Abstract
Maximum lifespan in birds and mammals varies strongly with body mass such that large species tend to live longer than smaller species. However, many species live far longer than expected given their body mass. This may reflect interspecific variation in extrinsic mortality, as life-history theory predicts investment in long-term survival is under positive selection when extrinsic mortality is reduced. Here, we investigate how multiple ecological and mode-of-life traits that should reduce extrinsic mortality (including volancy (flight capability), activity period, foraging environment and fossoriality), simultaneously influence lifespan across endotherms. Using novel phylogenetic comparative analyses and to our knowledge, the most species analysed to date (n = 1368), we show that, over and above the effect of body mass, the most important factor enabling longer lifespan is the ability to fly. Within volant species, lifespan depended upon when (day, night, dusk or dawn), but not where (in the air, in trees or on the ground), species are active. However, the opposite was true for non-volant species, where lifespan correlated positively with both arboreality and fossoriality. Our results highlight that when studying the molecular basis behind cellular processes such as those underlying lifespan, it is important to consider the ecological selection pressures that shaped them over evolutionary time.
Keywords: longevity, extrinsic mortality, MCMCglmm, volant, non-volant
1. Introduction
Lifespan, or longevity, is a fundamental life-history trait that exhibits considerable variation both within and among species. Maximum lifespan in vertebrates, for example, ranges from up to 211 years in the bowhead whale (Balaena mysticetus; [1]), down to just eight weeks in the pygmy goby (Eviota sigillata; [2]). Like most other life-history traits, lifespan varies strongly with body size such that large species tend to live longer than smaller species [3–6]. However, many species have far longer, or indeed shorter, lives than expected given their body mass (figure 1). Understanding the mechanisms underlying these deviations from predicted lifespan may reveal the secrets to treating and combating human ageing [7,8].
Figure 1.
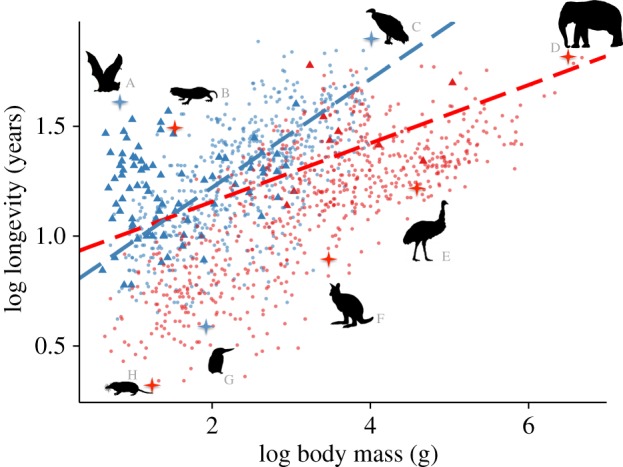
Relationships between body mass and maximum lifespan in birds and mammals. Silhouettes highlight a selection of species with much longer or shorter lifespans than expected given their body size. These species are (A) Myotis brandtii, Brandt's bat; (B) Heterocephalus glaber, naked mole rat; (C) Vultur gryphus, Andean condor; (D) Loxodonta Africana, African elephant; (E) Dromaius novaehollandiae, emu; (F) Dorcopsulus macleayi, Papuan forest-wallaby; (G) Ceryle rudis, pied kingfisher and (H) Myosorex varius, forest shrew. Blue points and line represent volant birds and mammals (n = 662; slope = 0.25, intercept = 0.73). Red points and line represent non-volant birds and mammals (n = 706; slope = 0.13, intercept = 0.89). Blue triangles represent bat species and red triangles represent non-volant bird species. Estimates of slopes and intercepts represent back transformed values from mean centred values given in table 1. (Online version in colour.)
One explanation for species living longer than expected, given their body size, is that low extrinsic mortality (i.e. low risk of death owing to external causes such as disease, predation, food shortages or accidents) will, on average, select for longer lifespans than when extrinsic mortality is high [9,10]. This is because when untimely death is more likely, investment in early and frequent reproduction is favoured rather than investment in long-term maintenance and survival. Therefore, species with adaptations that reduce the risks of extrinsic mortality should live longer than expected, given their body mass [11]. These ideas have led to myriad, taxon-specific hypotheses about traits that may reduce extrinsic mortality and result in increased lifespan (reviewed in [7]). However, there is little consensus about the general drivers of increased lifespan across clades.
