Abstract
Sperm chemoattraction, where sperm locate unfertilized eggs by following a concentration gradient of egg-derived chemoattractants, has been widely documented across numerous taxa. While marine invertebrates are favoured models for understanding the underlying mechanisms of sperm chemoattraction, the evolutionary forces underpinning the process remain enigmatic. Here, we show that in mussels (Mytilus galloprovincialis), chemically moderated gamete preferences promote assortative fertilizations between genetically compatible gametes. When offered the choice of egg clutches from two females, sperm exhibited consistent but differential ‘preferences’ for chemical cues secreted from conspecific eggs. Critically, our data reveal that the preferences shown by sperm during the egg-choice trials are highly predictive of early embryonic viability when eggs and sperm from the same individuals are mixed during standard (no-choice) fertilization assays. Moreover, we demonstrate that by experimentally separating chemoattractants from eggs, sperm swimming behaviour is differentially regulated by egg-derived chemoattractants, and that these changes in sperm behaviour are highly consistent with observed patterns of gamete preferences, fertilization and larval survival. Together, this integrated series of experiments reveals that the behaviour of sperm is fine-tuned to respond differentially to the chemical signals emitted from different conspecific eggs, and that these choices have measurable fitness benefits.
Keywords: sperm chemotaxis, cryptic female choice, genetic compatibility, gamete choice, sperm attractants, sperm–egg interaction
1. Introduction
Since Lintner [1] highlighted a role for chemical attractants in promoting mating aggregations more than a century ago, our understanding of the role of chemical signalling in the context of reproduction has grown beyond mate attraction to increasingly complex communication systems [2]. For example, chemical signals can provide information about the location of prospective mates as well as their intrinsic quality in a manner analogous to auditory or visual displays [3–5]. Furthermore, they can be used to communicate information about genetic compatibility [6], allowing selection for mates whose particular genotype is matched to the choosing sex to produce adaptive gene combinations in offspring [7]. Nevertheless, despite increasing evidence for the ecological and evolutionary advantages attributable to selection for genetic compatibility [6,8], and the pervasiveness of chemical signalling across many animal and plant taxa [9], the use of chemosensory mechanisms to select for genetically compatibility mates has so far been identified in only a handful of studies [10–15].
While olfactory-based signalling allows intraspecific communication at the organism level, chemically mediated gamete interactions provide a potentially important form of intraspecific communication at the cellular level. Sperm chemotaxis, for example, involves egg-derived chemical attractants (egg chemoattractants) that guide sperm towards unfertilized eggs, thus causing a change in sperm swimming direction towards the source of chemoattractants (eggs) [16]. Sperm chemokinesis, on the other hand, describes a change in the steady-state speed of sperm cells, independent of direction, which is governed by the concentration of the chemical stimulus [16]. Both processes (hereafter ‘sperm chemoattraction’) can simultaneously contribute to the accumulation of sperm around unfertilized eggs [17,18].
Sperm chemoattraction has been identified in a wide range of organisms [19], including humans [16,20], but it is best understood in broadcast spawning species where the simultaneous release of gametes from numerous individuals into the external environment provides opportunities for gamete-level interactions. Although traditionally explained as a mechanism for maximizing gamete encounter rates and ensuring conspecific fertilizations (reviewed in [21]), recent evidence suggests that sperm chemoattraction may also function to select compatible gametes. In the mussel Mytilus galloprovincialis, sperm that are simultaneously exposed to egg chemoattractants coming from two different females display consistent but differential ‘preferences’ for eggs that confer high fertilization rates in subsequent (no-choice) crosses [22]. Although this finding is consistent with the intriguing possibility that egg chemoattractants play a role in the selection of genetically compatible mates, the extent to which differential gamete preferences influence offspring survival has never been investigated. This question is highly pertinent because biases in fertilization success do not always equate to biases in offspring survival and therefore gamete compatibility may not be predictive of offspring viability [23–25]. Thus, an outstanding question is whether sperm chemoattraction moderates the selection of genetically compatible sperm that result in indirect fitness benefits for females [21].
Here, we explore the potential for egg chemoattractants to facilitate sexual selection for compatible mates in the mussel M. galloprovincialis. To address this question, we derive estimates of gamete ‘preferences’ using a series of cross-classified dichotomous choice tests in which we measure the chemically moderated responses of sperm to the eggs of different females [22]. We then obtained gametes from the same individual males and females used in the choice assays and used them in subsequent no-choice in vitro fertilization assays to test for main and interacting male and female effects on fertilization rates and larval survival (the proportion of fertilized eggs surviving to the veliger (‘d’) stage’ of larval development). The patterns of gamete preferences taken from the choice trials were then related to the estimates of fertilization success and larval survival in the no-choice trials to determine whether gamete preferences predicted fertilization rates and embryo viability. A second component of our study focused on how sperm behaviour changes in response to egg chemoattractants from different females, using the same males and females used in the choice and no-choice trials described above. In this way, we were able to track changes in sperm behaviour according to the specific combination of sperm and eggs and relate these to variation in egg choice, fertilization rates and larval survival. Furthermore, by examining sperm behavioural responses, we were able to provide clues into the putative mechanisms (chemotaxis and/or chemokinesis) that underlie differential sperm attraction in this species. Our results provide, to our knowledge, the first evidence that sperm chemoattraction is associated with variation in offspring fitness, thus revealing a putative mechanism of sexual selection that facilitates ‘choice’ for genetically compatible mates. Our results also reveal interrelationships between patterns of sperm behaviour in response to egg chemoattractants, gamete preferences, fertilization success and larval fitness. These latter findings expose clear linkages between different phases of the sperm–egg interaction and subsequent offspring fitness.
