Abstract
The extent to which size constrains the evolution of brain organization and the genesis of complex behaviour is a central, unanswered question in evolutionary neuroscience. Advanced cognition has long been linked to the expansion of specific brain compartments, such as the neocortex in vertebrates and the mushroom bodies in insects. Scaling constraints that limit the size of these brain regions in small animals may therefore be particularly significant to behavioural evolution. Recent findings from studies of paper wasps suggest miniaturization constrains the size of central sensory processing brain centres (mushroom body calyces) in favour of peripheral, sensory input centres (antennal and optic lobes). We tested the generality of this hypothesis in diverse eusocial hymenopteran species (ants, bees and wasps) exhibiting striking variation in body size and thus brain size. Combining multiple neuroanatomical datasets from these three taxa, we found no universal size constraint on brain organization within or among species. In fact, small-bodied ants with miniscule brains had mushroom body calyces proportionally as large as or larger than those of wasps and bees with brains orders of magnitude larger. Our comparative analyses suggest that brain organization in ants is shaped more by natural selection imposed by visual demands than intrinsic design limitations.
Keywords: mushroom bodies, ants, allometry
1. Introduction
Brain or brain component size has been positively correlated with cognitive performance, behavioural flexibility, sociality and ecological success in taxa as varied in body size and life history as honeybees, birds, bats and primates [1–5]. However, the degree to which the sensory processing and integrative abilities of brains are constrained by absolute size remains unclear, despite intensive recent analysis [6–13]. Small brains and body sizes may confer computational or metabolic advantages to miniaturized taxa (for example, by reducing the transmission distance between neurons [10]), and tiny animals with highly miniaturized cells (such as Drosophila) can have many more neurons—and thus potentially more computational power—than larger animals (such as Aplysia). Nevertheless, theory suggests that energetic and architectural costs of increasing nervous system miniaturization should eventually outweigh any benefits, limiting the information processing and behavioural capabilities of very small animals [10,12]. Yet, the behavioural complexity of small-bodied arthropods is often surprisingly similar in quality to that of vastly larger vertebrates [6,12]. In fact, extremely small arthropods that have brains smaller than some single-celled protists [9,13] are capable of behaviours similar in sophistication to those performed by larger bodied relatives [8]. Moreover, abstract cognitive abilities previously thought to be exclusive to the repertoires of large-brained vertebrates or some cephalopods [14] have recently been demonstrated in insects [15]. Neural network models suggest that increases in cognitive complexity that intuitively seem to require extensive neural expansion and thus large brains, such as those that accompany the evolution of sociality, may actually require few neurons and only subtle changes in the local neural circuitry underlying specific ancestral behaviours (reviewed in [16]). Therefore, size-related constraints on nervous system performance may be less stringent than previously assumed, allowing very small animals to evolve relatively complex behaviour.
Brains are composed of modular networks characterized by functional and anatomical integration, the size, architecture and behavioural roles of which can evolve with varying degrees of independence (‘mosaic brain evolution’ [17–19]). The relationship between absolute brain size and behavioural complexity may thus be further obscured by the likelihood that task performance abilities in animals spanning ants to primates are affected more by the size and/or architecture of specific brain subregions critical to those tasks, rather than, or in addition to, brain size per se [1,20–25]. Demonstrations of the association of brain composition and behaviour include correlations between the relative size of the neocortex and mammalian sociality [26–29], the relative size of the hippocampus and spatial memory in food-hoarding birds [30–32], and task–performance related differences among social hymenopteran (ant, bee and wasp) workers in the relative size of the mushroom bodies [21,33–39], which are key integrative centres of the insect brain functionally convergent with (and/or potentially deeply homologous to) higher forebrain regions in vertebrates [40,41]. Neuroethological studies of many insects, including social hymenopterans thought to be among the most cognitively advanced invertebrates, have appropriately focused on the mushroom bodies, given their well-characterized role as critical substrates for learning and memory, sensory integration and behavioural plasticity [42–45]. Yet, only very recently have brain- and mushroom body-scaling relationships been comparatively described across taxa varying in ecology, life history and size [46–49]. Even less attention has been given to the scaling of the major primary sensory input neuropils—the antennal (AL) and optic lobes (OL)—which process olfactory and visual afferent information, respectively, and supply the mushroom body calyces (C) in hymenopterans. Understanding how these aspects of brain composition affect worker behaviour and division of labour has been a prominent goal of social insect research, and significant interspecific variation in brain composition associated with task performance has begun to be identified in ants [21,33,47,50] and wasps [51].
Absolute brain size was recently proposed to constrain the evolution of the organization of the functional module comprising the C, AL and OL in polistine wasps [49]; interspecific comparisons suggested that small-brained wasps have lower ratios of C volume to the combined volume of the AL + OL (C/AO hereafter) owing to positive allometry between C and brain size and negative allometry between AL + OL and brain size. Absolute brain size has thus been argued to constrain relative neural investment in central processing (C) versus peripheral sensory neuropils (AL and OL) in this taxon and, by inference, in animals generally [49]. Here, we examine whether size-related developmental constraints have indeed significantly impacted the evolution of neuropil organization within the C/AL/OL module in a broad sample of social hymenopteran taxa (electronic supplementary material, figure S1) that vary in brain size over more than two orders of magnitude (figure 1). We include social bees and wasps in our analyses but focus on ants, given their marked evolutionary miniaturization: ant workers often possess bodies and brains significantly smaller than social bees and wasps, yet they display the highly sophisticated individual cognitive abilities [53,54] and adaptive collective behaviours [55,56] typical of the social Hymenoptera. Leveraging newly collected data and reanalysing published scaling relationships to provide broad taxonomic coverage, we show that the C/AO ratio is not significantly positively correlated with brain size within or among species or across the social Hymenoptera.
Figure 1.