The ability to fly, and thus more easily escape predation and unfavourable conditions, is perhaps the most effective way a terrestrial species can evolve to reduce its extrinsic mortality and increase its lifespan [11–13]. This is supported strongly by striking differences in the lifespan of volant (flying) and non-volant (non-flying) vertebrates; on average, bats live 3.5 times longer than similar-sized non-volant placental mammals [14,15], whereas birds live up to four times longer than similar-sized mammals [3,16]. However, flight may not be the only route to reducing extrinsic mortality and thereby increasing lifespan. Ecological factors may also be important. Previous studies have investigated the relationship between lifespan and various ecological variables, but most only investigated select groups of species and few considered multiple traits simultaneously (e.g. [17]).
Here, we investigate how multiple ecological and mode-of-life traits simultaneously influence maximum lifespan across birds and mammals. We generate clear, testable hypotheses (see below) about the relationships among lifespan and ecological and mode-of-life traits known to influence extrinsic mortality risk; including flight capability (volant or non-volant), activity period (diurnal, crepuscular (i.e. active at dawn and dusk), nocturnal or cathemeral (i.e. active both day and night)), foraging environment (terrestrial, semi-arboreal, arboreal, aerial or aquatic) and fossoriality (i.e. living in burrows; fossorial, semi-fossorial, non-fossorial). We then test these hypotheses across birds and mammals using, to our knowledge, the largest number of species to date (n = 589 birds and 779 mammals) and state-of-the-art phylogenetic comparative analyses (including using a distribution of 500 combined bird and mammal phylogenies) to control for the phylogenetic autocorrelation introduced by shared ancestry [18] and body mass [3]. Together, these novel features of our analyses allow unprecedented testing of these key hypotheses that underpin life-history evolution in endotherms.
We predict that, after controlling for body mass: (i) volant species will live longer than non-volant species, because they can more easily evade predators and unfavourable conditions [11–13]; (ii) nocturnal, crepuscular or cathemeral species will live longer than diurnal species, because species that are active at night or dusk are likely to be harder for predators to detect [12,19]; (iii) species which forage in non-terrestrial environments (i.e. species which feed in trees, water or aerially) will live longer than terrestrial foragers, because they will be more capable of escaping from predators than species that feed on the ground [13,17,20]; and (iv) fossorial (i.e. species that live in permanent burrows) and semi-fossorial species will live longer than purely terrestrial species, because they possess means to escape predation and unfavourable conditions through refuge [21].
We expect that the ecological factors which influence lifespan will vary among volant and non-volant species, because sources of extrinsic mortality will differ in these two groups. We therefore split species into volant (most birds and all bats) and non-volant (some birds and most mammals) subgroups, to discover general, broad-scale correlates of lifespan in endotherms, rather than separate correlates for birds and mammals. We then tested our hypotheses on volant and non-volant species separately. We find that, as predicted, after controlling for body mass and phylogeny, the most important factor enabling longer lifespan is the ability to fly. In addition, ecological correlates of lifespan varied among volant and non-volant species such that the longest lived volant species are nocturnal, cathemeral or diurnal, whereas the longest lived non-volant species are arboreal or fossorial.
2. Material and methods
(a). Data
We used maximum longevity as our measure of lifespan as it is thought to be the best available estimator of a species' ageing rate [5] and because of the amount of high-quality longevity data available. We obtained data on maximum longevity (years) and adult body mass (g) from the AnAge database [1,22]. In our main analysis, we excluded species with maximum longevity estimates based on fewer than 10 longevity records, or with low or questionable data quality as defined in the AnAge database [5]. As maximum values are dependent on sample size, we also ran a sensitivity analysis excluding species with maximum longevity estimated from fewer than 100 longevity records. This should show whether data quantity has a strong influence on our results, though it is worth remembering that data quality in a database such as AnAge is highly heterogeneous, and increasing sample size may not necessarily correlate with increased data quality. Note that longevity records for non-volant mammals tend to come from captive individuals, whereas data for bats and birds tend to come from wild caught individuals. Although we expect captive individuals to live longer than wild individuals, on average maximum longevity tends to remain unchanged between captive and wild populations [23]. Further, given that bats and birds live longer than non-volant mammals, this should make our analyses more conservative.
To test our hypotheses concerning the relationships between lifespan, mode-of-life and ecological traits, we collected data on the flight capability (volant or non-volant), activity period (diurnal, crepuscular, nocturnal or cathemeral), foraging environment (terrestrial, semi-arboreal, arboreal, aerial or aquatic) and fossoriality (fossorial, semi-fossorial or non-fossorial) of each species using Walker's Mammals of the World [24], the Handbook of Birds of the World series [25], the Handbook of the Birds of Europe, the Middle East and North Africa series [26] and some additional sources (electronic supplementary material, appendix 1, [27–29]). These categories are described in detail in the electronic supplementary material, appendix 1. We used the taxonomy of Wilson & Reeder [30] for mammals and Jetz et al. [31] for birds. We excluded purely aquatic mammals (Cetacea and Sirenia) from the analyses, because we expect selection pressures to be very different in these groups. We also excluded gliding mammals, because there were too few species (n = 9) to run a separate analysis and because this group could equally fit into either the volant or non-volant subgroups.