2. Material and methods
(a). Study species and spawning procedures
Mytilus galloprovincialis is a benthic broadcast spawning mollusc with a breeding season spanning from June to September. Early larval development is punctuated by the morphologically distinct stages of motile trochophores and veligers (or ‘d-stage’ larvae) before larvae attach to strata as pediveligers (which mature into adult mussels). Mussels were collected from a marine population off Woodman Point Jetty, Western Australia (32°14′03.6″ S, 115°76′25″ E) between June and August 2012, and left to acclimatize for at least 2 days in recirculating seawater tanks (kept at approx. 17°C to match ambient seawater temperatures) before use. Note that this marine population differs from the (estuarine) population tested previously to reveal differential sperm chemoattraction in this species [22]. Spawning was induced by temperature shock by transferring mussels from their holding tanks into 60 × 37 × 37 cm plastic containers filled with approximately 6 cm of seawater preheated to 30°C [26]. Once individual mussels commenced spawning, their gender was identified (based on production of eggs or sperm) and they were immediately removed from the plastic containers, rinsed in filtered seawater (FSW) to prevent contamination and placed individually into a plastic cup containing approximately 10 ml of FSW where they continued to spawn [22]. Upon reaching suitably high densities, gametes were transferred into lightly aerated plastic tubes containing 20 ml of FSW. The concentrations of these stock solutions were estimated using an improved Neubauer haemocytometer (sperm) or under a dissecting microscope (eggs) using a known volume and diluted as required to obtain the desired gamete concentrations for each experiment (see below).
(b). Experimental overview
We explored the role that egg chemoattractants play in influencing gamete ‘choice’, fertilization rates, larval survival and sperm swimming behaviour. Our experiment builds on the experimental design used by Evans et al. [22] to explore how variation in gamete preferences (mediated by egg chemoattractants) influence fertilization rates, but extends this by exploring: (i) the fitness consequences of chemically mediated gamete preferences, and (ii) the action of isolated egg chemoattractants on sperm behaviour. We conducted four fully crossed factorial experiments [27] simultaneously, using gametes from the same individual males and females crossed in identical combinations but under differing experimental conditions that enabled us to explore the role of egg chemoattractants in mediating: (i) gamete preferences; (ii) fertilization rates; (iii) juvenile survival; and (iv) sperm swimming behaviour. Each of these experiments was run as individual blocks (overall n = 14), with each block comprising gametes from two males (MA and MB) and two females (FA and FB) crossed for all four combinations (MA × FA; MA × FB; MB × FA; MB × FB).
(c). Egg-choice trials
Using the fully crossed factorial design described above, we performed factorial dichotomous choice trials, where each replicate comprised gametes from two males and two females. In each of these replicates (hereafter ‘blocks’), sperm from each of the two males were given the simultaneous choice of eggs from the two females in a dichotomous choice chamber (i.e. one in which MA was given the choice of chemoattractant cues from FA and FB, and the second in which MB was given the choice between FA and FB). These chambers were constructed as described previously [22] to offer the bidirectional choice of chemoattractant cues. Each choice trial was replicated (n = 2), with the position of eggs alternated between trials within each replicate to account for possible side biases. Egg concentrations were adjusted to 1 × 106 eggs ml–1; 2 ml from each of the two females was then gently pipetted into their respective wells and left for 1 h to establish a chemoattractant concentration gradient. We then added 1 ml of sperm to the centre of each chamber and left the sample for a further 3 h to allow sperm to travel along either side of the flume. Sperm concentrations were kept as dense as possible (by extracting sperm directly from each male's exhalent siphon) to maximize the numbers recovered after the 3 h choice trial. Maximizing concentrations in this way was essential to ensure we could detect sperm in as many trials as possible, but also meant that sperm density was not standardized among males or blocks (however, sperm densities were standardized for individual males within each block, such that the choice trials for each male involved the same number of sperm). For this reason, the significant male effects detected in the dichotomous choice assays were not interpreted in this study, as they were almost certainly attributable to differences in sperm densities among males (see §2f). After 3 h, 100 µl of water was collected from each distal well from a point 15 mm above the eggs to ensure that sperm were sampled en route to the eggs and not at the site of fertilization; sperm counts on these retrieved samples were performed using an improved Neubauer haemocytometer. The differences in absolute sperm numbers between the two wells provided a measure of gamete ‘preference’ expressed by an individual male's sperm for eggs from the two females. In this way, each male acts as his own control with respect to sperm numbers, because we are interested in the differences in sperm numbers (which in turn is expected to be influenced by the interacting effects of egg chemoattractants and sperm).
(d). No-choice trials: fertilization rate and larval survival
We used the same crossing design (n = 14 blocks of 2 × 2 crosses) comprising the same individual males and females for the fertilization and larval survival trials. For each trial, fertilization assays were performed in a 5.5 cm diameter Petri dish. Gamete concentrations for both sexes were standardized within and among blocks. Egg concentrations were calculated through extrapolation of observed counts within a known volume and adjusted to 2 × 105 eggs ml−1. Sperm density was estimated using an improved Neubauer haemocytometer and adjusted to 2 × 106 sperm ml−1. These gamete concentrations were determined during preliminary tests to ensure ceiling (100% fertilization rate) or basement (0% fertilization rate) effects did not hamper the interpretation of results. Sperm and egg solutions (each 10 ml) were gently mixed within a Petri dish and left for 1 h under light aeration. Samples were then mixed thoroughly and partitioned for subsequent fertilization and larval survival estimates. For the fertilization assays, 5 ml of each sample was fixed in a 10% buffered formalin solution and stored until required for the counts. Fertilization rates were estimated by examining 100 haphazardly selected eggs under a Leica DM1000 microscope (×400) and determining the proportion of eggs undergoing cleavage and/or with a visible polar body [28]. Fertilization rates were highly variable (mean ± s.e. = 64.54% ± 1.62; range = 15–99%, n = 112).
