemurs. In fact, there is a negative correlation between frequency of scent marking and call production in lemurs [23]. In our own observations, lemurs produced contact calls while marking only three times (figure 1b). Moreover, the contact calls we used frequently elicit (often simultaneous) antiphonal calls from multiple individuals [30], making it unlikely that lemurs hear only the vocalization of the conspecific whose scent they detect when they hear a vocalization. Such temporal dissociation between encountering an individual's scent and contact call suggests that the opportunities for learning to associate the two cannot be easily mediated by temporal contingency. Future empirical studies are needed to tease apart how lemurs learn to recognize familiar conspecifics. One possibility is that identity information in a lemur's scent and vocalization are learned at different times, and as a result, independently linked to the overall representation of that individual, creating a multisensory representation that makes olfactory–auditory matching possible in lemurs.
Remembering individuals' identities plays a critical role in the group dynamics of species living in complex social groups. Identity information from multiple modalities may be stored in memory together with information on age, sex, kinship, and possibly a history of social interactions [5,13], forming representations which bring together different types of information. Evidence which suggests that these representations also include multisensory information that aids in the recognition of others has so far been shown only in tests based on matching of visual and acoustic cues [2–8]. Our study, which demonstrates individual recognition through olfactory–auditory matching, provides further support for the presence of multisensory representations of familiar individuals.
Acknowledgements
We thank Duke Lemur Center, especially Dr Sarah Zehr and David Brewer. This is DLC publication no. 1264.
All sampling and testing procedures were approved by Duke University Institutional Animal Care and Use Committee (A121-10-05).
Data accessibility
The dataset supporting this article has been uploaded to Dryad (doi:10.5061/dryad.842nm).
Funding statement
This research was supported by Princeton University EEB Research Grant to I.G.K., National Science Foundation BCS-0547760 CAREER Award to A.A.G., National Science Foundation grant nos. IOS-0719003 and IOS-1021633 to C.M.D. and National Science Foundation grant nos. IBN-9874523, CNS-025214 and IOB-9874523 to D.I.R.
References
- 1.Rowe C. 1999. Receiver psychology and the evolution of multicomponent signals. Anim. Behav. 58, 921–931. ( 10.1006/anbe.1999.1242) [DOI] [PubMed] [Google Scholar]
- 2.Proops L, McComb K, Reby D. 2009. Cross-modal individual recognition in domestic horses (Equus caballus). Proc. Natl Acad. Sci. USA 106, 947–951. ( 10.1073/pnas.0809127105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondo N, Izawa E, Watanabe S. 2012. Crows cross-modally recognize group members but not non-group members. Proc. R. Soc. B 279, 1937–1942. ( 10.1098/rspb.2011.2419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adachi I, Hampton RR. 2011. Rhesus monkeys see who they hear: spontaneous cross-modal memory for familiar conspecifics. PLoS ONE 6, e23345 ( 10.1371/journal.pone.0023345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sliwa J, Duhamel JR, Pascalis O, Wirth S. 2011. Spontaneous voice-face identity matching by rhesus monkeys for familiar conspecifics and humans. Proc. Natl Acad. Sci. USA 108, 1735–1740. ( 10.1073/pnas.1008169108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lampe JF, Andre J. 2012. Cross-modal recognition of human individuals in domestic horses (Equus caballus). Anim. Cogn. 15, 623–630. ( 10.1007/s10071-012-0490-1) [DOI] [PubMed] [Google Scholar]