Rather than basing our analyses on just a single phylogenetic tree and assuming this tree was known without error, we instead used a distribution of trees. We extracted 500 bird trees from the posterior distribution of a recent bird phylogeny generated under a Bayesian inference framework [31], and used the 10 000 mammal trees constructed by Kuhn et al. [32]. Each individual mammal tree comprises one resolution of the polytomies of a previously published supertree [33]. We treat these as equivalent to a Bayesian posterior distribution of trees, because no such tree analysis exists for all mammals. As we needed a distribution of phylogenies containing both birds and mammals, we randomly selected one bird tree and one mammal tree (without replacement) and bound them to make a combined tree. The trees were bound with a root age of 315 million years, corresponding to the fossil calibration for all amniotes, i.e. Archerpeton anthracos (electronic supplementary material, appendix 1; [34]). We repeated this procedure 500 times to generate a distribution of 500 combined bird and mammal trees.
Many studies on vertebrate ageing have noted a strong correlation between maximum longevity and metabolic rate. Opinion is divided as to whether this is a causative relationship or merely confounded with the strong correlation between body mass and metabolic rate [7]. To determine whether our conclusions hold when we include metabolic rate in our models, we also compiled mass-specific basal metabolic rate (BMR; Wg−1) data (see the electronic supplementary material, appendix 1).
In total, our analyses used data from 589 birds (579 volant and 10 non-volant) and 779 mammals (83 volant and 696 non-volant; see the electronic supplementary material, appendix 3: table A1 for more details and appendix 2 for the complete dataset). This was reduced to 112 birds and 330 mammals when we include BMR in our models, and 474 birds and 435 mammals in the sensitivity analysis using only species with 100 or more longevity records.
(b). Analyses
To test our hypotheses, we fitted the following three models, with maximum longevity and body mass incorporated as continuous variables; flight capability, foraging environment, activity period and fossoriality as factors and with body mass : flight capability representing the interaction between body mass and flight capability.
-
(1) For all species (n = 1368):
maximum longevity = f(body mass + flight capability + body mass : flight capability).
-
(2) For volant species only (n = 662):
maximum longevity = f(body mass + foraging environment + activity period).
-
(3) For non-volant species only (n = 706):
maximum longevity = f(body mass + foraging environment + fossoriality + activity period).
All analyses were carried out in R v. 3.0.2 [35]. Maximum longevity and body mass (and BMR, see below) were log10 transformed to correct inherent skewness before being mean centred and expressed in units of standard deviation.
We fitted our models using Bayesian phylogenetic mixed models from the MCMCglmm package [36], to account for non-independence in species traits introduced as a result of common ancestry [18]. MCMCglmm uses a Markov chain Monte Carlo (MCMC) estimation approach and accounts for non-independence among closely related species by including the phylogenetic relationships among species as a random variable. We determined the number of iterations, thinning and the burn-in period for each model run across all trees using diagnostics in the coda package [37] and we checked for convergence between model chains using the Gelman-Rubin statistic, the potential scale reduction factor (PSR), with all models required to have a PSR below 1.1 [38]. Following the recommendations of Hadfield [36], we used an uninformative inverse-Wishart distribution (with variance, V, set to 0.5 and belief parameter, nu, set to 0.002) and a parameter expanded prior, with a half-Cauchy distribution (described by the parameters V = 0.5, nu = 1, the prior mean alpha.mu = 0, and alpha.V = 102, which represents the prior standard deviation with a scale of 10), for the random factor to improve mixing and decrease autocorrelation among iterations.
As noted earlier, rather than using one phylogenetic tree and assuming this tree was error free, we instead used a distribution of 500 combined bird and mammal trees and fitted each of our models to each of these trees. We then combined the resulting model outputs to give model estimates which incorporate the error across the 500 trees. As the posterior outputs of MCMC models are combinable, coefficient distributions were created by amalgamating each coefficient posterior.
Finally, to determine whether our conclusions held when we excluded species with fewer than 100 longevity records or when metabolic rate was included in our models, we repeated models 1–3 with either the reduced dataset of species with 100 or more longevity records or with BMR as an additional linear covariate. We also repeated models 2 and 3 for birds and mammals (rather than volant and non-volant species) separately to ensure that differences between the volant and non-volant subgroups were owing to differences in flight capability and were not simply representing the difference between mammals and birds. We calculated the deviance information criteria (DIC), a hierarchical generalization of the Akaike information criteria, for each bird and mammal paired models and compared it to the paired volant and non-volant models to compare model ‘fit’ of each approach.