For the larval survival assays, developing embryos were reared in 10 ml aerated plastic containers in a controlled temperature room (CTR) at 18°C for 48 h, allowing surviving fertilized eggs to develop into the veliger (d-cell) stage of development [28]. After the incubation period, the plastic tubes were removed from the CTR and a 100 µl sample was observed under ×100 magnification using a Leica DM1000 microscope. Larval survival was estimated from the number of live d-cell larvae counted within the sample. Larval counts were converted to a percentage of initially fertilized eggs by dividing the total number of surviving (d-cell) embryos in the sample by the total number of eggs used for fertilizations × proportion fertilized in the initial sample. In this way, offspring survival was adjusted for initial fertilization rate, but the initial density of developing embryos would have varied among samples (owing to variation in fertilization rates). Rearing density is expected to decrease larval survival at higher concentrations [29,30], and therefore our estimates of the correlation between larval survival and fertilization rates (predicted to be positive if fertilization is biased towards genetically compatible mates) are likely to be conservative. Larval survival (%) exhibited substantial variation (mean ± s.e. = 17.5% ± 1.5; range = 0.5–85.8%, n = 112).
(e). Characterizing sperm swimming behaviour
We measured sperm swimming characteristics using computer-assisted sperm analyses (CASA; Hamilton-Thorne CEROS). Each male's sperm was tested in the presence of chemoattractants from females with which they were paired during the egg choice and fertilization trials (i.e. for each of the 2 × 2 pairwise combinations tested across 14 blocks). For these trials, we initially removed eggs from the FSW in which they were incubated to test for main and interacting effects of males (sperm) and females (chemoattractants) without any potentially confounding effects of eggs themselves. We were then able to explore whether our observed patterns of gamete preferences, fertilization and larval survival covaried with chemically moderated changes in sperm swimming behaviour. To isolate chemoattractants from eggs, we first created a concentrated solution of eggs from the stock solution (1 × 106 eggs ml−1) for each female. These samples were aerated for 1 h to allow chemoattractants to diffuse into the FSW. This solution was then filtered using Whatman filter paper (11 µm retention size) to remove eggs and large particles from the water containing chemoattractants (hereafter referred to as ‘egg water’). Extreme care was taken to ensure that all eggs were intact and undamaged during this exercise. We then mixed 500 µl of sperm (adjusted to 1 × 106 sperm ml−1) with 500 µl of egg water in a 1.7 ml eppendorf tube and immediately extracted 2 µl of this sperm–egg water solution for CASA assays. Each sperm–egg water sample was tested in a 12-cell multi-test slide (MP Biomedicals) that had been pre-washed in 1% polyvinyl alcohol to prevent sperm sticking to the glass [31]. The CASA assays were always performed within 1 min of sperm and egg water mixing and each sample was tested twice. Following previous work on this species [26], we considered the following seven parameters: average path velocity (VAP), which measures the average velocity of sperm over a smoothed cell path; straight-line velocity (VSL), the average velocity on a straight line between the start- and endpoint of a sperm's path; curvilinear velocity (VCL), the actual velocity along the trajectory; straightness (STR), a ratio of straight-line velocity to curvilinear velocity; linearity (LIN), a ratio of smooth path velocity with curvilinear velocity; beat cross frequency (BCF), a measure of the flagella's beat rate; and the amplitude of lateral head displacement (ALH), which estimates the magnitude of displacement of the sperm head about its trajectory.
(f). Statistical analysis
We used principal components (PC) analysis to reduce the seven (highly correlated) sperm parameters to two PCs with eigenvalues more than 1. The first (PC1) was loaded positively by VAP, VSL, VCL, STR and LIN, while the second (PC2) was loaded positively by ALH and negatively by BCF (table 1). Thus, high PC1 values describe ejaculates with relatively high swimming velocity and low path curvature (i.e. faster and straighter swimming), while high values of PC2 correspond with high lateral head displacement and relatively low flagella beat rates.
Table 1.
Principal component analysis of sperm motility traits generated by computer-assisted sperm analysis in the mussel M. galloprovincialis.
| principal component |
||
|---|---|---|
| sperm trait | PC1 | PC2 |
| VAP: smooth path velocity | 0.87608 | 0.39298 |
| VSL: straight-line velocity | 0.98811 | 0.00151 |
| VCL: curvilinear path sperm | 0.71616 | 0.6076 |
| ALH: amplitude of lateral head displacement | −0.39848 | 0.77635 |
| BCF: beat cross frequency | −0.35469 | −0.72031 |
| STR: straightness (ratio of VSL/VCL) | 0.72094 | −0.55441 |
| LIN: linearity (ratio of VAP/VCL) | 0.92237 | −0.35489 |
| eigenvalue | 3.9119 | 2.0785 |
| per cent of cumulative variance explained | 55.884 | 85.577 |
In our initial analysis, we used a series of sequential two-way ANOVA models to estimate the main and interactive effects of males and females on patterns of egg choice, fertilization rates, larval survival and the two sperm parameters (PC1 and PC2). As we performed a series (n = 14) of factorial crosses, analyses were performed individually for each block before being combined into a final model for each parameter as prescribed for the block ‘North Carolina II’ design [27]. Note that we do not interpret the ‘male’ effect detected in the egg-choice assays (table 2) because sperm densities would have systematically varied among male subjects prior to their use in the egg-choice assays (see §2c). In this way, sperm counts for a given male would have been retrieved in higher or lower numbers from both distal wells in the choice trials purely as a consequence of there being variance among males in the initial concentration of sperm (thus generating variance explained by males and not the interaction between males and females which is the central focus of this study).