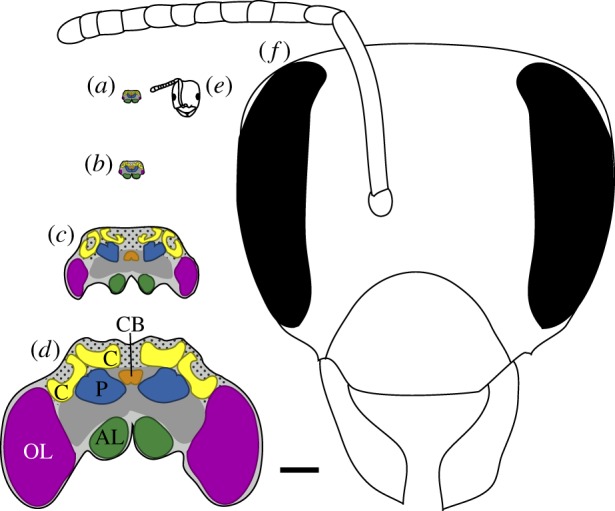
Stylized cross-sectional schematics of worker brains and heads, illustrating the approximate size range of taxa included in our study. Scale bar, 500 μm for all images. Brains of the ants (a) Brachymyrmex depilis and (b) Tapinoma sessile; (c) brain of the wasp Mischocyttarus mastigophorus; (d) brain of large worker of the bumblebee Bombus impatiens, with brain regions analysed in this study indicated; (e) B. depilis ant worker head; (f) B. impatiens large bumblebee worker head. Key to indicated brain regions (colour references refer to online figure): OL, optic lobe neuropil (purple); AL, antennal lobe neurophil (green); C, mushroom body calyx neuropil (yellow); P, mushroom body peduncle neuropil (blue); CB, central body neuropil (orange); other protocerebral neuropils, dark grey; Kenyon cell body region, stippled light grey. Schematics are based on images from this analysis, published studies [37,49,52] and online sources (discoverlife.org and antweb.org). (Online version in colour.)
2. Material and methods
(a). Neuropil investment in social hymenopteran species
We used brain composition data from four published studies of worker brain scaling in ants [57], honeybees and bumblebees [52], and social wasps [49,58] in our analyses, along with counts of the number of ommatidia per eye reported for the ant species [57]. We also collected new brain volumetrics and ommatidia counts from four additional ant species, using similar methods; our detailed protocol is described in the electronic supplementary material. Worker age was not precisely determined during the collection of these datasets. For Polistes fuscatus, Polistes dominulus and Bombus impatiens, brains were sampled from workers of unknown age; for the remaining species, relatively older workers were identified from their cuticular coloration and/or presence away from the nest and their brains were collected.
Quantification of neuropil investment in each case was based on standard conventions of immunohistochemistry and volumetric analysis, including fixation of brains in an aldehyde-based fixative, tissue staining with non-specific stains, dehydration, tissue embedding and sectioning, imaging of sections by microscopy, stereological measurements of the areas of brains and brain components in each section by camera lucida or with the aid of digital software and conversion of measured areas to volumes using section thicknesses. Additional details can be found in the electronic supplementary material, table S1, and full protocols are given in detail in the original references. We had direct access to three of the original databases from these studies and were able to extract additional measurements not reported in the initial publications [52,57,58]. For Neotropical polistine wasp brain scaling, we used only data reported in the original publication [49]; these measurements were corrected prior to analysis owing to unit scaling errors present in the original study, as described in the electronic supplementary material. Species names and original source publications of all studied taxa are listed in the electronic supplementary material, table S2.
(b). Quantification of brain size
For most bivariate brain scaling comparisons, brain size was calculated for that comparison as the total brain neuropil volume less the measured region or regions (‘rest of brain’, abbreviated RB). When we compared our data with previously published results from Neotropical polistine wasp brains [49], we instead used the brain size metric used in that study, as it was the only one available for those species—the combined volume of the mushroom body peduncle and lobes, the central body and the Kenyon cell body region (abbreviated PCK). PCK values could be positively correlated with C/AO ratios simply because a major contributor to PCK is the size of the mushroom body peduncle and lobes, which is probably strongly correlated with the size of the mushroom body calyces (C in C/AO). Thus, we used RB instead of PCK as a less biased brain size estimate whenever we performed comparisons that did not include the polistine wasp dataset. For honeybees, we estimated PCK volume by using the average Kenyon cell body region volume of foragers given by Withers et al. [59], along with our own neuropil volumetrics [52]. For the ant species measured in this study, we calculated PCK volume directly. PCK volume was not available for other ants or bumblebees that we included from previously published studies, accounting for sample size variation across analyses.
(c). Phylogenetic comparative analyses
To account for the effect of shared evolutionary history (phylogeny) on the correlation among the traits measured in this study, we performed phylogenetic comparative analyses following Felsenstein's independent contrasts [60] in the PDAP/PDTREE module [61] of Mesquite [62]. Phylogenetic relationships of the aculeate eusocial hymenopteran included in this study (electronic supplementary material, figure S1) were compiled from Wenzel & Carpenter [63] and Moreau & Bell [64], with placement of the ant genus Gigantiops following Astruc et al. [65]. Branch lengths were estimated from the molecular data and log transformed before analysis following Garland et al. [66].
(d). Statistical analyses
Allometric analyses were conducted on ln-transformed brain region volumes, with scaling slopes and their 95% confidence intervals calculated via standardized major axis (SMA) regression using the program (S)MATR 2.0, as described by Warton et al. [67]. The presence of linear relationships among brain volume ratios and brain size were assessed with Pearson's correlation or, for highly skewed data, with Spearman's correlation. Statistical analyses of phylogenetically independent contrasts (PICs) output by Mesquite were performed as linear Pearson's correlations with x-axis contrasts positivized and y-intercepts constrained to zero, as required to satisfy test assumptions [66].
3. Results and discussion
Our analysis of neuropil investment patterns in diverse eusocial hymenopteran species exhibiting great variation in body size does not support the hypothesis that brain size globally constrains the evolution of cognitive processing ability. The presentation of our findings is ordered according to taxon.
(a). Paper wasps
Using an extensive set of brain scaling data [58] from P. dominulus, Polistes flavus and P. fuscatus, which intraspecifically vary 1.5- to 2.5-fold in brain volume, we examined the relationship between C/AO and brain size within paper wasp species. Using as our proxy for brain size, the volume of brain neuropil less the volume of the measured structure or structures, which we term RB [33], bivariate relationships between the C, OL, AL and AL + OL volume with brain size were found to be moderate to strong (r2 range: 0.32–0.97), with log–log SMA slope point estimates ranging from 0.78 to 2.01 (electronic supplementary material, table S3). Scaling relationships for a given brain subdivision, based on SMA slope point estimates, were not consistent across species. The scaling coefficients (β) of AL + OL volume with RB, for example, were 0.80, 1.08 and 1.17 in P. dominulus, P. flavus and P. fuscatus, respectively. Isometry (scaling coefficient of 1.0) was statistically rejected in only five cases, for which the 95% confidence intervals of the slopes were above or below 1.0, indicating positive or negative allometry, respectively (electronic supplementary material, table S3). The significant positive allometry of C volume versus RB volume in P. fuscatus (β = 1.95 (1.46, 2.59)) and negative allometry of AL + OL volume versus RB volume in P. dominulus (β = 0.80 (0.73, 0.87)) were consistent with the predictions of O'Donnell et al. [49], whereas the significant positive allometries of AL volume versus RB volume in P. flavus (β = 2.01 (1.39, 3.15)) and P. fuscatus (β = 1.67 (1.25, 2.23)) were not (although, in the latter two cases, these did not result in significant deviations from isometry for the combined volume of the AL + OL).