3. Results
We found that volant species live longer than non-volant species of a similar body mass (table 1 and figure 1). In addition, for a given increase in body mass, the lifespans of volant species (modal slope estimate (after converting from mean-centred values) = 0.25; table 1) increase significantly more than the lifespans of non-volant species (modal slope estimate (after converting from mean-centred values) = 0.13; table 1).
Table 1.
Relationship between maximum longevity (years), body mass (g) and flight capability (volant or non-volant) in 1368 birds and mammals. (Estimates are modal estimates from 500 models. Lower CI = lower 95% confidence interval from 500 models. Upper CI = upper 95% confidence interval from 500 models. Posterior distribution = distribution of estimates from 500 models. Body mass : flight capability = interaction between body mass and flight capability. Notes: 24 000 000 iterations with 4 000 000 burn-in and thinning interval of 10 000.)
| estimate (β) | lower CI | upper CI | posterior distribution | |
|---|---|---|---|---|
| fixed terms | 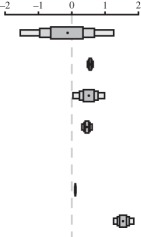 |
|||
| intercept | −0.145 | −1.544 | 1.260 | |
| body mass | 0.554 | 0.467 | 0.639 | |
| flight capability volant | 0.507 | 0.033 | 0.981 | |
| body mass: flight capability | 0.456 | 0.302 | 0.613 | |
| random terms | ||||
| residual variance | 0.107 | 0.090 | 0.127 | |
| phylogenetic variance | 1.542 | 1.264 | 1.871 | |
The relationships among our ecological variables and lifespan differed between the volant and non-volant subgroups. Within volant taxa, crepuscular species (i.e. those active at dusk and dawn) had significantly shorter lifespans than both diurnal and nocturnal species (table 2). By contrast, activity period was not associated with lifespan in non-volant species (table 3). Foraging environment did not influence lifespan significantly in volant species; bats and birds that forage on the ground do not have shorter lifespans than species that forage in the air or in trees (table 2). Within non-volant species, however, those foraging arboreally have longer lifespans than those foraging terrestrially, and fossorial (i.e. burrowing) species live longer than non-fossorial ones (table 3).
Table 2.
Relationship between maximum longevity (years), body mass (g), foraging environment and activity period in 662 volant birds and mammals. (Estimates are modal estimates from 500 models. Column heads explained same as given in table 1. Notes: 12 000 000 iterations with 2 000 000 burn-in and thinning interval of 5000.)
| estimate (β) | lower CI | upper CI | posterior distribution | |
|---|---|---|---|---|
| fixed terms | 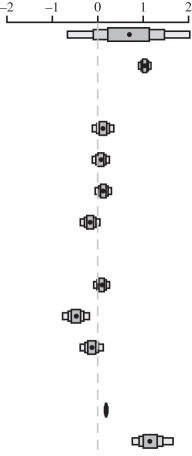 |
|||
| intercept | 0.668 | −0.664 | 2.028 | |
| body mass | 1.035 | 0.899 | 1.172 | |
| foraging environment | ||||
| aerial | 0.116 | −0.119 | 0.354 | |
| arboreal | 0.068 | −0.119 | 0.258 | |
| semi-arboreal | 0.124 | −0.056 | 0.301 | |
| aquatic | −0.166 | −0.383 | 0.049 | |
| activity period | ||||
| cathemeral | 0.085 | −0.088 | 0.261 | |
| crepuscular | −0.479 | −0.772 | −0.182 | |
| nocturnal | −0.131 | −0.385 | 0.122 | |
| random terms | ||||
| residual variance | 0.184 | 0.151 | 0.223 | |
| phylogenetic variance | 1.155 | 0.789 | 1.693 | |
Table 3.