Table 2.
Sources of variation for the direction of sperm migration (egg-choice assays), fertilization rate, larval survival and sperm motility (principal components from PC analysis) in the mussel M. galloprovincialis. (For each model, degrees of freedom (d.f.) and sums of squares (SS) were calculated individually for each block and then summed to estimate the mean squares (MSs) for each analysis. Levels of significance for male and female effects were evaluated by dividing their particular MSs by the interaction MS [27]. Significant effects are presented in italic. The formula Nmales Nfemales (n − 1), where Nmales = 2, Nfemales = 2 and n (number of replicate crosses) = 2 was used to calculate the d.f. related to the error variance for each block. As with d.f. associated with main effects, error d.f. was summed across blocks for the final analysis [27].)
| source of variation | d.f. | SS | MS | F | p |
|---|---|---|---|---|---|
| egg-choice assays | |||||
| male | 14 | 25227.73 | 1801.98 | 4.408 | 0.004 |
| female | 14 | 4235.797 | 302.5569 | 0.740 | 0.709 |
| interaction | 14 | 5722.655 | 408.7611 | 2.261 | 0.016 |
| error | 56 | 10123.21 | 180.7716 | ||
| fertilization rate | |||||
| male | 14 | 2130.2 | 152.1571 | 0.451 | 0.926 |
| female | 14 | 7704.769 | 550.3406 | 1.632 | 0.185 |
| interaction | 14 | 4721.216 | 337.2297 | 3.908 | <0.001 |
| error | 56 | 4832.5 | 86.29464 | ||
| larval survival | |||||
| male | 14 | 0.65855 | 0.047039 | 1.245 | 0.3447 |
| female | 14 | 0.767912 | 0.054851 | 1.452 | 0.247 |
| interaction | 14 | 0.528974 | 0.037784 | 1.905 | 0.045 |
| error | 56 | 1.110489 | 0.01983 | ||
| sperm motility (PC1) | |||||
| male | 14 | 60.37727 | 4.312662 | 1.375 | 0.280 |
| female | 14 | 37.2293 | 2.659236 | 0.848 | 0.619 |
| interaction | 14 | 43.91184 | 3.13656 | 7.434 | <0.001 |
| error | 56 | 23.62814 | 0.421931 | ||
| sperm motility (PC2) | |||||
| male | 14 | 25.97916 | 1.855655 | 0.816 | 0.646 |
| female | 14 | 39.60821 | 2.829158 | 1.244 | 0.344 |
| interaction | 14 | 31.83701 | 2.274072 | 2.152 | 0.022 |
| error | 56 | 59.17593 | 1.056713 | ||
Next, we explored the interrelationships between gamete preferences (from choice assays), fertilization rates, larval survival and sperm swimming behaviour. In this way, we were able to determine whether patterns of male-by-female interaction underlying gamete preferences correspond with fertilization rates and larval survival when dyads were tested during no-choice assays. Put crudely, we ask whether sperm smell their way to eggs that offer higher fertilization and survival returns. We also determined whether any interacting (and interrelated) effects of males and females during the gamete choice trials, fertilization and larval survival assays correspond with sperm swimming behaviour when ejaculates from individual focal males were tested in the different egg water samples. Again, in simple terms, we ask whether sperm swim differently (e.g. faster and straighter) in the presence of ‘attractive’ chemoattractants coming from eggs that offer greater fertilization and survival returns. To examine these potential interrelationships, we first calculated the means for each parameter from the replicate (n = 2) crosses performed within each block. We then calculated the difference in mean values for a given male and the two females to which he was assigned as a measure of a given male's relative ‘compatibility’ with each of the two females. For example, the difference between [MA × FA] − [MA × FB] for sperm counts in the choice assays provided a measure of male A's relative preference for eggs from the two females that could be correlated with the corresponding difference between, for example, larval survival (a measure of sperm–egg ‘compatibility’) and so on. As each male was represented twice within a block (i.e. each male was crossed with two females), we used a resampling procedure to extract data for just one male from each block at random to avoid pseudoreplication in our analysis of correlation coefficients. A total of 10 000 iterations of the resampling protocol were performed for the calculation of each distribution of correlation coefficients (each with n = 14 independent data points). We then calculated the mean and 95% confidence limits (CLs) for each distribution to assess the strength and significance of each correlation. Resampling procedures were carried out using PopTools v. 3.2 [32]. The complete dataset can be found in the electronic supplementary material.
3. Results
The analysis of sperm numbers recovered from the distal wells of dichotomous chambers (i.e. egg choice) revealed significant male-by-female interaction effects (table 2). Thus, consistent with previous findings for this species [22], the strength of chemically moderated gamete preferences was contingent on the specific identities of eggs and sperm present in each trial. Similarly, in the no-choice assays, fertilization rates, larval survival and the two composite measures of sperm motility (PC1 and PC2) were influenced by the interacting effects of male and female identities (table 2).