We also calculated C/AO ratios for these species and tested for a positive relationship with RB (electronic supplementary material, table S4). There was a significant positive correlation between these variables for P. dominulus (n = 22, Pearson's r20 = 0.56, p = 0.007), but not for the other two Polistes species sampled (P. flavus: n = 19, r17 = −0.32, p = 0.2; P. fuscatus: n = 22, r20 = 0.28, p = 0.2). Overall, these results indicate that brain size does not affect brain component scaling in the same way among Polistes species, suggesting that brain size is unlikely to be a general predictor of brain composition in the genus [49].
(b). Bumblebees and honeybees
Size variation among social bee workers has behavioural consequences particularly in bumblebees, which exhibit pronounced polymorphism and size-related division of labour, or alloethism [68,69]. Alloethism in social insects can be proximately generated at least in part by cognitive and neural differences among workers of different sizes [33,70,71], suggesting taxa with substantial intraspecific variation in body size and worker behaviour may be particularly informative systems for tests of size-related constraints on neural organization. In a recent analysis of bumblebee (B. impatiens) and honeybee (Apis mellifera) worker brains [52], honeybees showed approximately twofold variation in brain volume, while bumblebee workers varied approximately 12-fold in total brain volume. By comparison, the 10 species of polistine wasps examined by O'Donnell et al. [49] exhibited approximately fivefold interspecific variation in brain size. Despite robust sampling and substantial variation in brain size, however, Mares et al. [52] could not detect a significant correlation between the proportional sizes of the C, AL or OL and overall brain size for either bee species. These findings, like others (cf. [2]), indicate that most brain subregions in bumblebees and honeybees, including sensory input and processing regions, scale isometrically with brain size, resulting in similar patterns of brain organization across the entire polymorphic size distribution of workers in a colony.
We calculated C/AO for these bumblebee and honeybee brains and examined its scaling with brain size (electronic supplementary material, table S4). As expected given the isometric scaling of individual brain components, we did not find a significant correlation between the two variables in either case (bumblebees: n = 46, r44 = 0.04, p = 0.8; honeybees: n = 25, r23 = −0.18, p = 0.4), as RB explained virtually none of the intraspecific variation in C/AO for either species (r2 = 0.00 and 0.03, respectively). Therefore, we found no evidence that brain size constrains the organization of the C/AL/OL module in the social bees sampled, despite the presence of considerable intraspecific variation in brain, behaviour and body size in workers.
(c). Ants
The smallest eusocial hymenopterans are ants, whose workers can be orders of magnitude smaller than wasps and bees. The smallest ant workers, such as those of Carebara atoma (head width ca 0.3–0.4 mm), are dwarfed by those of the largest social wasps, such as the giant Asian hornet Vespa mandarinia (head width more than 9 mm). Ants therefore represent a critical group for testing hypotheses concerning the effects of miniaturization on brain composition and cognitive ability. We explored the relationship between C/AO and brain size in workers from a sample of 16 taxonomically, behaviourally and morphologically diverse ant species (from 16 separate genera in eight subfamilies, the Amblyoponinae, Myrmeciinae, Ponerinae, Cerapachyinae, Ectatomminae, Dolichoderinae, Myrmicinae and Formicinae). These workers approximate the size spectrum of ants, ranging from extremely small (e.g. Brachymyrmex depilis, head width ca 0.4 mm) to relatively large species (e.g. Gigantiops destructor, head width ca 2.5 mm) similar in body size to some paper wasps. We included data from a published morphometric study comparing visual and olfactory brain components and other anatomical parameters in ants [57], unpublished data from that study, and data from newly sampled ant species.
Interspecifically, bivariate relationships between C, OL, AL or OL + AL volume and brain size (RB) were strong for these ants (r2 range: 0.82–0.97). Interestingly, the allometries of these scaling relationships (electronic supplementary material, table S5) were opposite to those reported for polistine paper wasps [49]: SMA scaling coefficients of C volume versus RB volume were less than 1.0 (log–log β = 0.94), whereas scaling coefficients for AL + OL volume versus RB volume were above 1.0 (β = 1.19). For these two relationships, isometry was statistically rejected only for AL + OL scaling, indicating positive allometry (n = 16, r14 = 0.98, p < 0.0001, β = 1.19 (1.07, 1.32), r2 = 0.97). Using PICs, we confirmed this result was robust to possible biases introduced by shared evolutionary history. Results were similar to analyses of raw species data (electronic supplementary material, table S5): scaling coefficients for C volume contrasts versus RB volume contrasts were above 1.0 in the PIC analysis (β = 1.06), but the scaling of AL + OL volume contrasts versus RB volume contrasts remained the only relationship that departed significantly from isometry, with an elevated scaling slope indicating positive allometry (n = 16, r14 = 0.99, p < 0.0001, β = 1.16 (1.06, 1.28), r2 = 0.97). Owing to these allometries, there was a significant negative relationship between C/AO and RB among sampled ants (electronic supplementary material, table S4), whether tested with raw species values (figure 2a; n = 16, r14 = −0.65, p = 0.007) or PICs (figure 2b; n = 16, r14 = −0.70, p = 0.002). Unlike polistine wasps, larger brained ant species had proportionally smaller central processing neuropils (C) relative to the size of their sensory input neuropils (AL + OL).
Figure 2.
Brain scaling relationships within and among social insect taxa are not consistent with constraints on brain organization owing to body size miniaturization. (a) The ratio of central : peripheral sensory processing neuropils (C/AO) is significantly negatively, not positively, correlated with brain size in ants. (b) Results are similar for an analysis based on PICs. (c) C/AO is not significantly correlated with brain size across the social hymenoptera (semi-log plot of raw species values for ants (diamonds), honeybees (triangle) and polistine wasps (open circles)). Error bars indicate 95% confidence intervals for each ant species mean. The solid grey curve is the significant linear correlation reported for wasp data alone [49]. Dashed grey curves are extrapolations of this correlation into the brain size ranges of honeybees and ants. (d) Results are similar for analysis of PICs. (e) C/AO is significantly negatively correlated with eye size in ants. (f) This significant negative relationship persists after PIC analysis. Least-squares best-fit lines are shown for significant Pearson's linear correlations in (a,b,f), but not for the significant Spearman's non-parametric correlation in (e).