Relationship between maximum longevity (years), body mass (g), foraging environment, fossoriality and activity period in 706 non-volant birds and mammals. (Estimates are modal estimates from 500 models. Column heads explained same as given in table 1. Notes: 24 000 000 iterations with 4 000 000 burn-in and thinning interval of 10 000.)
| estimate (β) | lower CI | upper CI | posterior distribution | |
|---|---|---|---|---|
| fixed terms | 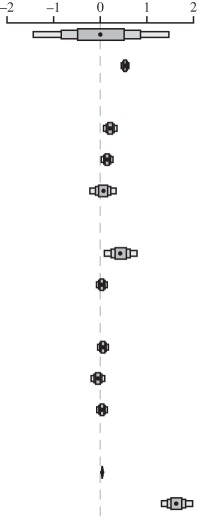 |
|||
| intercept | 0.013 | −1.433 | 1.467 | |
| body mass | 0.531 | 0.449 | 0.614 | |
| foraging environment | ||||
| arboreal | 0.213 | 0.070 | 0.358 | |
| semi-arboreal | 0.148 | 0.022 | 0.274 | |
| aquatic | 0.064 | −0.220 | 0.341 | |
| fossoriality | ||||
| fossorial | 0.437 | 0.088 | 0.785 | |
| semi-fossorial | 0.035 | −0.081 | 0.149 | |
| activity period | ||||
| cathemeral | 0.060 | −0.056 | 0.173 | |
| crepuscular | −0.050 | −0.194 | 0.096 | |
| nocturnal | 0.038 | −0.075 | 0.153 | |
| random terms | ||||
| residual variance | 0.042 | 0.031 | 0.059 | |
| phylogenetic variance | 1.627 | 1.319 | 1.985 | |
In the supplementary analysis with maximum longevity estimates based on 100 or more records, the models showed qualitatively comparable results to the findings in the main analysis (electronic supplementary material, appendix 3: tables A2–A4). When we included BMR as an additional linear covariate into models 1–3, the results showed similar general trends as those without BMR except with no significant effect of crepuscularity in volant species, no effect of semi-arboreality in non-volant species and a negative correlation between BMR and longevity in non-volant species (electronic supplementary material, appendix 3: tables A5–A7). We also repeated models 2 and 3 for birds and mammals (rather than volant and non-volant species) separately. The results were qualitatively identical apart from a predictable reduction in the phylogenetic residual term and also a lower combined DIC value for models 2 and 3 (modal volant and non-volant DIC = 1184) in comparison to a taxonomically split model (modal birds and mammals DIC = 1227) (electronic supplementary material, appendix 3: tables A8–A9). The phylogenetic residual term was high in all of our models (model 1: 1.542; model 2: 1.555; model 3: 1.627; tables 1–3) but was much lower in the taxonomically split bird and mammal models, as expected given their more restricted phylogenetic scope (birds: 0.371; mammals: 0.936; electronic supplementary material, appendix 3: table A10).
4. Discussion
As predicted, we found that volant species live longer than non-volant species of a similar body mass after accounting for phylogenetic relatedness. However, the effects of body mass on lifespan in the two groups differed: similar increases in body mass resulted in disproportionately greater increases in lifespan for volant compared with non-volant species. Additionally, after accounting for phylogeny and body size, the lifespans of volant species depended upon when species are active but not where that activity occurs, whereas the opposite was true for non-volant species. Thus, the longest-lived volant birds and mammals tended to be either diurnal or nocturnal, and the longest-lived non-volant species tended to be arboreal, semi-arboreal or fossorial.
The link between flight and long lifespan in vertebrates is well known. Among birds, flightless or weakly flying species (i.e. game birds) have the shortest lifespans [6,11,14]. Among mammals, bats live far longer than similar-sized non-volant mammals and gliding species also have greater lifespans than expected given their body mass [12]. This probably reflects the ability of flying species to escape sources of extrinsic mortality such as predation and unfavourable conditions, allowing greater investment in increased lifespan [11–13].
The relationships among our ecological variables and lifespan differed between the volant and non-volant subgroups, as expected given the different sources of extrinsic mortality they experience. Activity period was important in volant species, with crepuscular species possessing the shortest lifespans. This suggests that being crepuscular is a poor strategy for volant species, perhaps because they are exposed to both diurnal and nocturnal predators, resulting in higher extrinsic mortality. The scarcity of crepuscular volant species (n = 16) in our dataset also suggests that specialization to be active between nocturnal and diurnal periods is a relatively unsuccessful strategy. However, activity period was not related to lifespan in non-volant species, counter to our initial prediction that nocturnal, crepuscular and cathemeral species would be more long-lived, which assumed that diurnal species would be easier for predators to detect. However, there are many additional ways to avoid predation (see below) and many alternative reasons for becoming nocturnal, crepuscular or cathemeral. For example, many large mammals are crepuscular or cathemeral in order to avoid the intense heat of the day in tropical areas, while species such as wolves and hyenas may have become nocturnal to access more prey. Consequently, although nocturnality may decrease extrinsic mortality for some species, it may actually increase it for others.