Our correlation analyses based on resampled datasets revealed an overall significant positive correlation between gamete preferences in the egg-choice trials and fertilization rates in the no-choice trials (mean correlation coefficient r [95% CLs] = 0.53 [0.25–0.75]; figure 1a). Fertilization rate was also positively correlated with (fertilization-adjusted) larval survival (r = 0.51 [0.25–0.73]) despite the potential for density-dependent effects to counteract this pattern. Consistent with this finding, we also found that adjusted larval survival was significantly positively correlated with egg choice (r = 0.51 [0.17–0.77]; figure 1b). Taken together, these findings reveal that sperm preferentially swam towards eggs that yielded high fertilization rates and elevated offspring survival.
Figure 1.
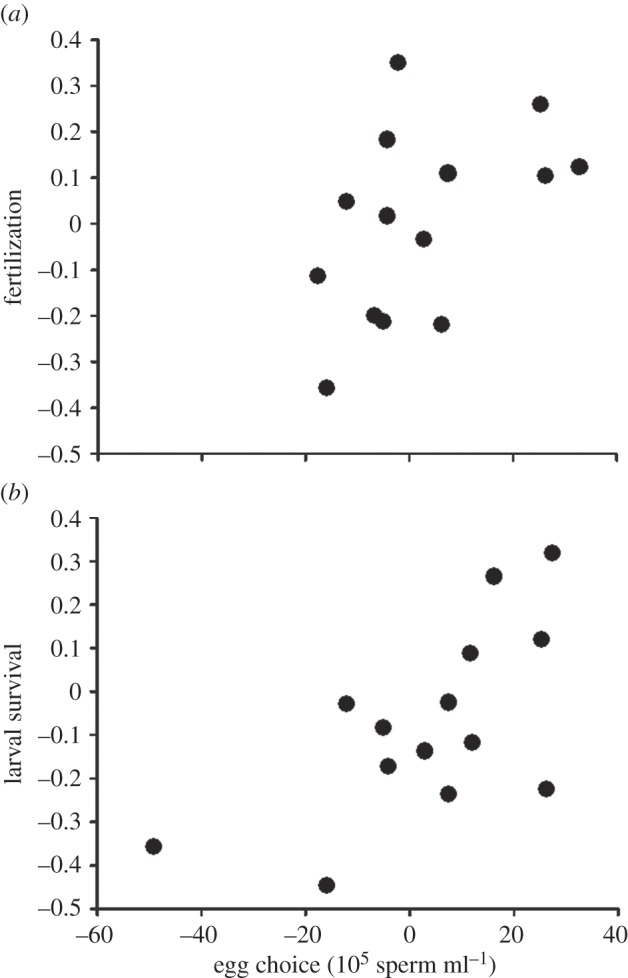
The relationship between sperm migration patterns within the egg-choice experiment (the difference in sperm numbers retrieved from distal wells of the dichotomous choice chambers) with (a) fertilization rate (the corresponding difference between the proportion of eggs fertilized for a given male and the two females within each block), and (b) larval survival (corresponding difference in proportion of offspring surviving to veliger larvae for a given male and the two females within each block). Scatterplots were generated using a dataset representative of the mean correlation coefficient obtained after 10 000 iterations of the resampling protocol.
The patterns of male-by-female interaction underlying the composite measure of sperm velocity and curvature (PC1) correlated positively with patterns of egg choice, fertilization and larval survival. Specifically, we found significant positive correlations between PC1 and egg choice (r = 0.54 [0.04–0.84), fertilization (r = 0.39 [0.003–0.71]) and larval survival (r = 0.64 [0.40–0.80]; figure 2). By contrast, we detected no significant correlations between PC2 and egg choice, fertilization or larval survival (r = 0.22 [−0.19–0.55]; r = 0.18 [−0.18–0.55]; r = 0.19 [−0.24–0.50], respectively).
Figure 2.
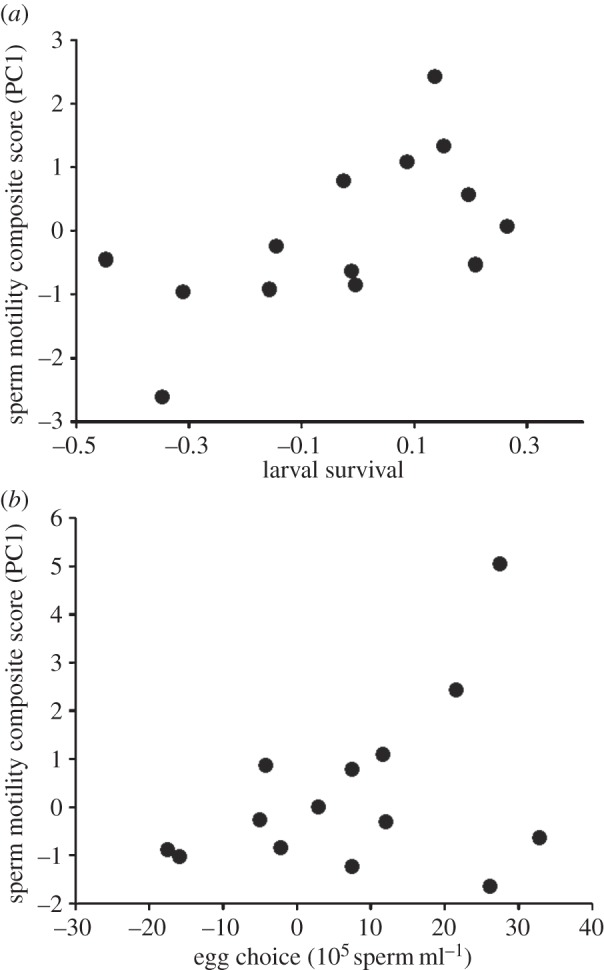
The relationship between sperm behaviour (difference in composite sperm motility measures between sperm and egg water for a given male and the two females within a block) with (a) sperm migration patterns within the egg-choice experiment (the difference in sperm density between distal wells of the dichotomous choice chambers), and (b) larval survival (corresponding difference in proportion of offspring surviving to veliger larvae for a given male and the two females within each block). Scatterplots were generated using a dataset representative of the mean correlation coefficient obtained after 10 000 iterations of the resampling protocol.