- 7.Proops L, McComb K. 2012. Cross-modal individual recognition in domestic horses (Equus caballus) extends to familiar humans. Proc. R. Soc. B 279, 3131–3138. ( 10.1098/rspb.2012.0626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adachi I, Kuwahata H, Fujita K. 2007. Dogs recall their owner's face upon hearing the owner's voice. Anim. Cogn. 10, 17–21. ( 10.1007/s10071-006-0025-8) [DOI] [PubMed] [Google Scholar]
- 9.Uy JAC, Safran RJ. 2013. Variation in the temporal and spatial use of signals and its implications for multimodal communication. Behav. Ecol. Sociobiol. 67, 1499–1511. ( 10.1007/s00265-013-1492-y) [DOI] [Google Scholar]
- 10.Bonadonna F, Mardon J. 2013. Besides colours and songs, odour is the new black of avian communication. In Chemical signals in vertebrates 12 (eds East ML, Dehnhard M.), pp. 325–339. Berlin, Germany: Springer. [Google Scholar]
- 11.Johnston RE. 2008. Individual odors and social communication: individual recognition, kin recognition, and scent over-marking. In Advances in the study of behavior (eds Brockmann J, Roper TJ, Naguib M, Wynne-Edwards KE, Barnard C, Mitani JC.), pp. 439–505. New York, NY: Academic Press. [Google Scholar]
- 12.Johnston RE, Peng A. 2008. Memory for individuals: hamsters (Mesocricetus auratus) require contact to develop multicomponent representations (concepts) of others. J. Comp. Psychol. 122, 121–131. ( 10.1037/0735-7036.122.2.121) [DOI] [PubMed] [Google Scholar]
- 13.Tibbetts EA, Dale J. 2007. Individual recognition: it is good to be different. Trends Ecol. Evol. 22, 529–537. ( 10.1016/j.tree.2007.09.001) [DOI] [PubMed] [Google Scholar]
- 14.Ghazanfar AA. 2013. Multisensory vocal communication in primates and the evolution of rhythmic speech. Behav. Ecol. Sociobiol. 67, 1441–1448. ( 10.1007/s00265-013-1491-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulahci IG, Ghazanfar AA. 2013. Multisensory recognition in vertebrates (especially primates). In Integrating face and voice in person perception (eds Belin P, Campanella S, Ethofer T.), pp. 3–27. New York, NY: Springer. [Google Scholar]
- 16.Partan S, Marler P. 1999. Communication goes multimodal. Science 283, 1272–1273. ( 10.1126/science.283.5406.1272) [DOI] [PubMed] [Google Scholar]
- 17.Muller-Schwarze D. 2006. Chemical ecology of vertebrates. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 18.Jolly A. 1966. Lemur social behavior and primate intelligence. Science 153, 501–506. ( 10.1126/science.153.3735.501) [DOI] [PubMed] [Google Scholar]
- 19.Petter JJ, Charles-Dominique P. 1979. Vocal communication in prosimians. In The study of prosimian behavior (eds Doyle GA, Martin RD.), pp. 247–304. New York, NY: Academic Press. [Google Scholar]
- 20.Schilling A. 1979. Olfactory communication in prosimians. In The study of prosimian behavior (eds Doyle GA, Martin RD.), pp. 461–542. New York, NY: Academic Press. [Google Scholar]
- 21.Kappeler PM. 1998. To whom it may concern: the transmission and function of chemical signals in Lemur catta. Behav. Ecol. Sociobiol. 42, 411–421. ( 10.1007/s002650050455) [DOI] [Google Scholar]
- 22.Scordato ES, Drea CM. 2007. Scents and sensibility: information content of olfactory signals in the ringtailed lemur, Lemur catta. Anim. Behav. 73, 301–314. ( 10.1016/j.anbehav.2006.08.006) [DOI] [Google Scholar]
- 23.Oda R. 1999. Scent marking and contact call production in ring-tailed lemurs (Lemur catta). Folia Primatol. 70, 121–124. ( 10.1159/000021684) [DOI] [PubMed] [Google Scholar]