Foraging environment did not influence lifespan significantly in volant species, but it was important in non-volant species where arboreal, semi-arboreal and fossorial species had longer lifespans than terrestrial species. The relationship between arboreality and extended lifespan in mammals has been shown previously [17], but to the best of our knowledge this is the first comparative analysis of lifespan in fossorial versus non-fossorial species. Our results may, therefore, offer a partial explanation for the exceptional longevity of naked mole rats (Heterocephalus glaber) which are completely fossorial and live 10 times longer than expected, given their body size [21].
Across all our models, body size was correlated strongly with lifespan, which is consistent with previous studies (e.g. [3,5,6]). However, our study is the first, to our knowledge, to demonstrate the general importance of body size in both volant and non-volant species concurrently (as opposed to traditional taxonomic groupings of birds, bats and terrestrial mammals separately). Large species are expected to live longer than small species, in part, because they should experience lower rates of extrinsic mortality and therefore invest more in long-term maintenance and survival. For example, large species are expected to suffer lower predation risk, and be better able to cope with temporary food shortages, climatic fluctuations and extreme weather compared with smaller species [15,39]. Also following from Peto's paradox, that cancer incidence does not correlate with body size despite the larger number of cells from which it can potentially develop [40], the increased lifespan afforded through decreased extrinsic mortality in large species can increase selection for molecular controls on senescence related diseases [11].
Previous studies generated similar slopes for the relationship between log lifespan and log body mass in birds (slope = 0.20) and mammals (slope = 0.22), but the bird slope had a much higher intercept [3,39]. By contrast, we found that, for a given increase in body mass, the lifespans of volant species (modal slope estimate (after converting from mean-centred values) = 0.25) increase significantly more than the lifespans of non-volant species (modal slope estimate (after converting from mean-centred values) = 0.13). This suggests that the similarities in bird and mammal slopes seen previously may have been the result of splitting species into taxonomic rather than ecological groups. The explanation for the steeper slope in volant species is unclear. However, we suggest that increasing body mass in volant species reduces their extrinsic mortality much more markedly than it does in non-volant species, favouring greater investment in long-term survival. Alternatively, this may reflect constraints on life-history optimization, particularly in bats, where the energetic and biomechanical requirements of flight restrict females to raising small litters [24]. This leaves few options for the optimization of life history in bats other than increasing lifespan, thereby increasing lifetime opportunities for reproduction.
For birds and mammals, the most important factor in reducing extrinsic mortality and allowing investment in a longer lifespan, after controlling for body size and phylogeny, is the ability to fly. This effect is so strong that it overshadows all other ecological variables explaining lifespan variation in volant species. However, there is still residual variation in all of our models. Much of this is related to phylogeny, particularly in volant species because this group contains a fairly even number of mammals (bats) and birds. On the other hand, owing to the paucity of lifespan data resulting in so few non-volant birds in our dataset (n = 10), the phylogenetic residuals in the non-volant model are unlikely to be attributed to the inclusion of birds species in the mammal dominated non-volant models. But even accounting for phylogeny (as we do in all our models), there is still substantial unexplained variation in the model for volant species (residual error = 0.18 in the model for volant species compared with 0.042 in the model for non-volant species; tables 2 and 3). We anticipated such unexplained variation would be related to bats because of their reputed extreme lifespans [14], yet, of the 32 species with the top 5% of residuals in our models of maximum lifespan against body size, foraging environment and activity period, less than half are bats. Instead, flying foxes (Pteropodidae) are scattered among the birds and fit the volant species regression line well, as do most microchiropteran bats. This suggests that the prolonged lifespans of most bats are not exceptional, given their volant mode-of-life. In our models, the exceptionally long-lived bats are mostly Vespertilionidae (Barbastella barbastellus, Myotis blythii, Myotis brandtii, Myotis daubentonii, Myotis evotis, Myotis lucifugus, Myotis myotis, Myotis mystacinus, Myotis nattereri, Myotis volans, Plecotus auritus and Plecotus austriacus) plus Desmodus rotundus, Rhinolophus ferrumequinum and Rhinolophus hipposideros. However, the species in the top 5% of residuals also include pelagic seabirds (Alca torda, Uria aalge, Pelecanus erythrorhynchos, Pelecanus occidentalis, Pelecanus onocrotalus, Diomedea epomophora, Diomedea exulans, Phoebastria immutabilis, Phoebastria nigripes, Thalassarche chrysostoma, Thalassarche melanophrys, Fulmarus glacialis, Puffinus puffinus, Fregata minor and Haliaeetus albicilla), parrots (Cacatua leadbeateri), flamingos (Phoenicopterus roseus), swans (Cygnus olor) and even diving ducks (Aythya fuligula). The mechanisms underlying the exceptional lifespans of these species are likely to be idiosyncratic, given the broad array of ecologies these birds exhibit. Thus, if we want to uncover the secrets of long life, we may need to expand our search beyond bats and naked mole rats [8,41]. When studying the molecular basis behind cellular processes such as those underlying longevity, it is important to consider the selection pressures that shaped them over evolutionary time.