4. Discussion
The key finding from our study is that sperm differentiate between intraspecific chemical cues to track eggs from genetically compatible females. Our present experiment on a marine population of M. galloprovincialis builds on previous work on a different (estuarine) population of the same species, which similarly revealed evidence for intraspecific gamete discrimination based on egg chemoattractants [22]. This study adds two key findings to these previous analyses. First, we show that there are measurable fitness benefits associated with these chemically moderated gamete preferences; sperm preferentially swim towards eggs that not only deliver higher fertilization rewards (this study and [22]) but also those that yield higher post-zygotic survival rates. In other contexts, previous studies have reported significant male-by-female interactions at fertilization [33,34], but the question of whether ‘compatible’ combinations of sperm and eggs yield high post-zygotic survival has received less attention, and some studies investigating this topic in other contexts have reported no significant association between fertilization and offspring viability [23–25]. Our study provides evidence that in M. galloprovincialis, the fertilization benefits of sperm chemoattraction extend ‘upstream’ to include enhanced offspring viability. Consequently, our study contributes to an increasing body of evidence demonstrating that postmating sexually selected mechanisms have the potential to generate fertilization biases in favour of compatible partners [35–37], although via a previously undocumented mechanism (sperm chemoattraction).
The second finding to emerge from this study is that patterns of male-by-female interaction (i.e. compatibility) underlying gamete preferences during the egg-choice trials are strongly consistent with those underlying sperm swimming behaviour when ejaculates were tested solely in the presence of (chemically infused) egg water from the same individuals. The significant sperm-by-egg water identity interactions underlying variation in sperm behaviour provide evidence that sperm swimming speeds are differentially upregulated according to variation in chemical signals emitted from eggs. Similar evidence for the differential upregulation of sperm velocity comes from salmonid fishes, where specific combinations of ovarian fluid (analogous to our egg water) and sperm result in non-transitive patterns of sperm behaviour across different male–female crosses, both within species [38–41] and between congeneric taxa [42]. This study similarly indicates that sperm respond differentially to chemoattractants in the absence of eggs, but also that such responses involve variations in both sperm swimming curvature (indicative of chemotaxis) and steady-state velocity (chemokinesis; see [16]). Moreover, our subsequent correlation analyses reveal clear associations between changes in sperm behaviour in response to egg chemoattractants and: (i) gamete preferences, (ii) fertilization, and (iii) larval fitness. These interrelationships strongly suggest that egg chemoattractants convey important information about sperm–egg compatibility in much the same way that olfactory odours can convey information about the compatibility of mating partners [13].
While our study exposes differential sperm chemoattraction as a putative mechanism underlying compatibility-based selection in mussels, it leaves open the question of what mechanistic process(es) underlie these patterns. Indeed, we presently lack studies that characterize intraspecific variation in egg chemoattractants, along with the associated signalling mechanisms that underlie sperm responses. Thus, we can only speculate on the mechanisms by which sperm fine-tune their chemotactic responses to chemoattractants from different conspecific females. Previous speculation on this issue surround possible links between the control of chemoattractants governing gamete attraction and mechanisms underlying gamete recognition proteins that mediate sperm–egg interactions upon gamete contact (reviewed in [21]). In this way, egg-derived chemical cues may prevent sperm from needlessly tracking the chemoattractants from eggs with which they are ultimately incompatible. We clearly require studies that focus on the biochemical and molecular mechanisms underlying intraspecific differences in chemically moderated gamete interactions to test these ideas.
We also require studies covering a broader range of taxa to determine the generality of our findings, particularly in broadcast spawning taxa where the opportunities for sexual selection are largely confined to gamete-level interactions [21]. We suspect that given the central importance of sperm chemoattraction in the sperm–egg interaction in marine broadcast spawners [16], the effects reported in this study may be widespread among these taxa [21]. However, sperm chemoattraction is not limited to broadcast spawners and occurs in several vertebrate species with internal fertilization [19], including mammals [20]. Given that mate choice for compatible individuals is known to yield fitness benefits in vertebrates [43], we would be surprised if chemically moderated gamete ‘preferences’ were not also involved in compatibility-based selection in these taxa.
Acknowledgement
We thank Cameron Duggin for assistance with animal collections, Elizabeth Harrison for laboratory assistance, Rowan Lymbery for help with data simulations, Paco Garcia-Gonzalez and Clelia Gasparini for advice and discussion, and two anonymous reviewers for comments on an earlier draft of the paper.
Funding statement
We thank the University of Western Australia (M.O.) and the Australian Research Council (J.P.E.) for financial support.