(d). Comparative analyses of ants, wasps and bees
All social hymenopterans are members of a monophyletic clade (Aculeata) and share a common, ancestral pattern of brain organization [72]. We thus examined whether the positive correlation between brain size and C/AO in polistine wasps [49] could apply across the social hymenoptera as a whole. Even though we detected the opposite pattern within sampled ants, we had to determine whether C/AO ratios were systematically lower in the smaller bodied ants than in the larger bodied paper wasps. In addition to 10 polistine paper wasp species [49], we included five other social hymenopterans for which we could calculate the same brain size metric available for the paper wasps (PCK volume). These were four small-bodied ant species representing four subfamilies (Amblyoponinae: Stigmatomma (=Amblyopone) pallipes; Myrmicinae: Pheidole moerens (minor workers); Formicinae: B. depilis; Dolichoderinae: Tapinoma sessile), as well as honeybees.
Brains of our sampled ant species were all substantially smaller than the wasp and honeybee brains analysed (figure 2c).1 In fact, the miniscule workers of P. moerens and B. depilis (head widths ca 0.4 mm) had brain PCK volumes nearly two orders of magnitude less than the smallest wasp brains previously studied (ca 6.0–7.0 × 10–4 mm3 versus ca 2.0 × 10−2 mm3 for the wasp Leipomeles dorsata (head width ca 1.9 mm)). However, these miniaturized ant brains had higher C/AO ratios than those of most of the wasps, including the three highest ratios in the analysis (S. pallipes, B. depilis and P. moerens). Consequently, when data from our four sampled ant species were added to the wasp scaling analysis, the significant positive relationship between C/AO and brain size reported for wasps alone [49] was not supported (electronic supplementary material, table S4; wasps + ants: n = 14, r12 = −0.20, p = 0.5).
To estimate PCK volume for honeybees, we combined data from two published studies that used somewhat different histological techniques [52,59]; the accuracy of this measurement is thus likely to be lower than that of the wasp and ant PCK values included in these comparisons. With this in mind, when we added honeybees to the wasp scaling analysis, the strength of the correlation between C/AO and PCK was lower than when honeybees were excluded (r9 = 0.47 for wasps + honeybee versus r8 = 0.77 for wasps alone), and the relationship no longer reached statistical significance (electronic supplementary material, table S4; p = 0.1).
Lastly, when we included both honeybees and the four ant species in the analysis with the wasps, we again failed to find support for a positive relationship between C/AO and PCK volume (figure 2c, electronic supplementary material, table S4; wasps + ants + honeybee: n = 15, r13 = −0.26, p = 0.4). As with raw species values, PICs of C/AO were never significantly correlated with PICs of PCK volume when either ants and/or honeybees were included in the analysis (electronic supplementary material, table S4; wasps + ants: n = 13, r12 = 0.50, p = 0.07; wasps + honeybee: n = 10, r9 = 0.54, p = 0.09; wasps + ants + honeybee (figure 2d): n = 14, r13 = 0.14, p = 0.6).
(e). Visual ecology and brain organization in ants
Our analyses do not support the hypothesis that the organization of the C, AL and OL is globally constrained by brain size in social insects: central processing regions, exemplified by the C, were not found to be relatively smaller in smaller brained individuals within or among species. What then accounts for the considerable variation in the ratio of central processing to peripheral sensory input regions, with Cs ranging from roughly 30 to 200% of the combined volume of the OLs and ALs across social hymenopteran species (figure 2c)?
Interspecific variation in sensory ecology in ants is evident in adaptions for the processing of visual information, probably driven by demands of habitat use (arboreal, above-/within-/below-litter-nesting species), foraging ecology and diel cycle [73,74]. Workers in some species possess large eyes and high visual acuity, whereas others are subterranean species with eyeless workers wholly reliant on olfaction and mechanosensation. We hypothesized that differences among ant species in visual ability could produce variation in C/AO ratios owing to developmental and functional linkages between visually guided behaviour, eye size and OL volume in association with selection for increased (or decreased) visually guided behaviour, resulting in mosaic evolution [18,33,51,75]. Under this hypothesis, workers could evolve larger (or smaller) eyes and OLs with little or no correlated change in AL or C size, although modifications of the C collar region, which receives visual input from the OLs, might be predicted. Highly visual ants would thus be predicted to have lower C/AO owing to the influence of OL volume on this ratio. If visual ability were positively correlated with brain and body size, this process would also lead to an apparent positive relationship between C/AO and brain size.
Workers in our sample of ants varied widely in visual ability, from blind subterranean species (Cerapachys sp.) to those with highly visual diurnal foragers (G. destructor). We thus used the number of ommatidia per eye, which is an important determinant of spatial resolution [76], as a quantitative proxy for visual acuity and examined variation among ant species in C/AO. The distribution of ommatidia number was highly skewed, and Cerapachys sp. had no ommatidia, precluding parametric analysis or log-transformation. We therefore used proportional brain structure sizes rather than allometric analyses to assess how organization of the C/AL/OL module scaled with ommatidia number. When analysing PICs, we used parametric correlation to satisfy test assumptions (constraining linear correlations through the origin), but when analysing raw species data we used non-parametric correlation to better account for skew in ommatidia number.