Acknowledgements
We thank the members of NERD club for insightful discussions, Gavin Thomas for assistance with the bird phylogeny, Luke McNally for suggestions on the analysis and the anonymous reviewers for their helpful suggestions.
Data accessibility
All data used in these analyses are available in the electronic supplementary material, appendix 2.
Funding statement
Funding was provided by the Earth and Natural Sciences (ENS) Doctoral Studies Programme, funded by the Higher Education Authority (HEA) through the Programme for Research at Third Level Institutions, Cycle 5 (PRTLI-5), co-funded by the European Regional Development Fund (ERDF) (K.H.); IRC Embark Initiative Postgraduate Scholarship (S.F.); Trinity College Dublin (T.G., A.K., S.K.), the EU INTERREG IVA Cross-border Programme-funded DOLMANT Project (ref. no: 002862; D.M.) and European Commission CORDIS Seventh Framework Programme (FP7) Marie Curie CIG grant (proposal no: 321696; N.C.). All calculations were performed on the Lonsdale cluster maintained by the Trinity Centre for High Performance Computing. This cluster was funded through grants from Science Foundation Ireland.
References
- 1.de Magalhães JP, Costa J. 2009. A database of vertebrate longevity records and their relation to other life-history traits. J. Evol. Biol. 22, 1770–1774. ( 10.1111/j.1420-9101.2009.01783.x) [DOI] [PubMed] [Google Scholar]
- 2.Depczynski M, Bellwood DR. 2005. Shortest recorded vertebrate lifespan found in a coral reef fish. Curr. Biol. 15, R288–R289. ( 10.1016/j.cub.2005.04.016) [DOI] [PubMed] [Google Scholar]
- 3.Lindstedt S, Calder W., III 1981. Body size, physiological time, and longevity of homeothermic animals. Q. Rev. Biol. 56, 1–16. ( 10.1086/412080) [DOI] [Google Scholar]
- 4.Promislow DEL. 1993. On size and survival: progress and pitfalls in the allometry of life span. J. Gerontol. 48, B115–B123. ( 10.1093/geronj/48.4.B115) [DOI] [PubMed] [Google Scholar]
- 5.de Magalhães JP, Costa J, Church GM. 2007. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J. Gerontol. Series A: Biol. Sci. Med. Sci. 62, 149–160. ( 10.1093/gerona/62.2.149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricklefs RE. 2010. Life-history connections to rates of aging in terrestrial vertebrates. Proc. Natl Acad. Sci. USA 107, 10 314–10 319. ( 10.1073/pnas.1005862107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricklefs RE. 2010. Insights from comparative analyses of aging in birds and mammals. Aging Cell 9, 273–284. ( 10.1111/j.1474-9726.2009.00542.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang G, et al. 2013. Comparative analysis of bat genomes provides insight into the evolution of flight and immunity. Science 339, 456–460. ( 10.1126/science.1230835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.Williams GC. 1957. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411. ( 10.2307/2406060) [DOI] [Google Scholar]
- 11.Partridge L, Barton NH. 1993. Optimality, mutation and the evolution of ageing. Nature 362, 305–311. ( 10.1038/362305a0) [DOI] [PubMed] [Google Scholar]
- 12.Holmes DJ, Austad SN. 1994. Fly now, die later: life-history correlates of gliding and flying in mammals. J. Mammal. 75, 224–226. ( 10.2307/1382255) [DOI] [Google Scholar]
- 13.Pomeroy D. 1990. Why fly? The possible benefits for lower mortality. Biol. J. Linnean Soc. 40, 53–65. ( 10.1111/j.1095-8312.1990.tb00534.x) [DOI] [Google Scholar]
- 14.Wilkinson GS, South JM. 2002. Life history, ecology and longevity in bats. Aging Cell 1, 124–131. ( 10.1046/j.1474-9728.2002.00020.x) [DOI] [PubMed] [Google Scholar]
- 15.Austad SN, Fischer KE. 1991. Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. J. Gerontol. 46, B47–B53. ( 10.1093/geronj/46.2.B47) [DOI] [PubMed] [Google Scholar]
- 16.Holmes D, Ottinger M. 2003. Birds as long-lived animal models for the study of aging. Exp. Gerontol. 38, 1365–1375. ( 10.1016/j.exger.2003.10.018) [DOI] [PubMed] [Google Scholar]