References
- 1.Lintner JA. 1882. A new principle in protection from insect attack. West NW Hortic. Soc. Proc. 27, 52–66. [Google Scholar]
- 2.Espmark Y, Amundsen T, Rosenqvist G. (eds). 2000 Animal signals: signalling and signal design in animal communication. Trondheim, Norway: Tapir Academic Press. [Google Scholar]
- 3.Wyatt TD. 2003. Pheremones and animal behaviour: communication by smell and taste. Cambridge, UK: Cambride University Press. [Google Scholar]
- 4.Veltsos P, Wicker-Thomas C, Butlin RK, Hoikkala A, Ritchie MG. 2012. Sexual selection on song and cuticular hydrocarbons in two distinct populations of Drosophila montana. Ecol. Evol. 2, 80–94. ( 10.1002/ece3.75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas ML, Simmons LW. 2009. Sexual selection on cuticular hydrocarbons in the Australian field cricket, Teleogryllus oceanicus. BMC Evol. Biol. 9, 162–174. ( 10.1186/1471-2148-9-162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mays HL, Hill GE. 2004. Choosing mates: good genes versus genes that are a good fit. Trends Ecol. Evol. 19, 554–559. ( 10.1016/j.tree.2004.07.018) [DOI] [PubMed] [Google Scholar]
- 7.Tregenza T, Wedell N. 2000. Genetic compatibility, mate choice and patterns of parentage: invited review. Mol. Ecol. 9, 1013–1027. ( 10.1046/j.1365-294x.2000.00964.x) [DOI] [PubMed] [Google Scholar]
- 8.Puurtinen M, Ketola T, Kotiaho JS. 2009. The good-genes and compatible-genes benefits of mate choice. Am. Nat. 174, 741–752. ( 10.1086/606024) [DOI] [PubMed] [Google Scholar]
- 9.Roelofs WL. 1995. The chemistry of sex attraction. In Chemical ecology: the chemistry of biotic interaction (eds Eisner T, Meinwals J.), pp. 103–117. Washington, DC: National Academy Press. [Google Scholar]
- 10.Aeschlimann PB, Haberli MA, Reusch TBH, Boehm T, Milinski M. 2003. Female sticklebacks Gasterosteus aculeatus use self-reference to optimize MHC allele number during mate selection. Behav. Ecol. Sociobiol. 54, 119–126. [Google Scholar]
- 11.Olsson M, Madsen T, Nordby J, Wapstra E, Ujvari B, Wittsell H. 2003. Major histocompatibility complex and mate choice in sand lizards. Proc. R. Soc. Lond. B 270, S254–S256. ( 10.1098/rsbl.2003.0079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potts WK, Manning CJ, Wakeland EK. 1991. Mating patterns in seminatural populations of mice influenced by MHC genotype. Nature 352, 619–621. ( 10.1038/352619a0) [DOI] [PubMed] [Google Scholar]
- 13.Wedekind C, Füri S. 1997. Body odour preferences in men and women: do they aim for specific MHC combinations or simply heterozygosity? Proc. R. Soc. Lond. B 264, 1471–1479. ( 10.1098/rspb.1997.0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamazaki K, Boyse EA, Mike V, Thaler HT, Mathieson BJ, Abbott J, Boyse J, Zayas ZA, Thomas L. 1976. Control of mating preferences in mice by genes in major histocompatibility complex. J. Exp. Med. 144, 1324–1335. ( 10.1084/jem.144.5.1324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parrott ML, Ward SJ, Temple-Smith PD. 2007. Olfactory cues, genetic relatedness and female mate choice in the agile antechinus (Antechinus agilis). Behav. Ecol. Sociobiol. 61, 1075–1079. ( 10.1007/s00265-006-0340-8) [DOI] [Google Scholar]
- 16.Eisenbach M. 1999. Sperm chemotaxis. Rev. Reprod. 4, 56–66. ( 10.1530/ror.0.0040056) [DOI] [PubMed] [Google Scholar]
- 17.Ralt D, et al. 1994. Chemotaxis and chemokinesis of human spermatozoa to follicular factors. Biol. Reprod. 50, 774–785. ( 10.1095/biolreprod50.4.774) [DOI] [PubMed] [Google Scholar]
- 18.Riffell JA, Krug PJ, Zimmer RK. 2004. The ecological and evolutionary consequences of sperm chemoattraction. Proc. Natl Acad. Sci. USA 101, 4501–4506. ( 10.1073/pnas.0304594101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller RL. 1985. Sperm chemo-orientation in the metazoa. In Biology of fertilization (eds Metz CB, Monroy A.), pp. 275–337. New York, NY: Academic Press. [Google Scholar]
- 20.Eisenbach M, Giojalas LC. 2006. Sperm guidance in mammals: an unpaved road to the egg. Nat. Rev. Mol. Cell Biol. 7, 276–285. ( 10.1038/nrm1893) [DOI] [PubMed] [Google Scholar]
- 21.Evans JP, Sherman CDH. 2013. Sexual selection and the evolution of egg-sperm interactions in broadcast-spawning invertebrates. Biol. Bull. 224, 166–183. [DOI] [PubMed] [Google Scholar]
- 22.Evans JP, Garcia-Gonzalez F, Almbro M, Robinson O, Fitzpatrick JL. 2012. Assessing the potential for egg chemoattractants to mediate sexual selection in a broadcast spawning marine invertebrate. Proc. R. Soc. B 279, 2855–2861. ( 10.1098/rspb.2012.0181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans JP, García-González F, Marshall DJ. 2007. Sources of genetic and phenotypic variance in fertilization rates and larval traits in a sea urchin. Evolution 61, 2832–2838. ( 10.1111/j.1558-5646.2007.00227.x) [DOI] [PubMed] [Google Scholar]