As predicted, ommatidia number was not significantly correlated with the proportional sizes of the C or the AL, suggesting that relative investment in these neuropils evolved independently of investment in visual acuity or temporal resolution. This result held with analyses of raw species values (Spearman's correlation; C: n = 16, ρ14 = −0.50, p = 0.0501; AL: n = 16, ρ14 = −0.20, p = 0.5) and PICs (Pearson's correlation; C contrasts: n = 16, r14 = −0.19, p = 0.5; AL contrasts: n = 16, r14 = −0.44, p = 0.09). Proportional OL volume, by contrast, showed a strong, highly significant positive correlation with ommatidia number in both raw and phylogenetically corrected analyses (raw species values: n = 16, ρ14 = 0.92, p < 0.0001; PICs: n = 16, r14 = 0.88, p < 0.0001): OLs made up a larger proportion of total brain volume in species more reliant on vision. There was no evidence of a direct trade-off between investment in peripheral visual and olfactory processing neuropils, which could have reduced variation in C/AO among species with different visual abilities: the relative sizes of the ALs and OLs (with respect to all other brain regions) were not significantly correlated across species (raw species values: n = 16, r14 = −0.31, p = 0.2; PICs: n = 16, r14 = −0.48, p = 0.06). Because of these patterns of brain region scaling, C/AO showed, as predicted, a moderate to strong negative correlation with ommatidia number, whether analysed as raw species values (figure 2e; n = 16, ρ14 = −0.81, p = 0.0002) or PICs (figure 2f; n = 16, r14 = −0.58, p = 0.02): visual ant species had smaller central processing regions (C) relative to their peripheral sensory input regions (AL + OL). Because ommatidia number was positively related to overall brain size in these ants (ommatidia number versus total brain volume: n = 16, r14 = 0.72, p = 0.002), differences in visual ability are probably sufficient to explain the negative relationship between C/AO and brain size in these taxa.
4. Conclusion
Brain scaling relationships within taxa may reflect selective optima or global constraints that apply across diverse groups, providing insight into general design principles. Alternatively, selective regimes may differ among related taxa, yielding diverse scaling relationships and more local rules. The positive correlation between C/AO and brain size in Neotropical paper wasps has been interpreted as supporting the hypothesis that ‘only species with large brains are capable of relatively heavy investment in (mushroom body) calyces’, and that ‘this pattern of constrained higher order neural investment may hold true across the vertebrate–invertebrate divide’, providing ‘evidence for absolute brain size as an important factor in cognitive evolution’ [49]. Our analyses show, however, that this prediction does not hold even within the eusocial Hymenoptera. Within species of three well-studied bee and wasp genera (Polistes, Apis and Bombus), there were no consistent relationships between C/AO and brain size. In fact, we found a strong negative correlation between C/AO and brain size among ant species from 16 diverse genera, which was opposite to the predicted pattern [49]. Indeed, ant workers with brains orders of magnitude smaller than those of honeybees and paper wasps had C/AO ratios as high or higher (figure 2c). We therefore conclude that C/AO is not universally constrained by developmental rules governing brain size in the eusocial Hymenoptera.
Our analyses of a diverse sample of ant species indicate that selection associated with sensory ecology, rather than developmental constraint, has a stronger influence on the evolution of C/AO ratio in this clade: more visual ants have lower C/AO ratios, which in concert with a positive correlation between body size and visual ability, probably drives the negative relationship between C/AO and brain size in these taxa. Our findings are consistent with the following evolutionary scenario: (i) selection for increased or decreased visual acuity results in the directional evolution of eye size and ommatidia number, with correlated investment in the OLs, largely independently of mushroom body and AL investment; (ii) selection for increased visual ability is more common in lineages with large-bodied, above-ground foragers, whereas selection for reduced visual ability generally occurs in small-bodied, litter-dwelling or subterranean lineages; (iii) larger bodied (and brained) ants therefore tend to have larger eyes and OLs; thus (iv) larger brained species tend to have lower C/AO ratios, driven by the effect of their relatively larger OL volumes. Our taxonomic sampling did not include potential exceptions such as large-bodied taxa with small eyes (e.g. Eciton) or small-bodied taxa with large eyes (e.g. Pseudomyrmex). Although such extremes appear uncommon, their inclusion would be predicted to reduce the apparent correlation between brain size and C/AO, further reinforcing the importance of ecological selection rather than size-related developmental constraints in shaping brain organization. Such species should be targeted for future studies of brain scaling.
Ratios of brain components (such as C/AO) commonly used in studies of brain scaling can vary interspecifically owing to myriad evolutionary events involving changes of different magnitudes or signs in one, several or all of the component measurements of the ratio, making interpretation challenging. Conversely, similar ratios across taxa may obscure biologically interesting neuroecological differences. In the case of C/AO, for example, species with large ALs and small OLs could have similar ratios to species with small ALs and large OLs, yet the sensory abilities of these taxa would be predicted to be quite different. Lastly, interpreting brain component ratios (such as C/AO) as reflecting relative investment in cognitive function versus sensory input [49] discounts the importance of peripheral regions to information processing and behavioural output. In social bees, for example, neither the OLs or the ALs simply relay sensory information to the Cs for processing: visual input is highly processed in the OLs prior to reaching the Cs [77–80], and plastic changes causally linked to the formation and retrieval of olfactory memories occur in the ALs, upstream of olfactory information processing by the Cs [81,82]. Insects that rely on fast, high-acuity vision (such as fast fliers or, among the ants, visual predators), for example, may be likely to have large OLs, whereas insects that rely heavily on visual or olfactory memory (such as honeybees or navigating desert ants) may invest disproportionately in mushroom body neuropil, including the Cs. However, such strategies are not mutually exclusive, nor necessarily correlated, suggesting variation in C/AO ratios among taxa is unlikely to be explained by a single underlying factor such as brain size. As in vertebrates, social insect brain organization probably evolves largely as a mosaic [17,18,33,83] in response to selection from ecology and life history. The diversity of eusocial insects provides exceptional opportunities to uncover neuroecological principles broadly applicable to social brain evolution.
Endnote
Wasp PCK values were corrected as described in the electronic supplementary material.
Funding statement
M.L.M. was supported by a Howard Hughes Medical Institute Postdoctoral Faculty Fellowship. This work was supported by NSF Collaborative Research grant nos. IOB 0725013 and 0724591 to J.F.A.T. and W.G., respectively.