- 17.Shattuck MR, Williams SA. 2010. Arboreality has allowed for the evolution of increased longevity in mammals. Proc. Natl Acad. Sci. USA 107, 4635–4639. ( 10.1073/pnas.0911439107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvey PH, Pagel MD. 1991. The comparative method in evolutionary biology. Oxford, UK: Oxford University Press. [Google Scholar]
- 19.Promislow DEL, Harvey PH. 1990. Living fast and dying young: a comparative analysis of life-history variation among mammals. J. Zool. 220, 417–437. ( 10.1111/j.1469-7998.1990.tb04316.x) [DOI] [Google Scholar]
- 20.Byrnes G, Spence AJ. 2011. Ecological and biomechanical insights into the evolution of gliding in mammals. Integr. Comp. Biol. 51, 991–1001. ( 10.1093/icb/icr069) [DOI] [PubMed] [Google Scholar]
- 21.Buffenstein R, Jarvis JUM. 2002. The naked mole rat: a new record for the oldest living rodent. Sci. Aging Knowl. Environ. 2002, pe7 ( 10.1126/sageke.2002.21.pe7) [DOI] [PubMed] [Google Scholar]
- 22.Tacutu R, Craig T, Budovsky A, Wuttke D, Lehmann G, Taranukha D, Costa J, Fraifeld VE, de Magalhães JP. 2013. Human ageing genomic resources: integrated databases and tools for the biology and genetics of ageing. Nucleic Acids Res. 41, D1027–D1033. ( 10.1093/nar/gks1155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricklefs RE, Scheuerlein A. 2001. Comparison of aging-related mortality among birds and mammals. Exp. Gerontol. 36, 845–857. ( 10.1016/S0531-5565(00)00245-X) [DOI] [PubMed] [Google Scholar]
- 24.Nowak RM. 1999. Walker‘s mammals of the world. Baltimore, MD: The Johns Hopkins University Press. [Google Scholar]
- 25.del Hoyo J, Elliott A, Sargatal J. 1992. Handbook of the birds of the world, vol. 1: ostrich to ducks. Barcelona, Spain: Lynx Edicions. [Google Scholar]
- 26.Cramp S. 1977. Handbook of the birds of Europe, the Middle East, and North Africa: the birds of the Western Palearctic, vol. 1, ostrich to ducks. Oxford, UK: Oxford University Press. [Google Scholar]
- 27.Fry HC, Fry K. 2010. Kingfishers, bee-eaters and rollers: a handbook. London, UK: A & C Black. [Google Scholar]
- 28.Juniper T, Parr M. 2003. Parrots: a guide to parrots of the world. London, UK: A&C Black. [Google Scholar]
- 29.Williams TD. 1995. The penguins: Spheniscidae. Oxford, UK: Oxford University Press. [Google Scholar]
- 30.Wilson DE, Reeder DAM. 2005. Mammal species of the world: a taxonomic and geographic reference, 3rd edn Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 31.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444–448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 32.Kuhn TS, Mooers AØ, Thomas GH. 2011. A simple polytomy resolver for dated phylogenies. Methods Ecol. Evol. 2, 427–436. ( 10.1111/j.2041-210X.2011.00103.x) [DOI] [Google Scholar]
- 33.Bininda-Emonds ORP, et al. 2007. The delayed rise of present-day mammals. Nature 446, 507–512. ( 10.1038/nature05634) [DOI] [PubMed] [Google Scholar]
- 34.Reisz RR, Müller J. 2004. Molecular timescales and the fossil record: a paleontological perspective. Trends Genet. 20, 237–241. ( 10.1016/j.tig.2004.03.007) [DOI] [PubMed] [Google Scholar]
- 35.R Development Core Team. 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 36.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22.20808728 [Google Scholar]
- 37.Plummer M, Best N, Cowles K, Vines K. 2006. CODA: convergence diagnosis and output analysis for MCMC. R News 6, 7–11. [Google Scholar]
- 38.Gelman A, Rubin DB. 1992. Inference from iterative simulation using multiple sequences. Statist. Sci. 7, 457–472. ( 10.1214/ss/1177011136) [DOI] [Google Scholar]
- 39.Hulbert A, Pamplona R, Buffenstein R, Buttemer W. 2007. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol. Rev. 87, 1175–1213. ( 10.1152/physrev.00047.2006) [DOI] [PubMed] [Google Scholar]
- 40.Peto R, Roe F, Lee P, Levy L, Clack J. 1975. Cancer and ageing in mice and men. Br. J. Cancer 32, 411–426. ( 10.1038/bjc.1975.242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian X, et al. 2013. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 499, 346–349. ( 10.1038/nature12234) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in these analyses are available in the electronic supplementary material, appendix 2.