- 24.Gilchrist AS, Partridge L. 1997. Heritability of pre-adult viability differences can explain apparent heritability of sperm displacement ability in Drosophila melanogaster. Proc. R. Soc. Lond. B 264, 1271–1275. ( 10.1098/rspb.1997.0175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García-González F, Simmons LW. 2007. Paternal indirect effects on offspring viability and the benefits of polyandry. Curr. Biol. 17, 32–36. ( 10.1016/j.cub.2006.10.054) [DOI] [PubMed] [Google Scholar]
- 26.Fitzpatrick JL, Simmons LW, Evans JP. 2012. Complex patterns of multivariate selection on the ejaculate of a broadcast spawning marine invertebrate. Evolution 66, 2451–2460. ( 10.1111/j.1558-5646.2012.01627.x) [DOI] [PubMed] [Google Scholar]
- 27.Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, UK: Sinauer Associates, Inc. [Google Scholar]
- 28.Sanchez-Lazo C, Martinez-Pita I. 2012. Effect of temperature on survival, growth and development of Mytilus galloprovincialis larvae. Aquac. Res. 43, 1127–1133. ( 10.1111/j.1365-2109.2011.02916.x) [DOI] [Google Scholar]
- 29.Johnson DW. 2008. Combined effects of condition and density on post-settlement survival and growth of a marine fish. Oecologia 155, 43–52. ( 10.1007/s00442-007-0882-0) [DOI] [PubMed] [Google Scholar]
- 30.Vermeij MJA, Smith JE, Smith CM, Thurber RV, Sandin SA. 2009. Survival and settlement success of coral planulae: independent and synergistic effects of macroalgae and microbes. Oecologia 159, 325–336. ( 10.1007/s00442-008-1223-7) [DOI] [PubMed] [Google Scholar]
- 31.Wilson-Leedy JG, Ingermann RL. 2007. Development of a novel CASA system based on open source software for characterization of zebrafish sperm motility parameters. Theriogenology 67, 661–672. ( 10.1016/j.theriogenology.2006.10.003) [DOI] [PubMed] [Google Scholar]
- 32.Hood GM. 2009. PopTools, version 3.1.1 See http://www.cse.csiro.au/poptools.
- 33.Clark AG, Begun DJ, Prout T. 1999. Female × male interactions in Drosophila sperm competition. Science 283, 217–220. ( 10.1126/science.283.5399.217) [DOI] [PubMed] [Google Scholar]
- 34.Evans JP, Marshall DJ. 2005. Male-by-female interactions influence fertilization success and mediate the benefits of polyandry in the sea urchin Heliocidaris erythrogramma. Evolution 59, 106–112. [PubMed] [Google Scholar]
- 35.Brekke P, Wang J, Bennett PM, Cassey P, Dawson DA, Horsburgh GJ, Ewen JG. 2012. Postcopulatory mechanisms of inbreeding avoidance in the island endemic hihi (Notiomystis cincta). Behav. Ecol. 23, 278–284. ( 10.1093/beheco/arr183) [DOI] [Google Scholar]
- 36.Gillingham MAF, Richardson DS, Lovlie H, Moynihan A, Worley K, Pizzari T. 2009. Cryptic preference for MHC-dissimilar females in male red junglefowl, Gallus gallus. Proc. R. Soc. B 276, 1083–1092. ( 10.1098/rspb.2008.1549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Firman RC, Simmons LW. 2008. Polyandry facilitates postcopulatory inbreeding avoidance in house mice. Evolution 62, 603–611. ( 10.1111/j.1558-5646.2007.00307.x) [DOI] [PubMed] [Google Scholar]
- 38.Dietrich GJ, Wojtczak M, Slowinska M, Dobosz S, Kuzminski H, Ciereszko A. 2008. Effects of ovarian fluid on motility characteristics of rainbow trout (Oncorhynchus mykiss Walbaum) spermatozoa. J. Appl. Ichthyol. 24, 503–507. ( 10.1111/j.1439-0426.2006.01130.x) [DOI] [Google Scholar]
- 39.Evans JP, Rosengrave P, Gasparini C, Gemmell NJ. 2013. Delineating the roles of males and females in sperm competition. Proc. R. Soc. B 280, 20132047 ( 10.1098/rspb.2013.2047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosengrave R, Gemmell NJ, Metcalf V, McBride K, Montgomerie R. 2008. A mechanism for cryptic female choice in chinook salmon. Behav. Ecol. 19, 1179–1185. ( 10.1093/beheco/arn089) [DOI] [Google Scholar]
- 41.Urbach D, Folstad I, Rudolfsen G. 2005. Effects of ovarian fluid on sperm velocity in Arctic charr (Salvelinus alpinus). Behav. Ecol. Sociobiol. 57, 438–444. ( 10.1007/s00265-004-0876-4) [DOI] [Google Scholar]
- 42.Yeates SE, Diamond SE, Einum S, Emerson BC, Holt WV, Gage MJG. 2013. Cryptic choice of conspecific sperm controlled by the impact of ovarian fluid on sperm swimming behavior. Evolution 67, 3523–3536. ( 10.1111/evo.12208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agbali M, Reichard M, Bryjova A, Bryja J, Smith C. 2010. Mate choice for nonadditive genetic benefits correlate with MHC dissimilarity in the rose bitterling (Rhodeus ocellatus). Evolution 64, 1683–1696. ( 10.1111/j.1558-5646.2010.00961.x) [DOI] [PubMed] [Google Scholar]