References
- 1.Shultz S, Dunbar RIM. 2010. Species differences in executive function correlate with hippocampus volume and neocortex ratio across nonhuman primates. J. Comp. Psychol. 124, 252–260. ( 10.1037/a0018894) [DOI] [PubMed] [Google Scholar]
- 2.Gronenberg W, Couvillon MJ. 2010. Brain composition and olfactory learning in honey bees. Neurobiol. Learn. Mem. 93, 435–443. ( 10.1016/j.nlm.2010.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ratcliffe JM, Fenton MB, Shettleworth SJ. 2006. Behavioral flexibility positively correlated with relative brain volume in predatory bats. Brain Behav. Evol. 67, 165–176. ( 10.1159/000090980) [DOI] [PubMed] [Google Scholar]
- 4.Sol D, Timmermans S, Lefebvre L. 2002. Behavioural flexibility and invasion success in birds. Anim. Behav. 63, 495–502. ( 10.1006/anbe.2001.1953) [DOI] [Google Scholar]
- 5.Dunbar RIM, Shultz S. 2007. Evolution in the social brain. Science 317, 1344–1347. ( 10.1126/science.1145463) [DOI] [PubMed] [Google Scholar]
- 6.Chittka L, Niven J. 2009. Are bigger brains better? Curr. Biol. 19, R995–R1008. ( 10.1016/j.cub.2009.08.023) [DOI] [PubMed] [Google Scholar]
- 7.Quesada R, Triana E, Vargas G, Douglass JK, Seid MA, Niven JE, Eberhard WG, Wcislo WT. 2011. The allometry of CNS size and consequences of miniaturization in orb-weaving and cleptoparasitic spiders. Arthropod Struct. Dev. 40, 521–529. ( 10.1016/j.asd.2011.07.002) [DOI] [PubMed] [Google Scholar]
- 8.Eberhard WG. 2007. Miniaturized orb-weaving spiders: behavioural precision is not limited by small size. Proc. R. Soc. B 274, 2203–2209. ( 10.1098/rspb.2007.0675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polilov AA. 2012. The smallest insects evolve anucleate neurons. Arthropod Struct. Dev. 41, 29–34. ( 10.1016/j.asd.2011.09.001) [DOI] [PubMed] [Google Scholar]
- 10.Niven JE, Farris SM. 2012. Miniaturization of nervous systems and neurons. Curr. Biol. 22, R323–R329. ( 10.1016/j.cub.2012.04.002) [DOI] [PubMed] [Google Scholar]
- 11.Chittka L, Skorupski P. 2011. Information processing in miniature brains. Proc. R. Soc. B 278, 885–888. ( 10.1098/rspb.2010.2699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns JG, Foucaud J, Mery F. 2011. Costs of memory: lessons from ‘mini’ brains. Proc. R. Soc. B 278, 923–929. ( 10.1098/rspb.2010.2488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Woude E, Smid HM, Chittka L, Huigens ME. 2013. Breaking Haller's rule: brain–body size isometry in a minute parasitic wasp. Brain Behav. Evol. 81, 86–92. ( 10.1159/000345945) [DOI] [PubMed] [Google Scholar]
- 14.Vitti J. 2013. Cephalopod cognition in an evolutionary context: implications for ethology. Biosemiotics 6, 393–401. ( 10.1007/s12304-013-9175-7) [DOI] [Google Scholar]
- 15.Avarguès-Weber A, Giurfa M. 2013. Conceptual learning by miniature brains. Proc. R. Soc. B 280, 20131907 ( 10.1098/rspb.2013.1907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lihoreau M, Latty T, Chittka L. 2012. An exploration of the social brain hypothesis in insects. Front. Physiol. 3, 442 ( 10.3389/fphys.2012.00442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hager R, Lu L, Rosen GD, Williams RW. 2012. Genetic architecture supports mosaic brain evolution and independent brain–body size regulation. Nat. Commun. 3, 1079 ( 10.1038/ncomms2086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barton RA, Harvey PH. 2000. Mosaic evolution of brain structure in mammals. Nature 405, 1055–1058. ( 10.1038/35016580) [DOI] [PubMed] [Google Scholar]
- 19.Kolb EM, Rezende EL, Holness L, Radtke A, Lee SK, Obenaus A, Garland T. 2013. Mice selectively bred for high voluntary wheel running have larger midbrains: support for the mosaic model of brain evolution. J. Exp. Biol. 216, 515–523. ( 10.1242/jeb.076000) [DOI] [PubMed] [Google Scholar]
- 20.Ott SR, Rogers SM. 2010. Gregarious desert locusts have substantially larger brains with altered proportions compared with the solitarious phase. Proc. R. Soc. B 277, 3087–3096. ( 10.1098/rspb.2010.0694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gronenberg W, Riveros AJ. 2009. Social brains and behavior: past and present. In Organization of insect societies: from genome to sociocomplexity (eds Gadau J, Fewell J.), pp. 377–401. Cambridge, MA: Harvard University Press. [Google Scholar]
- 22.Smith AR, Seid MA, Jiménez LC, Wcislo WT. 2010. Socially induced brain development in a facultatively eusocial sweat bee Megalopta genalis (Halictidae). Proc. R. Soc. B 277, 2157–2163. ( 10.1098/rspb.2010.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shultz S, Dunbar R. 2010. Encephalization is not a universal macroevolutionary phenomenon in mammals but is associated with sociality. Proc. Natl Acad. Sci. USA 107, 21 582–21 586. ( 10.1073/pnas.1005246107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shultz S, Dunbar RIM. 2010. Social bonds in birds are associated with brain size and contingent on the correlated evolution of life-history and increased parental investment. Biol. J. Linn. Soc. 100, 111–123. ( 10.1111/j.1095-8312.2010.01427.x) [DOI] [Google Scholar]
- 25.Catania KC. 2012. Tactile sensing in specialized predators: from behavior to the brain. Curr. Opin. Neurobiol. 22, 251–258. ( 10.1016/j.conb.2011.11.014) [DOI] [PubMed] [Google Scholar]
- 26.Finarelli JA, Flynn JJ. 2009. Brain-size evolution and sociality in Carnivora. Proc. Natl Acad. Sci. USA 106, 9345–9349. ( 10.1073/pnas.0901780106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunbar RIM. 2009. The social brain hypothesis and its implications for social evolution. Ann. Hum. Biol. 36, 562–572. ( 10.1080/03014460902960289) [DOI] [PubMed] [Google Scholar]
- 28.Perez-Barberia FJ, Shultz S, Dunbar RIM. 2007. Evidence for coevolution of sociality and relative brain size in three orders of mammals. Evolution 61, 2811–2821. ( 10.1111/j.1558-5646.2007.00229.x) [DOI] [PubMed] [Google Scholar]
- 29.Dunbar RIM. 1998. The social brain hypothesis. Evol. Anthropol. 6, 178–190. () [DOI] [Google Scholar]
- 30.Roth TC, II, Pravosudov VV. 2009. Hippocampal volumes and neuron numbers increase along a gradient of environmental harshness: a large-scale comparison. Proc. R. Soc. B 276, 401–405. ( 10.1098/rspb.2008.1184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Healy SD, de Kort SR, Clayton NS. 2005. The hippocampus, spatial memory and food hoarding: a puzzle revisited. Trends Ecol. Evol. 20, 17–22. ( 10.1016/j.tree.2004.10.006) [DOI] [PubMed] [Google Scholar]