- 24.Ramsay N, Giller P. 1996. Scent-marking in ring-tailed lemurs: responses to the introduction of ‘foreign’ scent in the home range. Primates 37, 13–23. ( 10.1007/BF02382916) [DOI] [Google Scholar]
- 25.Boulet M, Charpentier MJ, Drea CM. 2009. Decoding an olfactory mechanism of kin recognition and inbreeding avoidance in a primate. BMC Evol. Biol. 9, 281 ( 10.1186/1471-2148-9-281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mertl AS. 1975. Discrimination of individuals by scent in a primate. Behav. Biol. 14, 505–509. ( 10.1016/S0091-6773(75)90684-7) [DOI] [PubMed] [Google Scholar]
- 27.Palagi E, Dapporto L. 2006. Beyond odor discrimination: demonstrating individual recognition by scent in Lemur catta. Chem. Senses 31, 437–443. ( 10.1093/chemse/bjj048) [DOI] [PubMed] [Google Scholar]
- 28.Charpentier MJE, Crawford JC, Boulet M, Drea CM. 2010. Message ‘scent’: lemurs detect the genetic relatedness and quality of conspecifics via olfactory cues. Anim. Behav. 80, 101–108. ( 10.1016/j.anbehav.2010.04.005) [DOI] [Google Scholar]
- 29.Oda R. 1996. Effects of contextual and social variables on contact call production in free-ranging ringtailed lemurs (Lemur catta). Int. J. Primatol. 17, 191–205. ( 10.1007/BF02735447) [DOI] [Google Scholar]
- 30.Macedonia JM. 1986. Individuality in a contact call of the ringtailed lemur (Lemur catta). Am. J. Primatol. 11, 163–179. ( 10.1002/ajp.1350110208) [DOI] [PubMed] [Google Scholar]
- 31.Macedonia JM. 1993. The vocal repertoire of the ringtailed lemur (Lemur catta). Folia Primatol. 61, 186–217. ( 10.1159/000156749) [DOI] [PubMed] [Google Scholar]
- 32.Oda R. 2002. Individual distinctiveness of the contact calls of ring-tailed lemurs. Folia Primatol. 73, 132–136. ( 10.1159/000064785) [DOI] [PubMed] [Google Scholar]
- 33.Scordato ES, Dubay G, Drea CM. 2007. Chemical composition of scent marks in the ringtailed lemur (Lemur catta): glandular differences, seasonal variation, and individual signatures. Chem. Senses 32, 493–504. ( 10.1093/chemse/bjm018) [DOI] [PubMed] [Google Scholar]
- 34.Crawford JC, Boulet M, Drea CM. 2011. Smelling wrong: hormonal contraception in lemurs alters critical female odour cues. Proc. R. Soc. B 278, 122–130. ( 10.1098/rspb.2010.1203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boulet M, Crawford JC, Charpentier MJ, Drea CM. 2010. Honest olfactory ornamentation in a female-dominant primate. J. Evol. Biol. 23, 1558–1563. ( 10.1111/j.1420-9101.2010.02007.x) [DOI] [PubMed] [Google Scholar]
- 36.Seo HS, Hummel T. 2011. Auditory–olfactory integration: congruent or pleasant sounds amplify odor pleasantness. Chem. Senses 36, 301–309. ( 10.1093/chemse/bjq129) [DOI] [PubMed] [Google Scholar]
- 37.Drea CM, Scordato ES. 2008. Olfactory communication in the ringtailed lemur (Lemur catta): form and function of multimodal signals. Chem. Signals Ver. 11, 91–102. [Google Scholar]
- 38.Mertl AS. 1976. Olfactory and visual cues in social interactions of Lemur catta. Folia Primatol. 26, 151–161. ( 10.1159/000155736) [DOI] [PubMed] [Google Scholar]
- 39.Rothman RJ, Mech LD. 1979. Scent-marking in lone wolves and newly formed pairs. Anim. Behav. 27, 750–760. ( 10.1016/0003-3472(79)90010-1) [DOI] [Google Scholar]
- 40.Sharpe LL, Jooste MM, Cherry MI. 2012. Handstand scent marking in the dwarf mongoose (Helogale parvula). Ethology 118, 575–583. ( 10.1111/j.1439-0310.2012.02045.x) [DOI] [Google Scholar]
- 41.Wells MC, Bekoff M. 1981. An observational study of scent-marking in coyotes, Canis latrans. Anim. Behav. 29, 332–350. ( 10.1016/S0003-3472(81)80093-0) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting this article has been uploaded to Dryad (doi:10.5061/dryad.842nm).