- 32.Lucas JR, Brodin A, de Kort SR, Clayton NS. 2004. Does hippocampal size correlate with the degree of caching specialization? Proc. R. Soc. Lond. B 271, 2423–2429. ( 10.1098/rspb.2004.2912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muscedere ML, Traniello JFA. 2012. Division of labor in the hyperdiverse ant genus Pheidole is associated with distinct subcaste- and age-related patterns of worker brain organization. PLoS ONE 7, e31618 ( 10.1371/journal.pone.0031618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Withers GS, Fahrbach SE, Robinson GE. 1993. Selective neuroanatomical plasticity and division of labour in the honeybee. Nature 364, 238–240. ( 10.1038/364238a0) [DOI] [PubMed] [Google Scholar]
- 35.Farris SM, Robinson GE, Fahrbach SE. 2001. Experience- and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee. J. Neurosci. 21, 6395–6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fahrbach SE, Moore D, Capaldi EA, Farris SM, Robinson GE. 1998. Experience-expectant plasticity in the mushroom bodies of the honeybee. Learn. Mem. 5, 115–123. ( 10.1101/lm.5.1.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molina Y, O'Donnell S. 2008. Age, sex, and dominance-related mushroom body plasticity in the paperwasp Mischocyttarus mastigophorus. Dev. Neurobiol. 68, 950–959. ( 10.1002/dneu.20633) [DOI] [PubMed] [Google Scholar]
- 38.O'Donnell S, Donlan N, Jones T. 2007. Developmental and dominance-associated differences in mushroom body structure in the paper wasp Mischocyttarus mastigophorus. Dev. Neurobiol. 67, 39–46. ( 10.1002/dneu.20324) [DOI] [PubMed] [Google Scholar]
- 39.O'Donnell S, Donlan NA, Jones TA. 2004. Mushroom body structural change is associated with division of labor in eusocial wasp workers (Polybia aequatorialis, Hymenoptera: Vespidae). Neurosci. Lett. 356, 159–162. ( 10.1016/j.neulet.2003.11.053) [DOI] [PubMed] [Google Scholar]
- 40.Tomer R, Denes AS, Tessmar-Raible K, Arendt D. 2010. Profiling by image registration reveals common origin of annelid mushroom bodies and vertebrate pallium. Cell 142, 800–809. ( 10.1016/j.cell.2010.07.043) [DOI] [PubMed] [Google Scholar]
- 41.Farris SM. 2008. Structural, functional and developmental convergence of the insect mushroom bodies with higher brain centers of vertebrates. Brain Behav. Evol. 72, 1–15. ( 10.1159/000139457) [DOI] [PubMed] [Google Scholar]
- 42.Heisenberg M. 1998. What do the mushroom bodies do for the insect brain? An introduction. Learn. Mem. 5, 1–10. ( 10.1101/lm.5.1.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farris SM, Roberts NS. 2005. Coevolution of generalist feeding ecologies and gyrencephalic mushroom bodies in insects. Proc. Natl Acad. Sci. USA 102, 17 394–17 399. ( 10.1073/pnas.0508430102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farris SM. 2011. Are mushroom bodies cerebellum-like structures? Arthropod Struct. Dev. 40, 368–379. ( 10.1016/j.asd.2011.02.004) [DOI] [PubMed] [Google Scholar]
- 45.Fahrbach SE. 2006. Structure of the mushroom bodies of the insect brain. Annu. Rev. Entomol. 51, 209–232. ( 10.1146/annurev.ento.51.110104.150954) [DOI] [PubMed] [Google Scholar]
- 46.Wehner R, Fukushi T, Isler K. 2007. On being small: brain allometry in ants. Brain Behav. Evol. 69, 220–228. ( 10.1159/000097057) [DOI] [PubMed] [Google Scholar]
- 47.Riveros AJ, Seid MA, Wcislo WT. 2012. Evolution of brain size in class-based societies of fungus-growing ants (Attini). Anim. Behav. 83, 1043–1049. ( 10.1016/j.anbehav.2012.01.032) [DOI] [Google Scholar]
- 48.Seid MA, Castillo A, Wcislo WT. 2011. The allometry of brain miniaturization in ants. Brain Behav. Evol. 77, 5–13. ( 10.1159/000322530) [DOI] [PubMed] [Google Scholar]
- 49.O'Donnell S, Clifford M, Molina Y. 2011. Comparative analysis of constraints and caste differences in brain investment among social paper wasps. Proc. Natl Acad. Sci. USA 108, 7107–7112. ( 10.1073/pnas.1017566108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelber C, Rössler W, Roces F, Kleineidam CJ. 2009. The antennal lobes of fungus-growing ants (Attini): neuroanatomical traits and evolutionary trends. Brain Behav. Evol. 73, 273–284. ( 10.1159/000230672) [DOI] [PubMed] [Google Scholar]
- 51.Molina Y, Harris RM, O'Donnell S. 2009. Brain organization mirrors caste differences, colony founding and nest architecture in paper wasps (Hymenoptera: Vespidae). Proc. R. Soc. B 276, 3345–3351. ( 10.1098/rspb.2009.0817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mares S, Ash L, Gronenberg W. 2005. Brain allometry in bumblebee and honey bee workers. Brain Behav. Evol. 66, 50–61. ( 10.1159/000085047) [DOI] [PubMed] [Google Scholar]
- 53.Wehner R. 2003. Desert ant navigation: how miniature brains solve complex tasks. J. Comp. Physiol. A 189, 579–588. ( 10.1007/s00359-003-0431-1) [DOI] [PubMed] [Google Scholar]
- 54.Josens R, Eschbach C, Giurfa M. 2009. Differential conditioning and long-term olfactory memory in individual Camponotus fellah ants. J. Exp. Biol. 212, 1904–1911. ( 10.1242/jeb.030080) [DOI] [PubMed] [Google Scholar]
- 55.Dussutour A, Deneubourg JL, Beshers S, Fourcassie V. 2009. Individual and collective problem-solving in a foraging context in the leaf-cutting ant Atta colombica. Anim. Cogn. 12, 21–30. ( 10.1007/s10071-008-0165-0) [DOI] [PubMed] [Google Scholar]
- 56.Sasaki T, Pratt SC. 2011. Emergence of group rationality from irrational individuals. Behav. Ecol. 22, 276–281. ( 10.1093/beheco/arq198) [DOI] [Google Scholar]
- 57.Gronenberg W, Hölldobler B. 1999. Morphologic representation of visual and antennal information in the ant brain. J. Comp. Neurol. 412, 229–240. () [DOI] [PubMed] [Google Scholar]
- 58.Gronenberg W, Ash LE, Tibbetts EA. 2007. Correlation between facial pattern recognition and brain composition in paper wasps. Brain Behav. Evol. 71, 1–14. ( 10.1159/000108607) [DOI] [PubMed] [Google Scholar]
- 59.Withers GS, Fahrbach SE, Robinson GE. 1995. Effects of experience and juvenile hormone on the organization of the mushroom bodies of honey bees. J. Neurobiol. 26, 130–144. ( 10.1002/neu.480260111) [DOI] [PubMed] [Google Scholar]
- 60.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15. ( 10.1086/284325) [DOI] [Google Scholar]
- 61.Midford PE, Garland T, Maddison W. 2002. PDAP: PDTREE package for Mesquite, v. 1.00. See http://mesquiteproject.org.
- 62.Maddison WP, Maddison DR. 2006. Mesquite: a modular system for evolutionary analysis, v. 1.1 See http://mesquiteproject.org.
- 63.Wenzel JW, Carpenter JM. 1994. Comparing methods: adaptive traits and tests of adaptation. In Phylogenetics and ecology (eds Eggleton P, Vane-Wright RI.), pp. 79–101. London, UK: Academic Press. [Google Scholar]
- 64.Moreau CS, Bell CD. 2013. Testing the museum versus cradle tropical biological diversity hypothesis: phylogeny, diversification, and ancestral biogeographic range evolution of the ants. Evolution 67, 2240–2257. ( 10.1111/evo.12105) [DOI] [PubMed] [Google Scholar]
- 65.Astruc C, Julien JF, Errard C, Lenoir A. 2004. Phylogeny of ants (Formicidae) based on morphology and DNA sequence data. Mol. Phylogenet. Evol. 31, 880–893. ( 10.1016/j.ympev.2003.10.024) [DOI] [PubMed] [Google Scholar]
- 66.Garland T, Harvey PH, Ives AR. 1992. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 41, 18–32. [Google Scholar]
- 67.Warton DI, Wright IJ, Falster DS, Westoby M. 2006. Bivariate line-fitting methods for allometry. Biol. Rev. 81, 259–291. ( 10.1017/s1464793106007007) [DOI] [PubMed] [Google Scholar]
- 68.Jandt JM, Dornhaus A. 2009. Spatial organization and division of labour in the bumblebee Bombus impatiens. Anim. Behav. 77, 641–651. ( 10.1016/j.anbehav.2008.11.019) [DOI] [Google Scholar]
- 69.Goulson D, Peat J, Stout JC, Tucker J, Darvill B, Derwent LC, Hughes WOH. 2002. Can alloethism in workers of the bumblebee, Bombus terrestris, be explained in terms of foraging efficiency? Anim. Behav. 64, 123–130. ( 10.1006/anbe.2002.3041) [DOI] [Google Scholar]
- 70.Kuebler LS, Kelber C, Kleineidam CJ. 2010. Distinct antennal lobe phenotypes in the leaf-cutting ant (Atta vollenweideri). J. Comp. Neurol. 518, 352–365. ( 10.1002/cne.22217) [DOI] [PubMed] [Google Scholar]
- 71.Riveros AJ, Gronenberg W. 2009. Olfactory learning and memory in the bumblebee Bombus occidentalis. Naturwissenschaften 96, 851–856. ( 10.1007/s00114-009-0532-y) [DOI] [PubMed] [Google Scholar]
- 72.Farris SM, Schulmeister S. 2011. Parasitoidism, not sociality, is associated with the evolution of elaborate mushroom bodies in the brains of hymenopteran insects. Proc. R. Soc. B 278, 940–951. ( 10.1098/rspb.2010.2161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greiner B, Narendra A, Reid SF, Dacke M, Ribi WA, Zeil J. 2007. Eye structure correlates with distinct foraging-bout timing in primitive ants. Curr. Biol. 17, R879–R880. ( 10.1016/j.cub.2007.08.015) [DOI] [PubMed] [Google Scholar]
- 74.Moser JC, Reeve JD, Bento JMS, Della Lucia TMC, Cameron RS, Heck NM. 2004. Eye size and behaviour of day- and night-flying leafcutting ant alates. J. Zool. 264, 69–75. ( 10.1017/S0952836904005527) [DOI] [Google Scholar]
- 75.de Winter W, Oxnard CE. 2001. Evolutionary radiations and convergences in the structural organization of mammalian brains. Nature 409, 710–714. ( 10.1038/35055547) [DOI] [PubMed] [Google Scholar]
- 76.Land MF, Nilsson D-E. 2002. Animal eyes. New York, NY: Oxford University Press. [Google Scholar]
- 77.Paulk AC, Dacks AM, Phillips-Portillo J, Fellous J-M, Gronenberg W. 2009. Visual processing in the central bee brain. J. Neurosci. 29, 9987–9999. ( 10.1523/jneurosci.1325-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paulk AC, Dacks AM, Gronenberg W. 2009. Color processing in the medulla of the bumblebee (Apidae: Bombus impatiens). J. Comp. Neurol. 513, 441–456. ( 10.1002/cne.21993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paulk AC, Phillips-Portillo J, Dacks AM, Fellous J-M, Gronenberg W. 2008. The processing of color, motion, and stimulus timing are anatomically segregated in the bumblebee brain. J. Neurosci. 28, 6319–6332. ( 10.1523/jneurosci.1196-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paulk AC, Gronenberg W. 2008. Higher order visual input to the mushroom bodies in the bee, Bombus impatiens. Arthropod Struct. Dev. 37, 443–458. ( 10.1016/j.asd.2008.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hammer M, Menzel R. 1998. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn. Mem. 5, 146–156. [PMC free article] [PubMed] [Google Scholar]
- 82.Rath L, Giovanni Galizia C, Szyszka P. 2011. Multiple memory traces after associative learning in the honey bee antennal lobe. Eur. J. Neurosci. 34, 352–360. ( 10.1111/j.1460-9568.2011.07753.x) [DOI] [PubMed] [Google Scholar]
- 83.Iwaniuk AN, Dean KM, Nelson JE. 2004. A mosaic pattern characterizes the evolution of the avian brain. Proc. R. Soc. Lond. B 271, S148–S151. ( 10.1098/rsbl.2003.0127) [DOI] [PMC free article] [PubMed] [Google Scholar]



